Abstract
Background
Transforming growth factor-beta 1(TGF-β1) is involved in the development of acute rejection (AR) episodes in solid organ transplant recipients; and a number of studies have been conducted to investigate the combined effects of human TGF-β1 gene (TGFB1) +869 T/C and +915 G/C polymorphisms on AR risk. However, the results obtained are inconclusive.
Methods
Eligible studies that investigated the haplotypic association between TGFB1 +869 T/C and +915 G/C polymorphisms and AR risk were comprehensively searched in the PUBMED, EMBASE, China National Knowledge Infrastructure, and Wanfang Database. Statistical analyses were performed by using STATA 12.0 and Review Manager 5.0.
Results
Fourteen eligible studies with 565 AR cases and 1219 non-AR cases were included. Overall, a significantly decreased risk was detected in patients carried with intermediate producer (IP) haplotypes (T/C G/C, T/T G/C, and C/C G/G) and/or low producer (LP) haplotypes (C/C G/C, C/C C/C, T/T C/C, and T/C C/C) compared with high producer (HP) haplotypes (T/T G/G and T/C G/G; IP vs. HP: OR = 0.75, 95% CI, 0.58–0.96, P heterogeneity = 0.238; IP/LP vs. HP: OR = 0.77, 95% CI, 0.61–0.98, P heterogeneity = 0.144). In addition, subgroup analysis by transplant types demonstrated a similar association in patients receiving heart transplant (IP vs. HP: OR = 0.32, 95% CI, 0.14–0.73, P heterogeneity = 0.790; IP/LP vs. HP: OR = 0.41, 95% CI, 0.20–0.85, P heterogeneity = 0.320).
Conclusions
The current meta-analysis and systematic review indicated that recipient TGFB1 HP haplotypes were significantly associated with an increased risk for AR in solid organ transplant recipients, particularly patients receiving cardiac allograft.
Introduction
Transforming growth factor-beta 1 (TGF-β1) is a multifunctional cytokine ubiquitously produced by a wide variety of cells, including T lymphocytes, monocytes, vascular endothelium, and renal tubular cells [1]. Functionally, TGF-β1 has been proven to be of fundamental importance in the development of various disorders [2], including coronary heart disease [3], human cancers [4], rheumatoid arthritis [5], asthma [6] and transplant rejection [7], [8]. In the setting of solid organ transplants, TGF-β1 has been conventionally recognized as a guardian against acute rejection (AR), as higher level of TGF-β1 in the graft tissue and serum was found in non-AR recipients than those suffering AR [9]–[11]. However, several novel lines of evidence have challenged the beneficial effects of TGF-β1 on transplant recipients [12], [13]. Although the functional role of TGF-β1 in AR initiation remains elusive, this cytokine is believed to exert pivotal and complicated functions in AR episodes.
The human TGF-β1 gene (TGFB1) is mapped on the chromosome 19q13.1–13.3 with seven exons and six introns, whose regulation and expression is influenced by the presence of single nucleotide polymorphisms (SNPs) [14]. Among these SNPs, +869 T/C (also known as rs1800470, T29C, or Leu10Pro) and +915 G/C (also termed as rs1800471, G74C, or Arg25Pro) polymorphisms in the first exon of TGFB1 have been the focus of extensive researches and donor TGFB1 +869 T/C polymorphism has been proven to be significantly associated with AR risk [15]. These two SNPs together result in nine potential inherited haplotypes, which could be categorized into three groups according to the in vitro production levels: high producer (HP) (T/T G/G and T/C G/G), intermediate producer (IP) (T/C G/C, T/T G/C, and C/C G/G) and low producer (LP) (C/C G/C, C/C C/C, T/T C/C and T/C C/C) [16], [17]. Since the first study conducted by Pelletier et al to evaluate the haplotypic association of TGFB1 +869 T/C and +915 G/C polymorphisms with AR risk in kidney transplant recipients [18], numerous molecular epidemiological studies have been conducted in different solid organ transplants, including kidney transplants [19]–[26], liver transplants [9], [27], [28], and heart transplants [13], [29]. However, the results of these studies were inconclusive.
In this meta-analysis, we integrated the data from all eligible studies to explore 1) the combined effects of recipient TGFB1 +869 T/C and +915 G/C polymorphisms on AR risk after solid organ transplantation and 2) the potential influence of covariants such as ethnicity, transplantation types, and immunosuppressive protocols.
