Abstract
Two bioelectrochemical membrane bioreactors (MBRs) developed by integrating microbial fuel cell and MBR technology were operated under closed-circuit and open-circuit modes, and high-throughput 454 pyrosequencing was used to investigate the effects of the power generation on the microbial community of bio-anode and bio-cathode. Microbes on the anode under open-circuit operation (AO) were enriched and highly diverse when compared to those on the anode under closed-circuit operation (AC). However, among the cathodes the closed-circuit mode (CC) had richer and more diverse microbial community compared to the cathode under open-circuit mode (CO). On the anodes AO and AC, Proteobacteria and Bacteroidetes were the dominant phyla, while Firmicutes was enriched only on AC. Deltaproteobacteria affiliated to Proteobacteria were also more abundant on AC than AO. Furthermore, the relative abundance of Desulfuromonas, which are well-known electrogenic bacteria, were much higher on AC (10.2%) when compared to AO (0.11%), indicating that closed-circuit operation was more conducive for the growth of electrogenic bacteria on the anodes. On the cathodes, Protebacteria was robust on CC while Bacteroidetes was more abundant on CO. Rhodobacter and Hydrogenophaga were also enriched on CC than CO, suggesting that these genera play a role in electron transfer from the cathode surface to the terminal electron acceptors in the bioelectrochemical MBR under closed-circuit operation.
Introduction
Microbial fuel cells (MFCs) offer a promising technology for both organic waste treatment and simultaneous power generation, and have attracted attention in the past decade. However, the practical applications of MFCs are limited by disadvantages such as low treatment efficiency and poor effluent quality. Membrane bioreactor (MBR) is another promising wastewater treatment process that has achieved great advances in the past two decades. MBR is considered as an alternate technology for conventional activated sludge (CAS) systems due to its high treatment efficiency and good effluent quality [1]. However, its wide-spread application is hindered by the high energy consumption.
Recently, efforts have been made to integrate MFC and MBR for wastewater treatment. It is presumed that the power generated by MFC from wastewater will partially offset the energy requirement of MBR, which in turn could increase treatment efficiency and enhance effluent quality of MFC. In a recently developed MFC-MBR system the aeration tank of an MBR was used as the cathode chamber [2]. Stainless-steel mesh membrane module has also been used as the cathode in an MFC-MBR system, which achieved a maximum power density of 8.62 W/m3 [3]. In another study, removal of 92.4% chemical oxygen demand (COD) and 4.35 W/m3 power density has also been reported [4]. These studies demonstrate the potential of bioelectrochemical MBR created by MFC-MBR integration for simultaneous wastewater treatment and energy production.
In these integrated systems, microorganisms play critical roles in both power generation and also wastewater treatment. Power is generated when microorganisms on the anode act as catalysts to oxidize organic matters and transfer the generated electrons to the cathode though an external circuit while the microbes on the cathode function as biocatalysts and accept electrons. On the other hand, biological wastewater treatment is generally carried out in the cathode chamber (aerobic MBR basin), which also impacts the microbial community on the cathode. A number of studies have determined the microbial community in typical MFCs [5]–[7]. However, studies on the microbial communities in bioelectrochemical MBRs are limited. Due to the distinct differences between typical MFCs and integrated MFC-MBR systems, the microbes in these newer systems are expected to be unique. Therefore, understanding their composition and functions will allow optimization of these integrated systems.
The goal of this study was to characterize the microbial community in the integrated MFC-MBR systems. To achieve this goal, we developed two identical bioelectrochemical membrane bioreactors (MBRs) by integrating MFC and MBR technology and operated them under closed-circuit and open-circuit modes. We then compared the microbial communities between the two MBRs using high-throughput 454 pyrosequencing to investigate the effects of power generation on microbial community changes on the bio-anode and bio-cathode. The results of this study provide insights into the microbial communities and their functions in MFC-MBR systems, and will facilitate optimization of these integrated systems for efficient wastewater treatment and power generation.
Materials and Methods
Experimental set-up
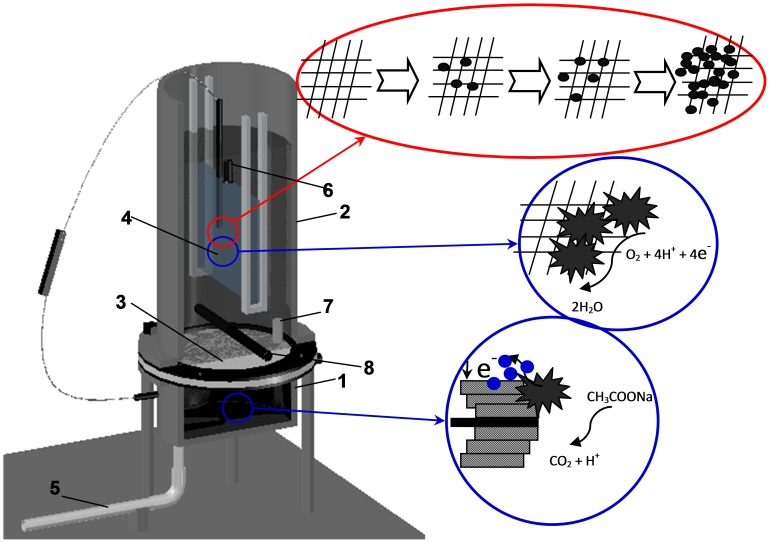
The schematic diagram of the experimental setup is shown in Fig. 1. The bioelectrochemical MBR was designed to have two cylinder compartments both with the same inner diameter of 10 cm, and designated as the anode and cathode chambers. The two chambers were separated by a cloth pretreated with polyvinylidene fluoride. A 3-cm long Plexiglas tube with 8 mm diameter was installed in the anode chamber to enable continuous upflow of wastewater from anode to the cathode.
