Abstract
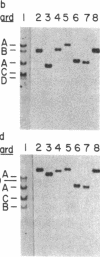
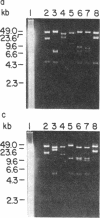
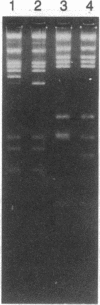
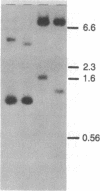
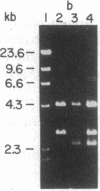
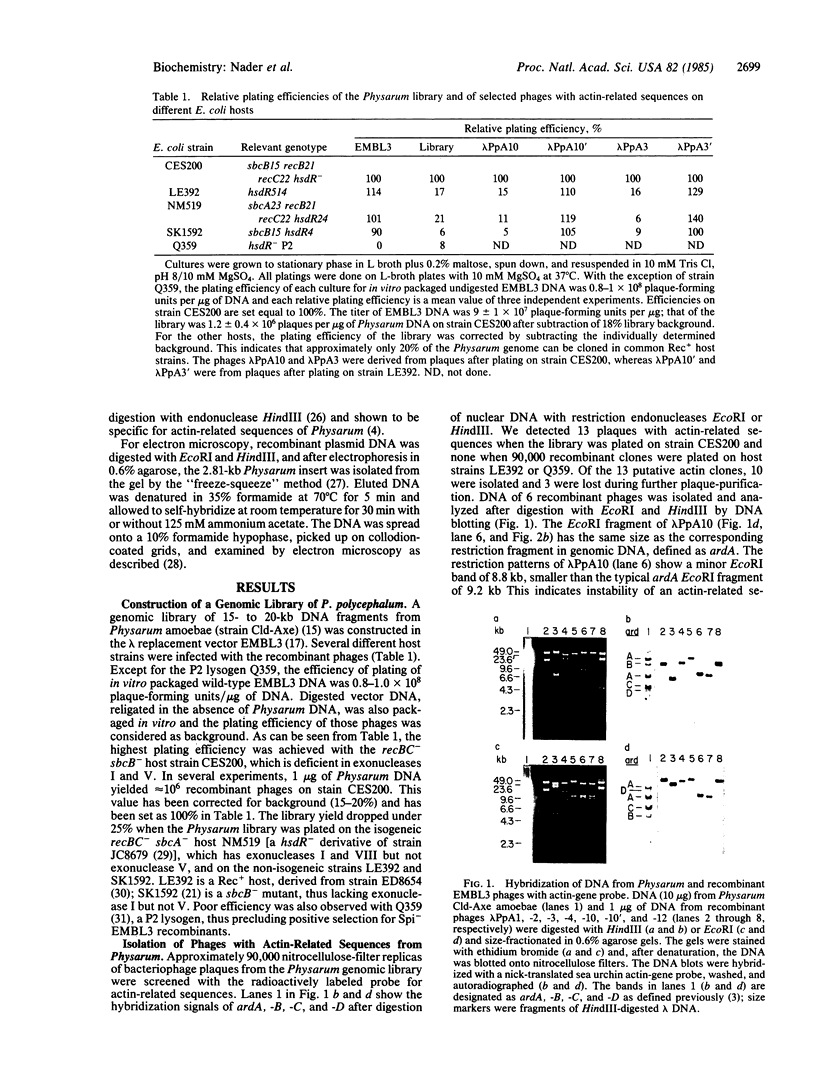
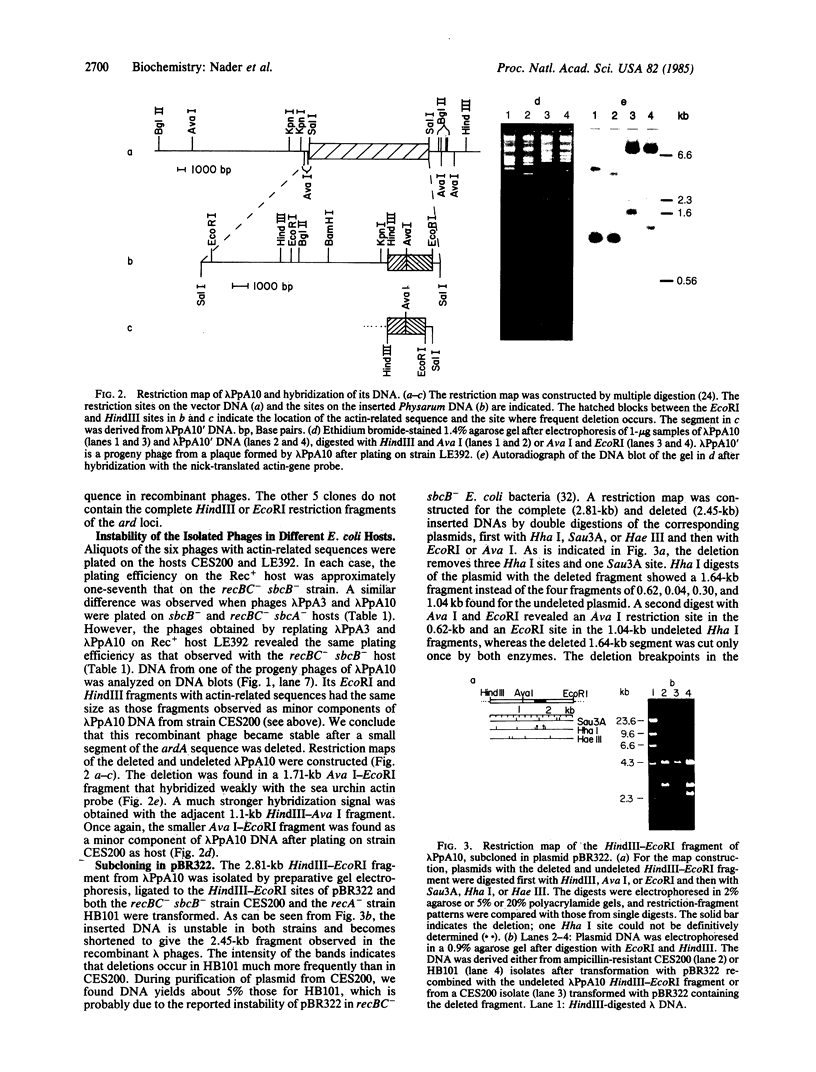
A genomic library of Physarum was constructed in the replacement vector EMBL3. Efficient propagation of the recombinant phages occurred only on the recBC-sbcB- host Escherichia coli CES200, which is deficient in the exonucleases I and V. Thirteen different recombinants with actin-related sequences were detected and 10 were purified from 90,000 plaques (the equivalent of 6 Physarum genomes) on strain CES200. Comparison of the plating efficiencies of the library and the actin-related isolates suggests that palindromic DNA sequences are responsible for the instability of Physarum DNA in E. coli. In one of these isolates, lambda PpA10, and in a 2.81-kilobase subclone of that isolate in plasmid pBR322, a deletion of 360 base pairs was detected that led to stable propagation of the recombinant DNA molecules in Rec+ E. coli. Electron microscopic analysis of the 2.81-kilobase fragment, after denaturation and self-hybridization, revealed secondary structures consistent with "foldback" structures. Restriction and DNA blot analysis of lambda PpA10 suggest that the unstable DNA segment is in close proximity to, if not part of, the previously defined actin-gene locus ardA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Progress toward a metabolic interpretation of genetic recombination of Escherichia coli and bacteriophage lambda. Genetics. 1974 Sep;78(1):259–271. doi: 10.1093/genetics/78.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Volckaert G., Nevers P. Precise and nearly-precise excision of the symmetrical inverted repeats of Tn5; common features of recA-independent deletion events in Escherichia coli. Gene. 1982 Jul-Aug;19(1):139–146. doi: 10.1016/0378-1119(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Dott P. J., Chuang C. R., Saunders G. F. Inverted repetitive sequences in the human genome. Biochemistry. 1976 Sep 7;15(18):4120–4125. doi: 10.1021/bi00663a032. [DOI] [PubMed] [Google Scholar]