Materials and Methods
Identification of eligible studies
This meta-analysis was conducted and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Checklist S1) [30]. To identify all eligible studies that investigated the haplotypic association of TGFB1 +869 T/C and +915 G/C polymorphisms with AR risk in solid organ transplantation, a comprehensive electronic search of PUBMED, EMBASE, China National Knowledge Infrastructure (CNKI), and Wanfang databases was performed until November 29, 2013. To search and include as many related studies as possible, we applied various combinations of the following medical subject headings and key words: transforming growth factor beta-1, TGFβ-1, or TGFB1; acute rejection, early allograft outcome, or graft rejection; transplantation or organ transplantation; polymorphisms or variants. Furthermore, the reference lists of reviews and retrieved articles were manually screened for additional studies No restrictions were placed on language, and only published studies with full-text articles were included.
Inclusion and exclusion criteria
The studies identified from the above mentioned databases were screened by two independent authors (Yu-Zheng Ge and Ran Wu) according to the following predesigned inclusion criteria: 1) case-control design; 2) evaluating the correlation of TGFB1 haplotypes (at position +869 and +915) with AR risk; and 3) providing sufficient data to calculate the odds ratio (OR) and its corresponding 95% confidence interval (CI). When several studies with overlapping data were eligible, those with smaller sample size or less reliability were excluded. In addition, studies without detailed information were excluded, after the efforts to extract data from the original paper or contact the corresponding authors failed.
Data extraction
Data of eligible studies were extracted by two reviewers (Yu-Zheng Ge and Tian-Ze Lu) independently and in duplicate according to the predesigned data-collection form. The following information was extracted: last name of first author, publication year, country of origin, ethnicity, transplantation type, immunosuppressive protocol, number of both ARs and non-ARs and phenotypic distribution in both groups. Different ethnic descents were categorized as Asian, Caucasian, African and Mixed (which included more than one ethnic descent). Transplantation types were characterized as renal, liver, and heart transplantation. Based on different calcineurin inhibitors (CNIs) within the immunosuppressive protocol, the studies were divided into two subgroups: Cyclosporine A (CsA) group and CsA/tacrolimus (FK506) group. Discrepancies occurring during the process of study inclusion and data extraction were resolved by discussion with a third reviewer (Wen-Cheng Li), and consensus on each item was achieved eventually.
Statistical analysis
Crude OR and its corresponding 95% CI were used to assess the strength of association between the haplotypes of +869 T/C and +915 G/C polymorphisms in TGFB1 gene and AR risk. The HP haplotypes (T/T G/G and T/C G/G) were used as the baseline for calculation of ORs in three different comparisons (LP vs. HP, IP vs. HP and LP/IP vs. HP). Stratified analyses were also conducted based on ethnicity, transplant types, and immunosuppressive protocols. The statistical significance of the pooled OR was assessed with the Z test, and P<0.05 was considered significant.
Chi-square based Q-test was performed to measure between-study heterogeneity, and the presence of heterogeneity was considered significant if P<0.10 [31]. When the heterogeneity was absent, the fixed-effect model (Mantel-Haenszel method) was used to pool the data from different studies [32]; otherwise, the random-effects model (DerSimonian and Laird method) was applied [33]. Sensitivity analyses were conducted to identify the effect of individual study on pooled results and to test the reliability of results by deleting a single study each time [34]. To determine the presence of publication bias, both Begg's funnel plot and Egger's linear regression test were conducted, and P<0.05 was considered significant [35], [36].
All statistical analyses were performed with STATA software (version 12.0; Stata Corporation, College Station, Texas, USA) and Review Manager (version 5.0; Cochrane Collaboration, Oxford, UK).
Results
Characteristics of eligible studies
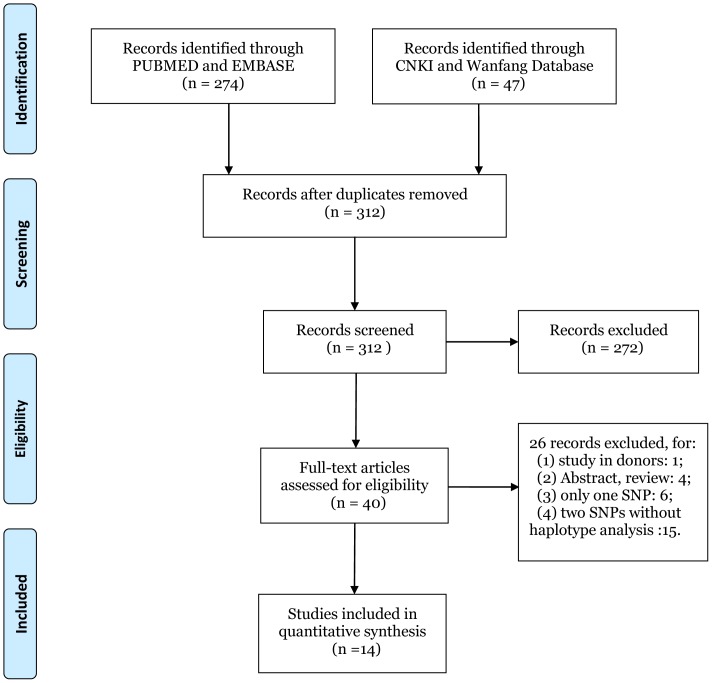
Fourteen eligible articles were identified according to the predesigned selection criteria, and the detailed screening process was shown in Figure 1. Among the 14 eligible studies, 9 were renal transplantation[18]–[26], 3 were liver transplantation [9], [27], [28], and 2 were heart transplantation[13], [29]. As for ethnicity, 7 were studies of Caucasians [9], [22], [23], [27], [28], [37], 3 studies were of Asians [19]–[21], and 4 studies of mixed ethnicity [13], [18], [26], [29]. Regarding the immunosuppressive protocols, patients were uniformly prescribed with CsA in 5 studies[18]–[20], [23], [29] while in the remaining 9 studies were applied with either CsA or FK506[9], [13], [21], [22], [24]–[28] (Table 1).