Figure 1. Schematic diagram of the bioelectrochemical MBR system.
(1) Anode chamber; (2) cathode chamber; (3) cloth separating the two chambers; (4) stainless-steel mesh membrane module; (5) influent pipe; (6) effluent pipe; (7) connecting pipe; (8) air diffuser.
The anode chamber was filled with graphite felts (10-cm diameter) that were cut into pieces of 1 cm×1 cm (width × thickness). Total volume of the anode chamber was 140 mL and the volume of water decreased to 37 mL after the installation of graphite felts. Then, a graphite rod (6 mm diameter) was inserted in the anode graphite felts and connected to the external circuit. The cathode was a flat-sheet membrane (4 cm×8 cm) made of stainless mesh (pore size 48 μm), and was installed in the cathode chamber with an effective volume of 1.25 L. Two graphite rods were fastened on each side of the membrane module using stainless steel wires. Two perforated Plexiglas tubes were mounted below the membrane module to supply oxygen and mix liquids with an aeration intensity of 0.67 L/min.
In the bioelectrochemical MBR under closed-circuit operation (MBRC), the graphite rods in both anode and cathode were connected with copper wires with 470 or 100 Ω external resistance, while the graphite rods remained open in the MBR under open-circuit operation (MBRO), which also served as a control reactor. This design enabled us to compare the differences in the microbial communities between the two systems, and analyze the impact of different modes of power generation on variations in the microbial communities.
Inoculation and operation
The anode chambers in both bioelectrochemical MBRs were inoculated with 10 mL sludge obtained from the anaerobic unit of an anaerobic/anoxic/oxic-MBR system, which is documented in a previous publication [8]. The mixed liquor suspended solid (MLSS) and mixed liquor volatile suspended solid (MLVSS) concentrations of the inoculated sludge were 16.2 g/L and 9.0 g/L, respectively. The cathode chambers were seeded with activated sludge from Shanghai Quyang municipal wastewater treatment plant in China, which had an MLSS concentration of 3.9 g/L and an MLVSS concentration of 2.8 g/L.
After inoculation, synthetic wastewater was fed into the anode chamber, and then allowed to flow into the cathode chamber. Composition of the synthetic wastewater was: CH3COONa·3H2O 640 mg/L, NH4Cl 57 mg/L, K2HPO4·3H2O 22 mg/L, CaCl2 11.5 mg/L, MgSO4 12 mg/L, and 10 mL of the trace element solution, which is similar to those used in previous studies [3], [9]. Both reactors were placed at room temperature (25±1°C) and sludge was not discharged during the operation.
At the end of the experimental period (75 days), biofilms were scraped from MBRC anode (AC), MBRC cathode (CC), MBRO anode (AO) and MBRO cathode (CO) to analyze the differences in the microbial communities using 454 pyrosequencing.
Analysis of the microbial communities
DNA extraction and PCR amplification
DNA was extracted from the microbial communities in AC, AO, CC and CO according to methods described previously [10]. To amplify the 16S rRNA from the samples the following universal primers were used: 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 533R (5′-TTACCGCGGCTGCTGGCAC-3′). Each PCR reaction was carried out in a 20 μL reaction volume containing 4 μL 5× FastPfu Buffer, 2 μL 2.5 mM dNTPs, 0.4 μL of each primer, 0.5 μL DNA and 0.4 μL FasrPfu Polymerase (TransStart FastPfu DNA Polymerase, TransGen, China). The thermocycling steps used for the PCR were: 95°C for 2 min, 25 cycles of 95°C for 30 sec followed by 55°C for 30 sec, and 72°C for 30 sec, and a final extension at 72°C for 5 min. After amplification, the amplicons were purified directly from the PRP mixture using the UNIQ-10 PCR purification Kit (Sangon, Shanghai, China) and quantified using TBS-380 (Turner BioSystems, Inc., USA).
454 pyrosequencing
A mixture of the purified amplicons was used for 454 pyrosequencing on a Roche massively parallel 454 GS-FLX Titanium sequencer (Roche 454 Life Sciences, Branford, CT, USA). To improve the quality of pyrosequencing data, defective reads were removed from the libraries; these included reads without a recognizable reverse primer, reads shorter than 150 bp, and those containing any ambiguous base call [10]. Then barcodes and primers were trimmed from the resulting sequences. The final pyrosequencing data contained 6381 (AO), 6854 (AC), 12243 (CO), 7097 (CC) high quality V1–V3 tags for the 16S rRNA-gene. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRA114114).
