Abstract
Dendrobium huoshanense is a valuable and versatile Chinese herbal medicine with the anecdotal claims of cancer prevention and anti-inflammation. However, its immunological activities are limited to in vitro studies on a few cytokines and immune cell functions. First, we investigated the effects of polysaccharides isolated from DH (DH-PS) on inducing a panel of cytokines/chemokines in mice in vivo and human in vitro. We found that DH polysaccharides (DH-PS) induced TH1, TH2, inflammatory cytokines and chemokines in mouse in vivo and human cells in vitro. Secondly, we demonstrated that DH-PS expanded mouse splenocytes in vivo including CD4+ T cells, CD8+ T cells, B cells, NK cells, NKT cells, monocytes/macrophages, granulocytes and regulatory T cells. Notably, DH-PS induced an anti-inflammatory molecule, IL-1ra, in mouse and human immune cells, especially monocytes. The serum level of IL-1ra elicited by the injection of DH-PS was over 10 folds of IL-1β, suggesting that DH-PS-induced anti-inflammatory activities might over-ride the inflammatory ones mediated by IL-1β. The signaling pathways of DH-PS-induced IL-1ra production was shown to involve ERK/ELK, p38 MAPK, PI3K and NFκB. Finally, we observed that IL-1ra level induced by DH-PS was significantly higher than that by F3, a polysaccharide extract isolated from another popular Chinese herbal medicine, Ganoderma lucidum. These results indicated that DH-PS might have potential applications for ameliorating IL-1-induced pathogenic conditions.
Introduction
Dendrobium huoshanense (DH), which is an herb of Orchidaceae family, has been used as a traditional Chinese herbal medicine for centuries with the anecdotal claims of cancer prevention and anti-inflammation. Polysaccharides isolated from Dendrobium huoshanense have been reported to induce TNF-α in peritoneal macrophages and IFN-γ in mouse splenocytes [1] and promote phagocytosis of macrophages [2]. To date, there have been no detailed studies on the systemic immune functions of DH-PS such as in vivo immune cell activations, inductions of comprehensive panel of cytokines/chemokines and anti-inflammatory molecules.
Among the cytokines, two forms of Interleukin-1 (IL-1α and IL-1β) are thought to play an important role in inflammation and involved in many pathological conditions including rheumatoid arthritis [3], [4]. They are produced primarily by mononuclear phagocytes, but also by a number of other cell types including skin keratinocytes [5]. These two cytokines are pro-inflammatory cytokines which can stimulate the expressions of genes associated with inflammation and autoimmune diseases. IL-1 exerts its functions by binding to type Ι IL-1 receptor and induces downstream signaling, leading to the expressions of numerous genes resulting in inflammation [6], [7], [8], [9]. A natural inhibitor of IL-1 activity, designated as secreted Interleukin-1 receptor antagonist (IL-1ra), was discovered and purified from the urine of the patients suffering from monocytic leukemia [7], [10]. IL-1ra, a 25 KD glycoprotein, is a member of IL-1 family that competes with IL-1 for the binding to IL-1 receptor, but unlike IL-1, this binding does not induce any signal transduction [11], [12], [13], [14]. IL-1ra is released in vivo during inflammation and immune-mediated diseases [15], which is thought to limit the deleterious effects brought by IL-1 [16], [17] and shown to be effective in the treatment of sepsis, graft-versus-host disease and rheumatoid arthritis in animal models [18], [19], [20], [21]. Additionally, IL-1ra (commercially produced as “anakinra”) has been used clinically to treat rheumatoid arthritis in which IL-1 plays a key role [22]. Many types of immune cells are reported to secrete IL-1ra including neutrophils, master cells, macrophages and monocytes [23], [24], [25] and several molecules have been shown to stimulate the secretion of IL-1ra including cytokines (IL-6 and IL-10, for example) and natural products [13], [26]. Polysaccharides isolated from Ganoderma Lucidum, another popular Chinese herbal medicine, have been shown to possess immune-modulating effects and reported to induce the production of IL-1ra [27]. Although DH-PS is believed to possess immune-modulating functions with the anecdotal claims of cancer prevention and anti-inflammation, comprehensive information regarding DH-PS on immune functions and its ability to stimulate the secretion of IL-1ra is still lacking.
In the present study, we evaluated the immune-modulating effects of DH-PS by investigating the profiles and kinetics of cytokine productions, as well as expansions and activations of immune cells induced by DH-PS in both human and mouse system. Moreover, we demonstrated that DH-PS induced IL-1ra production through the activation of PI3K, p38 MAPK and NFκB. Finally, we compared the IL-1ra production induced by DH-PS and F3, the polysaccharide extract from another popular Chinese herbal medicine, Ganoderma lucidum.