- Durica D. S., Schloss J. A., Crain W. R., Jr Organization of actin gene sequences in the sea urchin: molecular cloning of an intron-containing DNA sequence coding for a cytoplasmic actin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5683–5687. doi: 10.1073/pnas.77.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlind T. D., Ihler G. M. Electron microscopic mapping of complementary sequences on single strands of bacteriophage lambda DNA. J Mol Biol. 1982 Sep;160(1):23–39. doi: 10.1016/0022-2836(82)90129-2. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gerrie L. M., Humphreys J., Peoples O. P., Hardman N. Sequence organisation in nuclear DNA from Physarum polycephalum. Arrangement of highly-repeated sequences. Biochim Biophys Acta. 1983 Nov 17;741(2):214–223. doi: 10.1016/0167-4781(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Hardman N., Jack P. L., Brown A. J., McLachlan A. Distribution of inverted repeat sequences in nuclear DNA from Physarum polycephalum. Eur J Biochem. 1979 Feb 15;94(1):179–187. doi: 10.1111/j.1432-1033.1979.tb12884.x. [DOI] [PubMed] [Google Scholar]
- Hardman N., Jack P. L., Fergie R. C., Gerrie L. M. Sequence organisation in nuclear DNA from Physarum polycephalum. Interspersion of repetitive and single-copy sequences. Eur J Biochem. 1980 Jan;103(2):247–257. doi: 10.1111/j.1432-1033.1980.tb04309.x. [DOI] [PubMed] [Google Scholar]
- Hardman N., Jack P. L. Periodic organisation of foldback sequences in Physarum polycephalum nuclear DNA. Nucleic Acids Res. 1978 Jul;5(7):2405–2423. doi: 10.1093/nar/5.7.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Leach D. R., Stahl F. W. Viability of lambda phages carrying a perfect palindrome in the absence of recombination nucleases. 1983 Sep 29-Oct 5Nature. 305(5933):448–451. doi: 10.1038/305448a0. [DOI] [PubMed] [Google Scholar]