Figure 1. Flow diagram for study selection.
Description: A total of 14 studies were included in this meta-analysis and systematic review after a comprehensive study selection.
Table 1. Characteristics and haplotypes distribution of eligible studies included in the meta-analysis.
| Author | Year | Country | Ethnicity | Transplant types | CNIs | AR/non-AR | AR cases | Non-AR | ||||
| HP | IP | LP | HP | IP | LP | |||||||
| Pelletier | 2000 | USA | Mixed | RTx | CsA | 25/46 | 7 | 17 | 1 | 15 | 28 | 3 |
| Tambur | 2001 | USA | Caucasian | LTx | CsA/FK506 | 33/30 | 24 | 8 | 1 | 16 | 12 | 2 |
| Tian | 2001 | China | Asian | RTx | CsA | 19/72 | 16 | 3 | 0 | 56 | 16 | 0 |
| Tian | 2002 | China | Asian | RTx | CsA | 26/89 | 23 | 3 | 0 | 68 | 21 | 0 |
| Gu | 2003 | China | Asian | RTx | CsA/FK506 | 23/74 | 19 | 4 | 0 | 66 | 7 | 1 |
| Karasu | 2004 | Turkey | Caucasian | LTx | CsA/FK506 | 26/17 | 19 | 7 | 0 | 11 | 6 | 0 |
| Gourley | 2004 | USA | Mixed | HTx | CsA | 42/50 | 32 | 6 | 4 | 32 | 17 | 1 |
| Lacha | 2005 | Czech | Caucasian | RTx | CsA/FK506 | 117/228 | 33 | 65 | 19 | 82 | 121 | 25 |
| Di Filippo | 2006 | USA | Mixed | HTx | CsA/FK506 | 39/72 | 36 | 3 | 0 | 54 | 16 | 2 |
| Gómez-Mateo | 2006 | Spain | Caucasian | LTx | CsA/FK506 | 42/108 | 32 | 9 | 1 | 74 | 27 | 7 |
| Canossi | 2007 | Italy | Caucasian | RTx | CsA | 25/61 | 19 | 5 | 1 | 37 | 21 | 3 |
| Kocierz | 2011 | Poland | Caucasian | RTx | CsA/FK506 | 49/150 | 43 | 5 | 1 | 113 | 32 | 5 |
| Seyhun | 2012 | Turkey | Caucasian | RTx | CsA/FK506 | 19/71 | 14 | 4 | 1 | 39 | 17 | 15 |
| Dhaouadi | 2013 | Tunisia | Mixed | RTx | CsA/FK506 | 80/151 | 55 | 17 | 8 | 105 | 38 | 8 |
Abbreviations: RTx, renal transplantation; LTx, liver transplantation; HTx, heart transplantation; CNIs, calcineurin inhibitors; CsA, cyclosporine A; FK506, tacrolimus; AR, acute rejection; LP, low producer; IP, intermediate producer; HP, high producer.