Results
DH-PS induced the secretions of multiple cytokines and chemokines in vivo
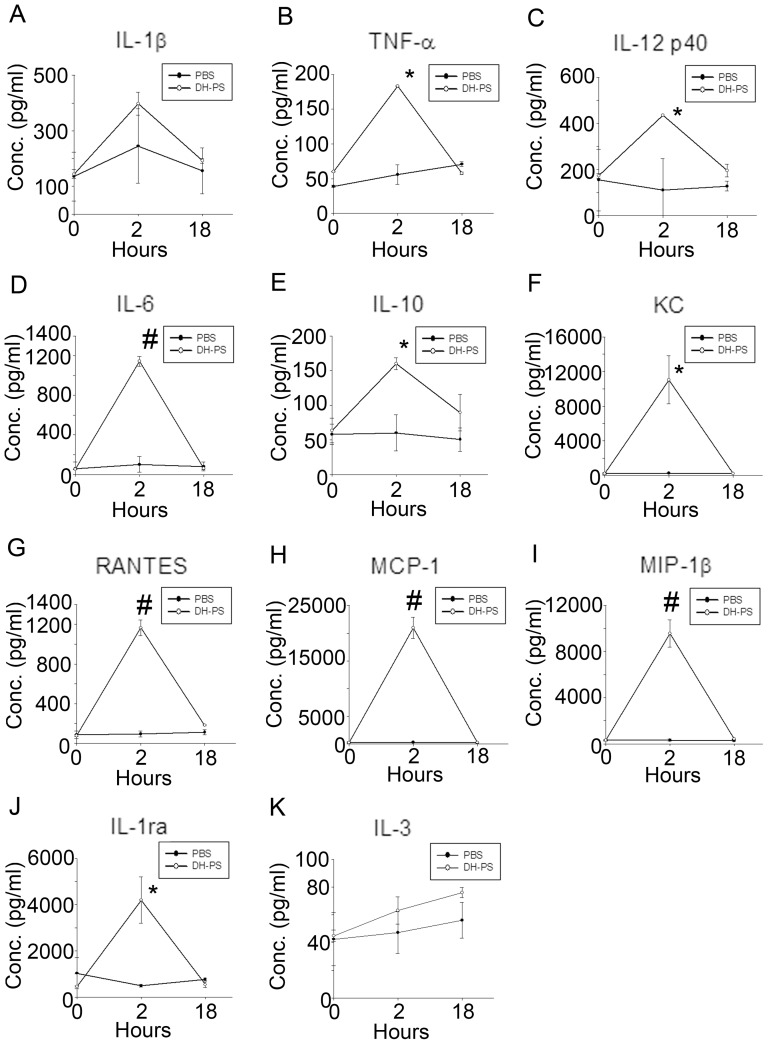
In order to understand the immunological activities of DH-PS, we investigated the profiles of cytokines and chemokines induced by DH-PS in mice. BALB/c mice (n = 3 for DH-PS and phosphate buffered saline (PBS) group) were injected intraperitoneally (IP) with DH-PS (300 μg/mouse) in PBS or PBS only and the sera were collected at 0 (before injection), 2 and 18 hrs after the injection of DH-PS for the detection of cytokines and chemokines, which were quantified by Beadlyte Mouse 21-plex Cytokine Detection system and ELISA kits (for IL-1ra). Compared with PBS group shown in Figure 1, the increase of TNF-α, IL-12 p40, IL-6, IL-10 and RANTES were observed at 2 hrs, along with robust inductions of KC, MCP-1 and MIP-1β. An apparent increase in IL-1β at 2 hrs was noted but it did not reach statistical significance. These results suggested that DH-PS induced Th1 (IL-12 p40), Th2 (IL-6, IL-10), inflammatory cytokines (TNF-α) and chemokines (KC, RANTES, MCP-1, MIP-1β), which might further modulate the downstream activations of immune cells. Furthermore, an anti-inflammatory molecule, IL-1ra, was increased from 465 to 4199 pg/ml (∼9 folds of PBS control group) at 2 hrs. This indicated that the amounts of IL-1ra induced were 10 folds more than IL-1β (increased from 144 to 397 pg/ml, ∼1.6 folds of PBS control group), suggesting that DH-PS-induced IL-1ra might over-ride the activity induced by IL-1β.
Figure 1. DH-PS elicited the productions of cytokines and chemokines in vivo.
BALB/c mice (n = 3 for DH-PS and PBS group) were injected intraperitoneally with DH-PS (300 μg/mouse) or PBS only. Sera collected at 0 (before injection), 2 and 18 hours were used for the measurements of cytokines and chemokines. Y-axis represented the mean concentrations (Conc.) of cytokines/chemokines with error bars showing the standard deviation of three mice. Statistically significant difference: * compared with PBS-treated group, p<0.01. # compared with PBS-treated group, p<0.001.