Classification of the microbial communities
Pyrosequencing reads were clustered into operational taxonomic units (OTUs) with an average length of 390 bp. Then the OTUs were further clustered using the MOTHUR program (http://www.Mothur.org/wiki/Main-Page) with sequence distances set at 0.03 or 0.05. Based on these clusters the following parameters were calculated for each sample: Shannon diversity index (http://www.mothur.org/wiki/Shannon), Chao1 richness (http://www.mothur.org/wiki/Chao), abundance-based coverage using the abundance coverage estimator (ACE) (http://www.mothur.org/wiki/Ace), Good's coverage (http://www.mothur.org/wiki/Coverage), and the rarefaction curves at α 0.03 and 0.05. Based on MOTHUR and SILVA106 database, representative reads from the clusters were classified with a confidence threshold of 80% at the levels of both phyla and genera.
Results and Discussion
Performance of the bioelectrochemical MBRs
In the bioelectrochemical MBR under closed-circuit operation, the stainless-steel membrane module with biofilm formation not only serves as a biocathode for the MFC but also as a dynamic membrane for efficient solid/liquid separation [4]. In both MBR systems, effluent turbidity of 0.8 NTU (Nephelometric turbidity unit) was reached within 24 h. In addition, the average efficiencies of chemical oxygen demand (COD) and NH4 +-N removal during the operation were 86.1% and 97.5% for MBRC, and 84.9% and 96.4% for MBRO. Maximum power density of the MBR under closed-circuit operation was as high as 8.6 W/(m3 anode net volume), indicating that the biochemical MBR not only achieved efficient wastewater treatment but also power production [4]. Experimental results also demonstrated that the MFC (the anodic chamber under closed-circuit operation) could increase the degradation rate of organic matters by 7.0–13.0% compared with the anode chamber in the open-circuit mode, and the Coulombic efficiencies of the MFC under closed-circuit operation were 1.9%, 3.2%, 4.5% and 12.3% in the four runs, respectively [4].
Microbial richness and diversity
In our study, four 16S rDNA gene libraries were constructed by high-throughput sequencing of microbial communities from AO, AC, CO and CC samples. After trimming, sorting and quality control, 6381(AO), 6854(AC), 12243(CO) and 7097(CC) high-quality sequence tags (average length - 390 bp) were clustered into 1859 (AO), 1488 (AC), 1997 (CO) and 1856 (CC) operational taxonomic units (OTUs) at 3% distance thresholds, and 1602 (AO), 1211 (AC), 1583 (CO) and 1493 (CC) OTUs at 5% distance thresholds (Table 1). The abundance-based coverage estimator (ACE), Chao1, Shannon and Good Coverage are also presented in Table 1. Higher numbers of OTUs were estimated for AO sample (4719 and 7816 in AO, 3424 and 5712 in AC, respectively) with infinite sampling at 3% distance by Chao1 estimator and ACE. This indicated that the richness of the bacterial communities in the MFC anode operated under open circuit was higher than that under closed circuit mode. However, for the cathode samples, microbial richness of Cc was greater than Co (Table 1). The Shannon diversity index values for AO and CC at 0.03/0.05 distance were higher than AC and CO, respectively, suggesting higher diversity in the microorganisms in AO than AC, while similar higher diversity was observed in CC when compare to CO. Since the anodes and cathodes of the two bioelectrochemical MBRs were initially inoculated with the same sludge, the observed differences in the microbial richness and diversity between the bioelectrodes could be attributed to power generation.
Table 1. Similarity based OTUs and species richness estimates of the bacterial phylotypes in the four samples.
| Cluster distance 0.03 | Cluster distance 0.05 | |||||||||
| OUT | ACE | Chao1 | Shannon | Coverage | OTU | ACE | Chao1 | Shannon | Coverage | |
| AO | 1895 | 7816 | 4719 | 6.34 | 0.81 | 1602 | 5950 | 3780 | 6.07 | 0.85 |
| AC | 1488 | 5712 | 3424 | 5.73 | 0.87 | 1211 | 3846 | 2510 | 5.41 | 0.90 |
| CO | 1997 | 7981 | 4597 | 4.98 | 0.90 | 1583 | 4964 | 3143 | 4.64 | 0.93 |
| CC | 1856 | 8993 | 5204 | 5.63 | 0.82 | 1493 | 5443 | 3450 | 5.32 | 0.87 |
Note: Species richness was estimated using the program MOTHUR as described in Materials and Methods.