Quantitative data synthesis
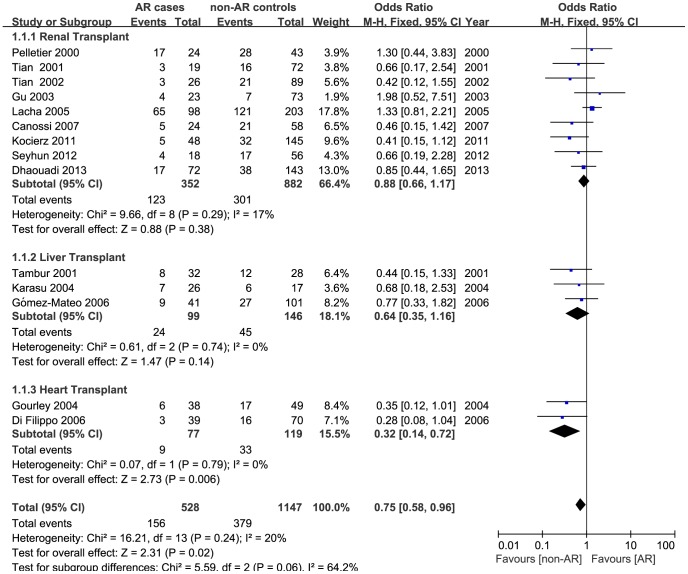
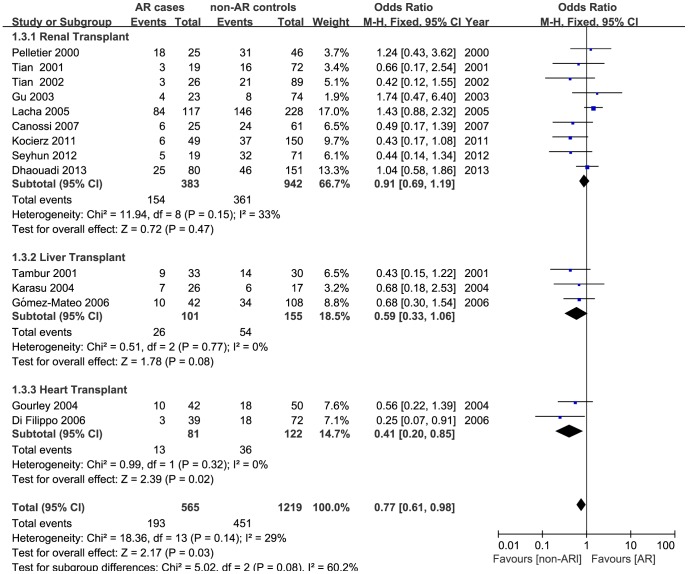
A total of 14 studies including 565 AR and 1219 non-AR cases were identified to assess the haplotypic association of TGFB1 +869 T/C and +915 G/C polymorphisms with AR risk of solid organ transplantation. Overall, a significantly decreased risk was detected in two comparisons (IP vs. HP: OR = 0.75, 95% CI, 0.58–0.96, P heterogeneity = 0.238, Figure 2; IP/LP vs. HP: OR = 0.77, 95% CI, 0.61–0.98, P heterogeneity = 0.144, Figure 3). Subgroup analyses based on ethnicity, transplant types, and immunosuppressive protocols were subsequently conducted, and the results demonstrated a remarkably decreased risk in heart transplant recipients (IP vs. HP: OR = 0.32, 95% CI, 0.14–0.73, P heterogeneity = 0.790, Figure 2; IP/LP vs. HP: OR = 0.41, 95% CI, 0.20–0.85, P heterogeneity = 0.320, Figure 3) and in patients uniformly administrated with CsA (IP vs. HP: OR = 0.57, 95% CI, 0.34–0.94, P heterogeneity = 0.491). Table 2 represents the strength of association between TGFB1 haplotypes (at position +869 and +915) and AR risk in transplant recipients.
Figure 2. Forest plot for risk of acute rejection associated with TGFB1 haplotypes (IP vs. HP) stratified by transplant types.
For each study, the estimate of OR and its 95% CI is plotted with a box and a horizontal line. Filled diamond pooled OR and its 95% CI.
Figure 3. Forest plot for risk of acute rejection associated with TGFB1 haplotypes (LP/IP vs. HP) stratified by transplant types.
For each study, the estimate of OR and its 95% CI is plotted with a box and a horizontal line. Filled diamond pooled OR and its 95% CI.
Table 2. Stratification analyses of combined effects of TGFB1 +869 T/C and +915 G/C polymorphisms on acute rejection risk.