DH-PS expanded and activated subpopulations of immune cells in vivo
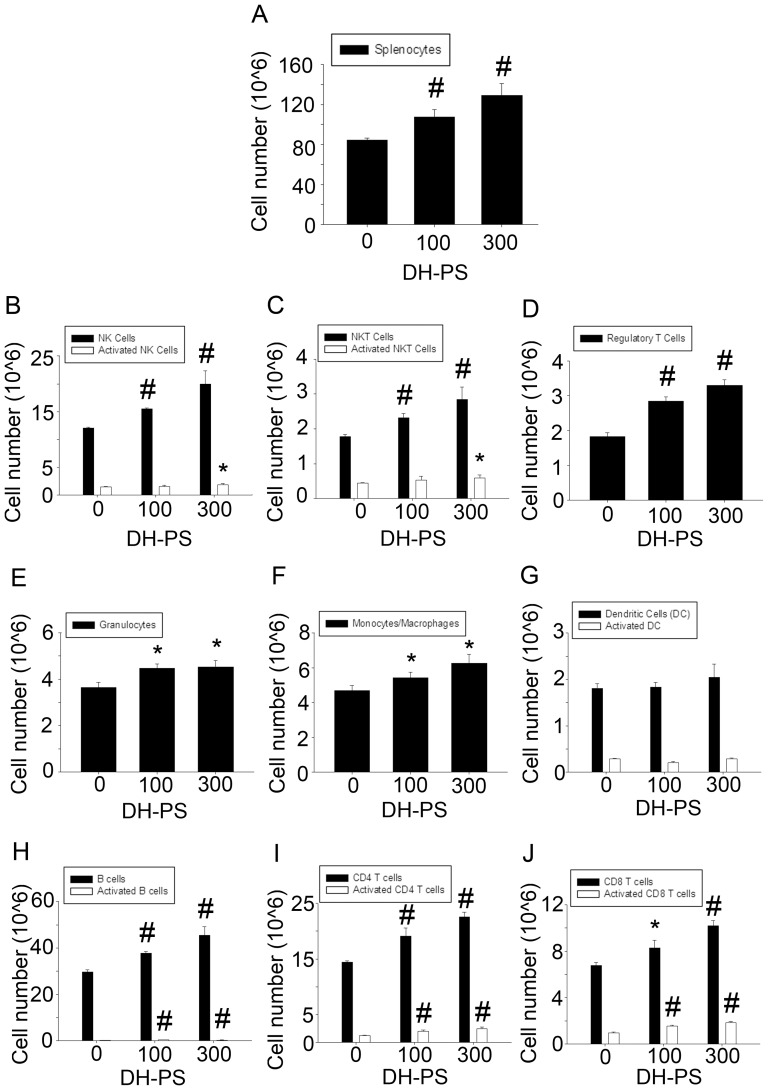
In order to investigate the effects of DH-PS on immune cells, we further analyzed whether DH-PS expanded and/or activated the subpopulations of mouse splenocytes. BALB/c mice (n = 3 for DH-PS and PBS group) were injected intraperitoneally with DH-PS (100 μg or 300 μg) or PBS (DH-PS = 0) only and sacrificed at 72 hrs for the harvest of splenocytes. Cells were counted and analyzed for markers characteristic of specific subpopulations of splenocytes by flow cytometry to determine the expansions/activations of various immune effector cells. As shown in Figure 2A, in comparison with PBS control group, the number of splenocytes was increased after the administration of DH-PS in a dose-dependent manner. Subpopulations of splenocytes examined included innate immune cells such as natural killer cells (NK-1.1+/CD3−)/activated natural killer cells (NK-1.1+/CD3−/CD69+), natural killer T cells (NK-1.1+/CD3+)/activated natural killer T cells (NK-1.1+/CD3+/CD69+), regulatory T cells (CD4+/CD25+/Foxp3+), granulocytes (Ly6G+), monocytes and macrophages (CD11b+) and dendritic cells (CD11c+/CD80+/CD86+) and adaptive immune cells such as B cells (B220+CD23+)/activated B cells (B220+CD23+CD69+), CD4+ T cells (CD3+CD4+)/activated CD4+ T cells (CD3+CD4+CD69+), CD8+ T cells (CD3+CD8+)/activated CD8+ T cells (CD3+CD8+CD69+). Overall, DH-PS induced modest increases (up to 2.08 fold) in all subpopulations examined. Compared with PBS group, we found that DH-PS induced 1.3 and 1.7 fold increases of NK cells at 100 μg and 300 μg, respectively. A mild increase (1.24 folds) of activated NK cells was also observed at 300 μg (Fig.2B). DH-PS induced 1.3 and 1.6 fold increases of NKT cells at 100 μg and 300 μg, respectively. An increase (1.37 folds) of activated NKT cells was also observed at 300 μg (Fig.2C). DH-PS also induced 1.55 and 1.79 fold rise of regulatory T cells at 100 μg and 300 μg, respectively (Fig.2D) and a trend in increases of granulocytes and monocytes/macrophages (Fig.2E, F). However, there was no significant effect of DH-PS on dendritic cells (Fig.2G). For the adaptive immune cells, DH-PS induced 1.27 and 1.53 fold increases of B cells and 2.08 and 1.91 fold rise of activated B cells at 100 μg and 300 μg, respectively (Fig.2H). As to CD4+ T cells (Fig.2I), DH-PS induced 1.32 and 1.56 fold increases at 100 μg and 300 μg, respectively. Activated CD4+ T cells were also augmented to 1.64 and 1.98 folds, respectively. For CD8+ T cells (Fig.2J), DH-PS induced 1.22 and 1.50 fold increases and activated CD8+ T cells to 1.63 and 1.96 folds at 100 μg and 300 μg, respectively. These findings indicated that DH-PS modestly expanded and/or activated many types of immune cells in mice.