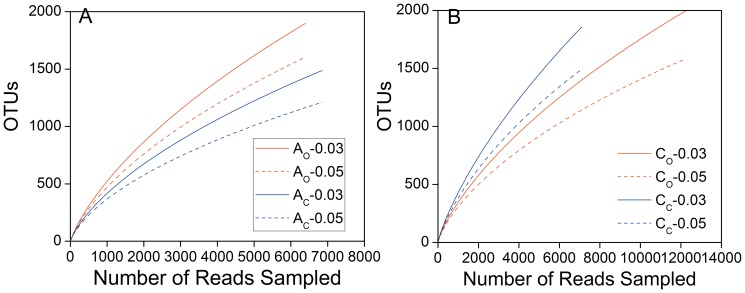
The rarefaction curves of all four samples at 3% and 5% distance thresholds are shown in Fig. 2. Evidently none of the rarefaction curves reached a plateau in this study, and new OTUs continued to emerge even after 12000 reads were sampled with pyrosequencing. The results indicated that pyrosequencing could successfully reveal the higher diversity of bacterial communities in MFC compared to other conventional molecular biological methods, such as DGGE and clone library [11]–[13]. Different slopes observed in the rarefaction curves represented diversity of the samples, and steeper slopes such as in AO indicated higher sample diversity when compared to AC and CC. Among all four samples CO was the least diverse.
Figure 2. Rarefaction curves based on 16S rDNA sequences of the bacterial communities from anode (A) and cathode (B) samples.
The OTUs are calculated based on 3% and 5% distances.
Taxonomic complexity of the bacterial communities
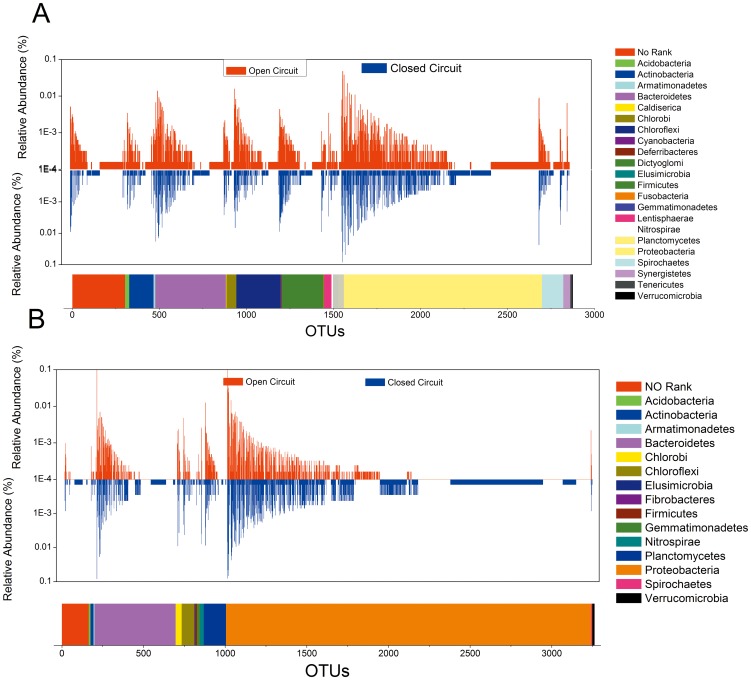
Phylogenetic analysis was used to characterize the anode and cathode microbial community structure and composition in the MBRs under open and closed circuit modes. A comparison of the relative bacterial community abundance at the phylum level for the anode and cathode samples is shown in Fig. 3. The number of phyla present in the AO and AC samples was nearly identical except for the minor phyla (Deferribacteres and Gemmatimonadetes), which accounted for less than 0.1% of the total community in the AO sample.
Figure 3. Relative abundance of bacterial reads retrieved from the anodes (A) and cathodes (B) in the open and closed circuit MFC models (Phylum level).
In the anode samples, 22 phyla were identified. Deferribacteres and Gemmatimonadetes were detected in AO while they were not present in AC. Among the total reads, 7.1% from AO and 5.3% from AC were not classified at the phylum level, indicating that at least some bacteria in the anode biofilms were not cultured. Proteobacteria and Bacteroidetes (45.2% and 15.5% for AO; 55.0% and 14.8% for AC) were the most abundant in both anode samples (Fig. 3), which is consistent with previous studies on MFCs anode biofilm analyzed by 454 pyrosequencing [14]–[16]. It should be noted that Proteobacteria was less abundant in AO than in AC, which could be associated with the differential abundance in Deltaproteobacteria (25.1% in AO and 38.9% in AC). The reason for this could be that some of the known exoelectrogenic bacteria (e.g., Desulfuromonas) belong to the phylum Deltaproteobacteria [17]. We also found that Chloroflexi (10.6%) was another major phylum represented in AO sample. Previous studies had reported that the predominant bacteria in the anaerobic digester sludge was Chloroflexi [18], which is capable of assimilating N-acetyl-D-glucosamine, a major structural component in bacterial cells [19]. In the AC sample, the third major phylum was Firmicutes (8.14%) besides the most abundant phyla Proteobacteria and Bacteroidetes. Previous studies have shown that Firmicutes was predominant in glucose-fed MFCs [16], [20], and the phylum could not be identified when MFC was switched to acetate-fed mode [20]. However, Firmicutes was detected in both anode samples in the present study, suggesting that Firmicutes could have originated from the inoculum and/or by symbiotic relationship among bacteria in the anodes. Moreover, Firmicutes was enriched in AC than AO, which could be related to its ability to transfer extracellular electrons and it is likely that the metabolites produced by Pseudomonas (also enriched in AC) enabled Firmicutes (Gram-positive) to achieve this extracellular electron transfer function [21].