| Category | LP vs. HP | IP vs. HP | LP/IP vs. HP | |||||||||
| Na | OR(95% CI) | Pb | Pc | Na | OR(95% CI) | Pb | Pc | Na | OR(95% CI) | Pb | Pc | |
| Total | 11 | 1.10(0.71,1.71) | 0.665 | 0.373 | 14 | 0.75(0.58,0.96) d | 0.021 d | 0.238 | 14 | 0.77(0.61,0.98) d | 0.030 d | 0.144 |
| Ethnicity | ||||||||||||
| Asian | 1 | 1.14(0.05,29.0) | 0.938 | — | 3 | 0.76(0.36,1.60) | 0.466 | 0.242 | 3 | 0.74(0.35,1.55) | 0.425 | 0.303 |
| Caucasian | 6 | 0.93(0.54,1.61) | 0.807 | 0.165 | 7 | 0.80(0.58,1.10) | 0.174 | 0.251 | 7 | 0.78(0.58,1.06) | 0.112 | 0.094 |
| Mixed | 4 | 1.58(0.72,3.49) | 0.258 | 0.507 | 4 | 0.65(0.42,1.03) | 0.064 | 0.163 | 4 | 0.77(0.51,1.16) | 0.214 | 0.160 |
| Transplantation Types | ||||||||||||
| RTx | 7 | 1.23(0.75,2.01) | 0.406 | 0.401 | 9 | 0.88(0.66,1.17) | 0.377 | 0.290 | 9 | 0.91(0.69,1.19) | 0.473 | 0.154 |
| LTx | 2 | 0.33(0.07,1.69) | 0.185 | 0.996 | 3 | 0.64(0.35,1.16) | 0.142 | 0.737 | 3 | 0.59(0.33,1.06) | 0.076 | 0.774 |
| HTx | 2 | 1.50(0.34,6.57) | 0.594 | 0.180 | 2 | 0.32(0.14,0.73) d | 0.006 d | 0.790 | 2 | 0.41(0.20,0.85) d | 0.017 d | 0.320 |
| Immunosuppressive protocol | ||||||||||||
| CsA | 3 | 1.37(0.41,4.59) | 0.607 | 0.462 | 5 | 0.57(0.34,0.94) d | 0.027 d | 0.491 | 5 | 0.62(0.38,1.01) | 0.055 | 0.694 |
| CsA/FK506 | 8 | 1.07(0.66,1.71) | 0.793 | 0.229 | 9 | 0.82(0.62,1.09) | 0.169 | 0.194 | 9 | 0.83(0.64,1.08) | 0.162 | 0.059 |
Abbreviations: RTx, renal transplantation; LTx, liver transplantation; HTx, heart transplantation; CsA, cyclosporine A; FK506, tacrolimus; LP, low producer; IP, intermediate producer; HP, high producer.
—, cannot be calculated;
Number of eligible studies;
P value of Z-test for significance of OR;
P value of Q-test for heterogeneity test;
Statistically significant results (in bold).
Heterogeneity test and sensitivity analysis
No significant between-study heterogeneity was observed in this meta-analysis (Table 2). Sensitivity analysis was also performed to explore the potential influence of each individual study on the overall results by deleting one single study each time from the pooled analysis. NO substantial change was demonstrated in the overall studies, indicating that no individual study could affect the pooled OR significantly (data not shown).
Publication bias
To examine the publication bias of the currently available literature, both Begg's funnel plot and Egger's test were conducted. The shape of the funnel plots did not reveal any evidence of obvious asymmetry in all comparison models (Figures S1, S2, S3). Then, the Egger's test was used to provide statistical evidence for funnel plot symmetry. The results also did not show any evidence of publication bias (Table S1).
Discussion
Organ transplantation has been recommended as the most optimal treatment choice for patients suffering end-stage disease. However, AR still remains a crucial determining factor which influences the short-term function and long-term outcome of both recipients and allografts [38]–[40]. To the best of our knowledge, this is the first meta-analysis focusing on the combined effects of TGFB1 +869 T/C and +915 G/C polymorphisms on AR risk. In the current study, we provide evidence that patients carried with TGFB1 HP haplotypes (T/T G/G and T/C G/G) are more likely to suffer from AR after solid organ transplantation (specifically heart transplantation), which could be utilized to identify patients predisposed to AR and potentially benefiting from tailored immunosuppressive protocol.
After pooling the data from 14 eligible studies, we shown that patients with TGFB1 IP and/or LP haplotypes were less likely to suffer from AR than those with HP haplotypes (IP vs. HP: OR = 0.75, 95% CI, 0.58–0.96; IP/LP vs. HP: OR = 0.77, 95% CI, 0.61–0.98). Considering that ethnic background could influence the frequency of genotypes and haplotypes in some context, we conducted subgroup analysis based on ethnicities but failed to detect any significant association. Further stratified analysis by transplant types demonstrated a remarkably decreased AR risk for heart transplant recipients carried with TGFB1 IP and/or LP haplotypes, which was in agreement with the study conducted in a cohort of 111 pediatric cardiac transplant recipients [13]. With respect to various CNIs applied in suppressive protocols, CsA and FK506 have been proven to influence the serum level of TGF-β1 differentially [41], [42]; thus, we intended to investigate the various impacts of different CNIs on the relationship between the TGFB1 haplotypes and AR risk. However, FK506 has never been administrated as a unique CNI in any included studies. Therefore, we divided the studies into two subgroups: CsA arm and CsA/FK506 arm, and then conducted a subgroup analysis. As a result, patients with TGFB1 IP haplotypes were less likely to be affected by AR in CsA arm (IP vs. HP: OR = 0.57, 95% CI, 0.34–0.94), which could be interpreted as a clue that the recipients with TGFB1 IP haplotypes can benefit from CsA after organ transplantation.