- Liu L. S., Lark K. G. The Red function of phage lambda mediates the alteration of an interspersed repeated DNA sequence from the kangaroo rat Dipodomys ordii. Mol Gen Genet. 1982;188(1):27–36. doi: 10.1007/BF00332992. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Mohberg J. Nuclear DNA content and chromosome numbers throughout the life cycle of the Colonia strain of the myxomycete, Physarum polycephalum. J Cell Sci. 1977 Apr;24:95–108. doi: 10.1242/jcs.24.1.95. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Burck K. B., Davidson N. Sequence arrangement and biological activity of cloned feline leukemia virus proviruses from a virus-productive human cell line. J Virol. 1981 May;38(2):688–703. doi: 10.1128/jvi.38.2.688-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Peoples O. P., Robinson A. C., Whittaker P. A., Hardman N. Sequence organisation in nuclear DNA from Physarum polycephalum. Genomic organisation of DNA segments containing foldback sequences. Biochim Biophys Acta. 1983 Nov 17;741(2):204–213. doi: 10.1016/0167-4781(83)90060-x. [DOI] [PubMed] [Google Scholar]
- Pierron G., Durica D. S., Sauer H. W. Invariant temporal order of replication of the four actin gene loci during the naturally synchronous mitotic cycles of Physarum polycephalum. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6393–6397. doi: 10.1073/pnas.81.20.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schedl T., Dove W. F. Mendelian analysis of the organization of actin sequences in Physarum polycephalum. J Mol Biol. 1982 Sep;160(1):41–57. doi: 10.1016/0022-2836(82)90130-9. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Manning J. E., Davidson N. Inverted repeat sequences in the Drosophila genome. Cell. 1975 Jun;5(2):159–172. doi: 10.1016/0092-8674(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Wilhelm M. L., Wilhelm F. X. A transposon-like DNA fragment interrupts a Physarum polycephalum histone H4 gene. FEBS Lett. 1984 Mar 26;168(2):249–254. doi: 10.1016/0014-5793(84)80256-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]