In the cathode samples, only 15 phyla were identified in total. Fibrobacteres were absent in CO, while Armatimonadetes and Spirochaetes were not found in CC. Only 1.08% of the total sequences in CO and 1.06% in CC were not identified at the phylum level, which were lesser than those in the anode samples. The dominant phyla were Proteobacteria (64.85% in CO and 71.61% CC) and Bacteroidetes (25.2% in Co and 19.2% in CC) (Fig. 3), followed by Planctomycetes (4.75%) and Chloroflexi (1.45%) in CO, and Planctomycetes (2.92%) and Chlorobi (1.70%) in CC. Although the majority of phyla in both cathode samples were similar, CO had abundant Bacteroidetes, while CC had more Protebacteria, which comprised of Alphaproteobacteria (CO - 5.7%, CC - 15.3%), Betaproteobacteria (CO - 49.2%, Cc - 45.3%), Deltaproteobacteria (CO - 3.1%, Cc - 3.1%), Gammaproteobacteria (CO - 6.1%, Cc - 6.4%), and Epsilonproteobacteria (CO - 0.1%, Cc - 0.01%) classes. Betaproteobacteria is a well-known ammonia-oxidizing bacteria, and was discovered as a low abundant class in cathode biofilms from ammonia-lacking MFC [22], and the predominant class in ammonia-containing MFCs [14], [23]. The enrichment of Betaproteobacteria in both cathode samples was consistent with the high ammonia removal efficiency of MBRC and MBRO (97.5% and 96.4%). Further, Alphaproteobacteria was robust in CC than CO, suggesting that the cathode in the bioelectrochemical MBR under closed-circuit operation facilitated the growth of Alphaproteobacteria [24]. In comparison with traditional dynamic MBR, the microbial community compositions in the bioelectrochemical MBR are also different. For instance, Chlorobi, Chloroflexi and Planctomycetes with relative abundance >1% were detected in our system, while they were absent in the dynamic MBRs reported by others [25]. This might indicate that the bioelectrochemical MBR owns its special community possibly due to the power generation.
Changes in the microbial community in bioelectrochemical MBRs under open and closed circuit modes
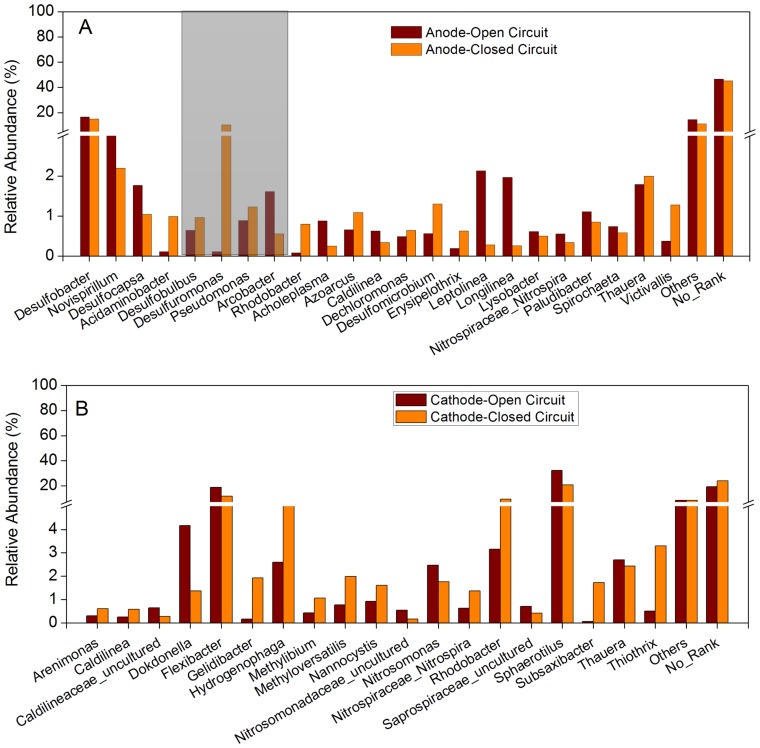
To further elucidate the differences among microbial communities between the two systems, all four samples were compared at the genus level. Relative abundance (>0.5%) of the most dominant taxa in AO and AC is depicted in Fig. 4A. Interestingly, Desulfobacter, which has not been reported in any bioelectrochemical systems (BES) thus far, was the most abundant bacteria (16.6% in AO and 15.0% in AC) in the anodes. This could be attributed to the fact that the inoculum used in this study was collected from the anaerobic unit of an anaerobic/anoxic/oxic-MBR with a longer sludge retention time (SRT = 60 d) and indeed, Desulfobacter was reported previously in this MBR [26]. Although both systems had the same dominant genus (Desulfobacter), the microbial communities between the two anode samples were quite different at the genus level. The relative abundance of Desulfuromonas, which is a known electrogenic bacteria, was much higher in AC (10.2%) when compared to AO (0.11%). This indicated that the power generation impacts microbial metabolism and that closed-circuit operation acclimated more electrogenic bacteria.