TGF-β1 is a pleiotropic and multifunctional cytokine with immunosuppressive and fibrogenic properties, which may play a central role in both the initiation and propagation of AR and chronic rejection (CR) [7], [8]. The expression of TGF-β1 has been proven to be regulated by the two SNPs (+869 T/C and +915 G/C polymorphisms), whose combination could be divided into three groups (LP, IP, and HP). However, the results obtained have challenged the conventional concept that TGF-β1 may inhibit the initiation of AR episodes. Our team previously demonstrated that donor TGFB1 +869 T/C HP genotype (TT) was significantly associated with decreased AR risk [15]. Considering the obvious discrepancies, several questions have been raised: 1) The impact of various haplotypes of TGFB1 +869 T/C and +915 G/C polymorphisms on actual production levels of TGF-β1. The effects of the nine potential haplotypes on TGF-β1 expression levels have been previously studied in vitro and among patients without organ transplant [16], [43], [44]. However, no significant association between TGFB1 SNPs and TGF-β1 plasma levels or intragraft mRNA levels was detected in a cohort of renal transplant recipients [45]. 2) The influence of various immunosuppressive protocols on the expression level of TGF-β1. CsA and FK506 were found to influence the serum level of TGF-β1 differentially [41], [42], [45], while the impact of other immunosuppressive regimens still remains elusive. 3) The potential effects of donor and/or donor-recipient pair TGFB1 haplotypes on AR risk. The current study is confined to recipient SNPs; however, in some context, donor polymorphisms may contribute much more to AR risk than recipient SNPs [15], [46]. 4) The exact etiology of AR episodes and the biological functions of TGF-β1 in the development of AR still remain unclear.
The current meta-analysis focused on the combined effects of TGFB1 +869 T/C and +915 G/C polymorphisms rather than one single SNP on AR risk [15], which could help to derive a precise estimation of the roles of TGFB1 SNPs in the development of AR episodes. However, several limitations should be considered when interpreting the results. First, considering the unavailability of other detailed information, we did not conduct stratified analysis based on some cofactors such as follow-up time, gender, age, panel reactive antibodies level, human leukocyte antigens mismatch and donor source, which may influence the results. Second, the limited number of AR cases and non-AR cases may lead to a relatively small power. Third, only published studies with sufficient data were included, thus, publication bias may have occurred even though results of both Begg's test and Egger's test did not detect it. Last but not least, the meta-analysis is retrospective due to the methodological limitations.
In summary, this meta-analysis suggested that recipient TGFB1 HP haplotypes of +869 T/C and +915 G/C polymorphisms (T/T G/G and T/C G/G) might be a possible genetic susceptibility locus for AR after solid organ transplantation, which could be utilized to identify patients predisposed to AR and potentially benefiting from personalized immunosuppressive protocol. In addition, monitoring TGFB1 could help manage CR to some extent, as TGFB1 triggers fibrogenesis linked to chronic rejection (CR). Further well-designed and unbiased studies with larger sample size, diverse ethnicities, donor-recipient pairing and various applications of CNIs should be conducted to verify our findings. Furthermore, functional studies of TGFB1 gene polymorphism are warranted to understand the underlying mechanisms.
Supporting Information
Begg's funnel plot for publication bias test (LP vs. HP for TGFB1 haplotypes).
(TIF)
Begg's funnel plot for publication bias test (IP vs. HP for TGFB1 haplotypes).
(TIF)
Begg's funnel plot for publication bias test (LP/IP vs. HP for TGFB1 haplotypes).
(TIF)
PRISMA checklist.
(DOC)
Statistical analyses of publication bias for TGFB1 haplotypes at +869 T/C and +915 G/C polymorphisms.
(DOC)
Funding Statement
This project was supported by grants from the National Natural Science Foundation of China (81070597, 81370853), Science and Education Development Program of the Jiangsu Province Health Board (LJ201107), Six Talent Peaks of the Jiangsu Province Health Bureau (2011-WS-093) and Research and Innovation Program for Graduates of Jiangsu Province (CXZZ13_0583). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Li MO, Flavell RA (2008) TGF-beta: a master of all T cell trades. Cell 134: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. N Engl J Med 342: 1350–1358. [DOI] [PubMed] [Google Scholar]
- 3. Edgley AJ, Krum H, Kelly DJ (2012) Targeting fibrosis for the treatment of heart failure: a role for transforming growth factor-beta. Cardiovasc Ther 30: e30–40. [DOI] [PubMed] [Google Scholar]
- 4. Massague J (2008) TGFbeta in Cancer. Cell 134: 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hussein YM, Mohamed RH, El-Shahawy EE, Alzahrani SS (2014) Interaction between TGF-beta1 (869C/T) polymorphism and biochemical risk factor for prediction of disease progression in rheumatoid arthritis. Gene 536: 393–397. [DOI] [PubMed] [Google Scholar]
- 6. Gagliardo R, Chanez P, Gjomarkaj M, La Grutta S, Bonanno A, et al. (2013) The role of transforming growth factor-beta1 in airway inflammation of childhood asthma. Int J Immunopathol Pharmacol 26: 725–738. [DOI] [PubMed] [Google Scholar]
- 7. Hutchinson IV (1999) The role of transforming growth factor-beta in transplant rejection. Transplant Proc 31: 9S–13S. [DOI] [PubMed] [Google Scholar]
- 8. Morris-Stiff G (2005) TGFbeta-1 and the development of chronic graft nephropathy: relative roles of gene, mRNA and protein. Ann R Coll Surg Engl 87: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tambur AR, Ortegel JW, Ben-Ari Z, Shabtai E, Klein T, et al. (2001) Role of cytokine gene polymorphism in hepatitis C recurrence and allograft rejection among liver transplant recipients. Transplantation 71: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 10. Pribylova-Hribova P, Kotsch K, Lodererova A, Viklicky O, Vitko S, et al. (2006) TGF-beta1 mRNA upregulation influences chronic renal allograft dysfunction. Kidney Int 69: 1872–1879. [DOI] [PubMed] [Google Scholar]
- 11. Benza RL, Coffey CS, Pekarek DM, Barchue JP, Tallaj JA, et al. (2009) Transforming growth factor-beta polymorphisms and cardiac allograft rejection. J Heart Lung Transplant 28: 1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann S, Park J, Jacobson LM, Muehrer RJ, Lorentzen D, et al. (2004) Donor genomics influence graft events: the effect of donor polymorphisms on acute rejection and chronic allograft nephropathy. Kidney Int 66: 1686–1693. [DOI] [PubMed] [Google Scholar]
- 13. Di Filippo S, Zeevi A, McDade KK, Bastien O, Webber SA (2006) Impact of TGFbeta1 gene polymorphisms on acute and chronic rejection in pediatric heart transplant allografts. Transplantation 81: 934–939. [DOI] [PubMed] [Google Scholar]
- 14. Shah R, Rahaman B, Hurley CK, Posch PE (2006) Allelic diversity in the TGFB1 regulatory region: characterization of novel functional single nucleotide polymorphisms. Hum Genet 119: 61–74. [DOI] [PubMed] [Google Scholar]
- 15.Ge YZ, Yu P, Jia RP, Wu R, Ding AX, et al. (2014) Association between transforming growth factor beta-1 +869T/C polymorphism and acute rejection of solid organ allograft: A meta-analysis and systematic review. Transpl Immunol http://dx.doi.org/10.1016/j.trim.2014.01.001. [DOI] [PubMed]
- 16. Awad MR, El-Gamel A, Hasleton P, Turner DM, Sinnott PJ, et al. (1998) Genotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation 66: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 17. Perrey C, Pravica V, Sinnott PJ, Hutchinson IV (1998) Genotyping for polymorphisms in interferon-gamma, interleukin-10, transforming growth factor-beta 1 and tumour necrosis factor-alpha genes: a technical report. Transpl Immunol 6: 193–197. [DOI] [PubMed] [Google Scholar]
- 18. Pelletier R, Pravica V, Perrey C, Xia D, Ferguson RM, et al. (2000) Evidence for a genetic predisposition towards acute rejection after kidney and simultaneous kidney-pancreas transplantation. Transplantation 70: 674–680. [DOI] [PubMed] [Google Scholar]
- 19. Tian Y, Ma W, Zhang Y (2001) [Influence of cytokine gene polymorphism on renal transplantation]. Zhonghua Yi Xue Za Zhi 81: 719–721. [PubMed] [Google Scholar]
- 20. Tian Y, Ma W, Zhang Y (2002) [Influence of cytokine gene polymorphism on renal transplantation]. Zhonghua Wai Ke Za Zhi 40: 256–258. [PubMed] [Google Scholar]
- 21. Gu XW, Zhao M, Li LY, Li M, Qian J (2003) [Cytokine gene polymorphism in sensitized kidney transplant recipients and its association with acute rejection episodes]. Di Yi Jun Yi Da Xue Xue Bao 23: 1211–1213. [PubMed] [Google Scholar]