Figure 4. Relative abundance of the predominant groups in anode (A) and cathode (B) samples at the genus level.
Relative abundance is defined as the number of sequences affiliated to a particular taxon divided by the total number of sequences per sample (%). Genera with relative abundance less than 0.5% in both libraries are defined as “others”.
Microbial groups with electrochemical activities were observed in the anodes (gray shaded region in Fig. 4A) and included Desulfobulbus, Desulfuromonas, Pseudomonas and Arcobacter (0.64%, 0.11%, 0.89% and 1.6% in AO; 0.96%, 10.2%, 1.2% and 0.55% in AC, respectively). Desulfobulbus is reported to not only use soluble electron-acceptors such as sulfate and Fe (III) but also uses the electrode surface as electron acceptor when pyruvate, lactate, propionate or hydrogen are provided as electron donors without exogenous electron-shuttling compounds for electricity production [27]. Desulfuromonas can also oxidize ethanol, propanol, and butanol with Fe (III) as electron acceptor [28]. However, Bond et al. reported that Desulfuromonas can conserve energy to support their growth by oxidizing organic compounds such as acetate and benzoate with an electrode acceptor [29]. Pseudomonas can produce a soluble redox metabolite, pyocyanin, which mediates the transfer of electrons between bacteria and the anode in an MFC [30]. Besides, Arcobacter can associate with the electrode and rapidly generate a strong electronegative potential in the presence of acetate [31]. In the present study, the high abundance of Desulfuromonas and low abundance of Desulfobulbus and Pseudomonas is likely due to the use of acetate as electron donor. However, Arcobacter, which uses acetate as the electron donor and an electrode as the electron acceptor, was abundant in AO than AC presumably due to the competition between Desulfuromonas and Arcobacter for the electron donor (acetate). In addition to these, AC also contained some minor bacterial populations (relative abundance <1%) such as Clostridium, Comamonas and Geobacter all of which have the capacity to generate electricity [17].
Leptolinea and Longilinea were represented in larger fractions in AO (2.1% and 2.0%) than in AC (0.28% and 0.26%) (Fig. 4A), suggesting that these two genera might not grow well in the closed-circuit anode biofilm. Such limitation in Ac could be due to the fact that both are strict filamentous anaerobes used in fermenting carbohydrates, and are in general isolated from thermophilic or mesophilic sludge [32]. Notably, Rhodobacter, a well-known photofermentative bacteria that produces hydrogen from many organic substrates [33], [34] was enriched in AC (1.61%) than AO (0.55%). Previously, Rhodobacter was shown to be directly involved in power production [35].
Interestingly, the microbial community structure between CO and CC samples was very different (Fig. 4B). Sphaerotilus was the most dominant bacteria in both CO and CC samples with a relative abundance of 32.2% and 20.8% respectively, followed by Flexibacter (18.9%), and Dokdonella (4.2%) in CO, and Flexibacter (11.7%), and Rhodobacter (9.3%) in CC. Both Sphaerotilus and Flexibacter were the dominant genera shared by both CO and CC samples, and these genera have been reported to be frequently responsible for filamentous bulking in activated sludge [36], [37]. However, in Cc a decrease in their abundance demonstrated that the closed-circuit conditions can alleviate filamentous bulking of activated sludge. Rhodobacter, which is a hydrogen producing bacteria [33] and Hydrogenophaga, an autotrophic H2-oxidizing bacteria that utilizes hydrogen as the energy source [38], were accumulated in the CC biofilm (9.3% - Rhodobacter and 5.0% - Hydrogenophaga) (Fig. 4B). However, they only accounted for 3.2% and 2.6% in CO, respectively. Thrash et al. demonstrated that microorganisms could accept electrons from a solid-state electrode via the cathodic production of hydrogen, which mediates the transfer of electrons [39]. Therefore, both Rhodobacter and Hydrogenophaga could be presumed to play a role in electron transfer from the cathode surface to the terminal electron acceptor.
Through 454 pyrosequencing, the functional microbes for bioelectricity generation are clarified. The present work also shows that the microbial community structure in the cathode is different. These results present the potential for further improving the system performance through regulation of electrogenic microbes as microorganisms play a key role in organic matter degradation and power generation. The enrichment of functional microbes for enhancing the power density needs further studying since now the optimization of the system mainly relies on reactor configuration, electrode materials and separators.
Conclusions
High-throughput 454 pyrosequencing revealed that the bioelectrochemical MBRs operated under open and closed circuit conditions could result in diverse microbial community structures. Chao1 estimators and Shannon diversity indexes indicated that the microorganisms in AO was richer and highly diverse than those in AC. However, among the cathodes CC revealed a richer and more diverse microbial community when compared to CO. The microbial community composition in the anode samples revealed that Proteobacteria and Bacteroidetes were the dominant phyla in Ao and Ac, while Firmicutes was enriched in AC. Deltaproteobacteria affiliated to Proteobacteria were also more abundant in AC than AO. In addition, the relative abundance of Desulfuromonas, which are well-known electrogenic bacteria, was much higher in AC compared to AO, indicating that closed-circuit operation can acclimate more electrogenic bacteria. In the cathode samples, Protebacteria were robust in CC while Bacteroidetes were more abundant in CO. Rhodobacter and Hydrogenophaga were represented more in CC than CO, suggesting that the two genera could play a role in electron transfer from the surface of cathode to the terminal electron acceptors in the bioelectrochemical MBR under closed-circuit operation. Changes in the microbial community between the two bioelectrochemical MBRs demonstrated that the power generation affected the microbial community structure in both anode and cathode biofilms, facilitating the selection of functional microbes in the closed-circuit operation system for power generation.