- 22. Lacha J, Hribova P, Kotsch K, Brabcova I, Bartosova K, et al. (2005) Effect of cytokines and chemokines (TGF-beta, TNF-alpha, IL-6, IL-10, MCP-1, RANTES) gene polymorphisms in kidney recipients on posttransplantation outcome: influence of donor-recipient match. Transplant Proc 37: 764–766. [DOI] [PubMed] [Google Scholar]
- 23. Canossi A, Piazza A, Poggi E, Ozzella G, Di Rocco M, et al. (2007) Renal allograft immune response is influenced by patient and donor cytokine genotypes. Transplant Proc 39: 1805–1812. [DOI] [PubMed] [Google Scholar]
- 24. Kocierz M, Siekiera U, Kolonko A, Karkoszka H, Chudek J, et al. (2011) -174G/C interleukin-6 gene polymorphism and the risk of transplanted kidney failure or graft loss during a 5-year follow-up period. Tissue Antigens 77: 283–290. [DOI] [PubMed] [Google Scholar]
- 25. Seyhun Y, Mytilineos J, Turkmen A, Oguz F, Kekik C, et al. (2012) Influence of cytokine gene polymorphisms on graft rejection in Turkish patients with renal transplants from living related donors. Transplant Proc 44: 1670–1678. [DOI] [PubMed] [Google Scholar]
- 26. Dhaouadi T, Sfar I, Bardi R, Jendoubi-Ayed S, Abdallah TB, et al. (2013) Cytokine gene polymorphisms in kidney transplantation. Transplant Proc 45: 2152–2157. [DOI] [PubMed] [Google Scholar]
- 27. Karasu Z, Ulukaya S, Ayanoglu HO, Basturk B, Ulukaya E, et al. (2004) Cytokine gene polymorphism and early graft rejection in liver transplant recipients. Transplant Proc 36: 2791–2795. [DOI] [PubMed] [Google Scholar]
- 28. Gomez-Mateo J, Marin L, Lopez-Alvarez MR, Moya-Quiles MR, Miras M, et al. (2006) TGF-beta1 gene polymorphism in liver graft recipients. Transpl Immunol 17: 55–57. [DOI] [PubMed] [Google Scholar]
- 29. Gourley IS, Denofrio D, Rand W, Desai S, Loh E, et al. (2004) The effect of recipient cytokine gene polymorphism on cardiac transplantation outcome. Hum Immunol 65: 248–254. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 33. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 34. Thakkinstian A, McElduff P, D'Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24: 1291–1306. [DOI] [PubMed] [Google Scholar]
- 35. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 36. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seyhun Y, Mytilineos J, Turkmen A, Oguz F, Kekik C, et al. (2012) Influence of cytokine gene polymorphisms on graft rejection in Turkish patients with renal transplants from living related donors. Transplant Proc 44: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 38. Opelz G, Dohler B (2008) Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation 85: 661–666. [DOI] [PubMed] [Google Scholar]
- 39. Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, et al. (2012) The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant 31: 1052–1064. [DOI] [PubMed] [Google Scholar]
- 40. Thurairajah PH, Carbone M, Bridgestock H, Thomas P, Hebbar S, et al. (2013) Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation 95: 955–959. [DOI] [PubMed] [Google Scholar]
- 41. Dai J, Michineau S, Franck G, Desgranges P, Becquemin JP, et al. (2011) Long term stabilization of expanding aortic aneurysms by a short course of cyclosporine A through transforming growth factor-beta induction. PLoS One 6: e28903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pakosz K, Zakliczynski M, Krol W, Pyka L, Zakliczynska H, et al. (2012) Association of transforming growth factor beta1 (TGF- beta1) with gingival hyperplasia in heart transplant patients undergoing cyclosporine-A treatment. Ann Transplant 17: 45–52. [DOI] [PubMed] [Google Scholar]
- 43. Yamada Y, Miyauchi A, Goto J, Takagi Y, Okuizumi H, et al. (1998) Association of a polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to osteoporosis in postmenopausal Japanese women. J Bone Miner Res 13: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 44. Suthanthiran M, Li B, Song JO, Ding R, Sharma VK, et al. (2000) Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci U S A 97: 3479–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hueso M, Navarro E, Moreso F, Beltran-Sastre V, Ventura F, et al. (2006) Relationship between subclinical rejection and genotype, renal messenger RNA, and plasma protein transforming growth factor-beta1 levels. Transplantation 81: 1463–1466. [DOI] [PubMed] [Google Scholar]
- 46. Lv R, Hu X, Bai Y, Long H, Xu L, et al. (2012) Association between IL-6 -174G/C polymorphism and acute rejection of renal allograft: evidence from a meta-analysis. Transpl Immunol 26: 11–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Begg's funnel plot for publication bias test (LP vs. HP for TGFB1 haplotypes).
(TIF)
Begg's funnel plot for publication bias test (IP vs. HP for TGFB1 haplotypes).
(TIF)
Begg's funnel plot for publication bias test (LP/IP vs. HP for TGFB1 haplotypes).
(TIF)
PRISMA checklist.
(DOC)
Statistical analyses of publication bias for TGFB1 haplotypes at +869 T/C and +915 G/C polymorphisms.
(DOC)