Funding Statement
This work was financially supported by the Science and Technology Commission of Shanghai Municipality project (12230707000) and by the Fundamental Research Funds for the Central Universities. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Meng F, Chae S, Drews A, Kraume M, Shin H, et al. (2009) Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res 43: 1489–1512. [DOI] [PubMed] [Google Scholar]
- 2. Wang YP, Liu XW, Li WW, Li F, Wang YK, et al. (2012) A microbial fuel cell-membrane bioreactor integrated system for cost-effective wastewater treatment. Appl Energy 98: 230–235. [Google Scholar]
- 3. Wang Z, Huang J, Zhu C, Ma J, Wu Z (2013) A bioelectrochemically-assisted membrane bioreactor (BEAMBR) for simultaneous wastewater treatment and energy production. Chem Eng Technol 36: 2044–2050. [Google Scholar]
- 4. Wang YK, Sheng GP, Li WW, Huang YX, Yu YY, et al. (2011) Development of a novel bioelectrochemical membrane reactor for wastewater treatment. Environ Sci Technol 45: 9256–9261. [DOI] [PubMed] [Google Scholar]
- 5. Logan BE, Hamelers R, Rozendal R, Schröder U, Keller J, et al. (2006) Microbial fuel cells: Methodology and technology. Environ Sci Technol 40: 5181–5192. [DOI] [PubMed] [Google Scholar]
- 6. Jiang D, Li B (2009) Novel electrode materials to enhance the bacterial adhesion and increase the power generation in microbial fuel cells (MFCs). Water Sci Technol 59: 557–563. [DOI] [PubMed] [Google Scholar]
- 7. Rismani-Yazdi H, Chisty AD, Carver SM, Yu Z, Dehority BA (2011) Effect of external resistance on bacterial diversity and metabolism in cellulose-fed microbial fuel cells. Bioresource Technol 102: 278–283. [DOI] [PubMed] [Google Scholar]
- 8. Wang P, Wang ZW, Wu ZC, Mai SH (2011) Fouling behaviours of two membranes in a submerged membrane bioreactor for municipal wastewater treatment. J Membr Sci 382: 60–69. [Google Scholar]
- 9. Boeije G, Corstanje R, Rottiers A, Schowanek D (1999) Adaptation of CAS test system and synthetic sewage for biological nutrient removal, Part I: Development of a new synthetic sewage. Chemosphere 4: 699–709. [DOI] [PubMed] [Google Scholar]
- 10. Ma J, Wang Z, Yang Y, Mei X, Wu Z (2013) Correlating microbial community structure and composition with aeration intensity in submerged membrane bioreactors by 454 high-throughput pyrosequencing. Water Res 47: 859–769. [DOI] [PubMed] [Google Scholar]
- 11. Aelterman P, Rabaey K, Pham HT, Boon N, Verstraete W (2006) Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol 40: 3388–3394. [DOI] [PubMed] [Google Scholar]
- 12. Logan BE, Murano C, Scott K, Gray ND, Head IM (2005) Electricity generation from cysteine in a microbial fuel cell. Water Res 39: 942–952. [DOI] [PubMed] [Google Scholar]
- 13. Rabaey K, Read ST, Clauwaert P, Freguia S, Bond PL, et al. (2008) Cathodic oxygen reduction catalyzed by bacteria in microbial fuel cells. ISME J 2: 519–527. [DOI] [PubMed] [Google Scholar]
- 14. Sayess RR, Saikaly PE, Li D, Semerjian L (2013) Reactor performance in terms of COD and nitrogen removal and bacterial community structure of a three-stage rotation bioelectrochemical contactor. Water Res 47: 881–894. [DOI] [PubMed] [Google Scholar]
- 15. Zhang G, Zhao Q, Jiao Y, Wang K, Lee D, et al. (2012) Efficient electricity generation from sewage sludge using biocathode microbial fuel cell. Water Res 46: 43–45. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Min B, Huang L, Angelidaki I (2011) Electricity generation and microbial community response to substrate changes in microbial fuel cell. Bioresource Technol 102: 1166–1173. [DOI] [PubMed] [Google Scholar]
- 17. Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7: 375–381. [DOI] [PubMed] [Google Scholar]
- 18. Nelson MC, Morrison M, Yu Z (2011) A meta-analysis of the microbial diversity observed in anaerobic digesters. Bioresource Technol 102: 3730–3739. [DOI] [PubMed] [Google Scholar]
- 19. Zang K, Kurisu F, Kasuga I, Furumai H, Yagi O (2008) Analysis of the phylogenetic diversity of estrone-degrading bacteria in activated sewage sludge using microautoradiography-fluorescence in situ hybridization. Syst Appl Microbiol 31: 206–214. [DOI] [PubMed] [Google Scholar]
- 20. Chae K, Choi M, Lee J, Kim K, Kim IS (2009) Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresource Technol 100: 3518–3525. [DOI] [PubMed] [Google Scholar]
- 21. Goud RK, Mohan SV (2013) Prolonged applied potential to anode facilitate selective enrichment of bio-electrochemically active Proteobacteria for mediating electron transfer: Microbial dynamics and bio-catalytic analysis. Bioresource Technol 137: 160–170. [DOI] [PubMed] [Google Scholar]
- 22. Wang Z, Zheng Y, Xiao Y, Wu S, Wu Y, et al. (2013) Analysis of oxygen reduction and microbial community of air-diffusion biocathode in microbial fuel cells. Bioresource Technol 144: 74–79. [DOI] [PubMed] [Google Scholar]
- 23. Wang HP, Jiang SC, Wang Y, Xiao B (2013) Substrate removal and electricity generation in a membrane-less microbial fuel cell for biological treatment of wastewater. Bioresource Technol 138: 109–116. [DOI] [PubMed] [Google Scholar]
- 24. Li C, Ding L, Cui H, Zhang L, Xu K, et al. (2012) Application of conductive polymers in biocathode of microbial fuel cells and microbial community. Bioresource Technol 116: 459–465. [DOI] [PubMed] [Google Scholar]
- 25. Chu H, Dong B, Zhang Y, Zhou X, Yu Z (2012) Pollutant removal mechanisms in a bio-diatomite dynamic membrane reactor for micro-polluted surface water purification. Desalination 293: 38–45. [Google Scholar]
- 26. Ma J, Wang Z, Zou X, Feng J, Wu Z (2013) Microbial communities in an anaerobic dynamic membrane bioreactor (AnDMBR) for municipal wastewater treatment: Comparison of bulk sludge and cake layer. Process Biochem 48: 510–516. [Google Scholar]
- 27. Holmes DE, Bond DR, Lovley DR (2004) Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl Environ Mcrobiol 70: 1234–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JR, Jung SH, Regan JM, Logan BE (2007) Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresource Technol 98: 2568–2577. [DOI] [PubMed] [Google Scholar]
- 29. Bond DR, Holmes DE, Tender LM, Lovley DR (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295: 483–485. [DOI] [PubMed] [Google Scholar]
- 30. Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70: 5373–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fedorovich V, Knighton MC, Pagaling E, Ward BF, Free A, et al. (2009) Novel electrochemically active bacterium phylogenetically related to Arcobacter butzler, isolated from a microbial fuel cell. Appl Environ Microbiol 75: 7326–7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reddy MV, Mohan SV (2012) Influence of aerobic and anoxic microenvironments on polyhydroxyalkanoates (PHA) production from food waste and acidogenic effluents using aerobic consortia. Bioresource Technol 103: 313–321. [DOI] [PubMed] [Google Scholar]
- 33. Kim M, Kim D, Cha J (2012) Culture conditions affecting H2 production by phototrophic bacterium Rhodobacter sphaeroider KD131. Int J Hydrog Energy 37: 14055–14061. [Google Scholar]
- 34. Kobayashi J, Hasegawa S, Itou K, Yoshimune K, Komoriya T, et al. (2012) Expression of aldehyde dehydrogenase gene increases hydrogen production from low concentration of acetate by Rhodobacter sphaeroides. Int J Hydrog Energy 37: 9602–9609. [Google Scholar]
- 35. Cho YK, Donohue TJ, Tejidor I, Anderson MA, McMahon KD, et al. (2008) Development of a solar-powered microbial fuel cell. J Appl Microbiol 104: 640–650. [DOI] [PubMed] [Google Scholar]
- 36. Esposito A, Pagnanelli F, Lodi A, Solisio C, Veglió F (2001) Biosorption of heavy metals by Sphaerotilus natans: equilibrium study at different pH and biomass concentrations. Hydrometallurgy 60: 129–141. [Google Scholar]
- 37. Samaras P, Papadimitriou CA, Vavoulidou D, Yiangou M, Sakellaropoulos GP (2009) Effect of hexavalent chromium on the activated sludge process and on the sludge protozoan community. Bioresource Technol 100: 38–43. [DOI] [PubMed] [Google Scholar]
- 38. Yoon K, Sakai Y, Tsukada N, Fujisawa K, Nishihara H (2009) Purification and biochemical characterization of a membrane-bound [NiFe]-hydrogenase from a hydrogen-oxidizing, lithotrophic bacterium, Hydrogenophaga sp. AH-24. FEMS Microbiol Lett 290: 114–120. [DOI] [PubMed] [Google Scholar]
- 39. Thrash JC, Trump JI, Weber KA, Miller E, Achenbach LA, et al. (2007) Electrochemical stimulation of microbial perchlorate reduction. Environ Sci Technol 41: 1740–1746. [DOI] [PubMed] [Google Scholar]