Figure 2. DH-PS expanded subpopulations of splenocytes.
BALB/c mice (n = 3 for DH-PS and PBS group) were injected intraperitoneally with DH-PS (100 μg or 300 μg) or PBS only (DH-PS = 0) and sacrificed at 72 hrs for the harvest of splenocytes. Cell populations were characterized by the following markers: NK cells (NK-1.1+CD3−), activated NK cells (NK-1.1+CD3−CD69+), NKT cells (NK-1.1+CD3+), activated NKT cells (NK-1.1+CD3+CD69+), regulatory T cells (CD4+CD25+FOXP3+), granulocytes (Ly6G+), monocytes and macrophages (CD11b+), dendritic cells (CD11c+), activated dendritic cells (CD11C+CD80+CD86+), B cells (B220+CD23+), activated B cells (B220+CD23+CD69+), CD4+ T cells (CD3+CD4+), activated CD4+ T cells (CD3+CD4+CD69+), CD8+ T cells (CD3+CD8+) and activated CD8+ T cells (CD3+CD8+CD69+). Results were presented as mean values with error bars showing the standard deviation of three mice. Results were converted to log value before the determination of statistical significance. Statistically significant difference: * compared with PBS-treated group, p<0.05. # compared with PBS-treated group, p<0.01.
DH-PS induced the productions of multiple cytokines and chemokines in human immune cells
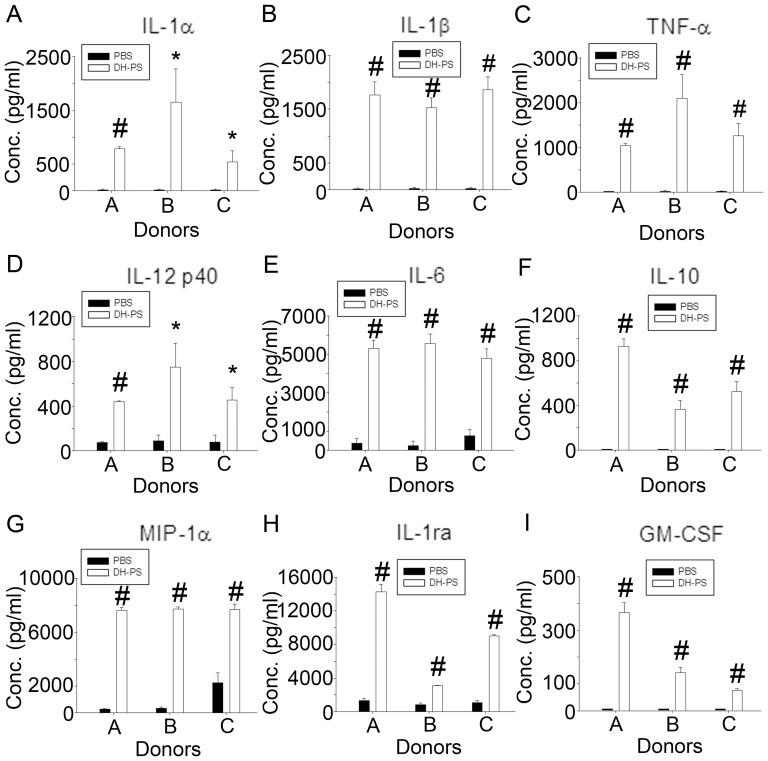
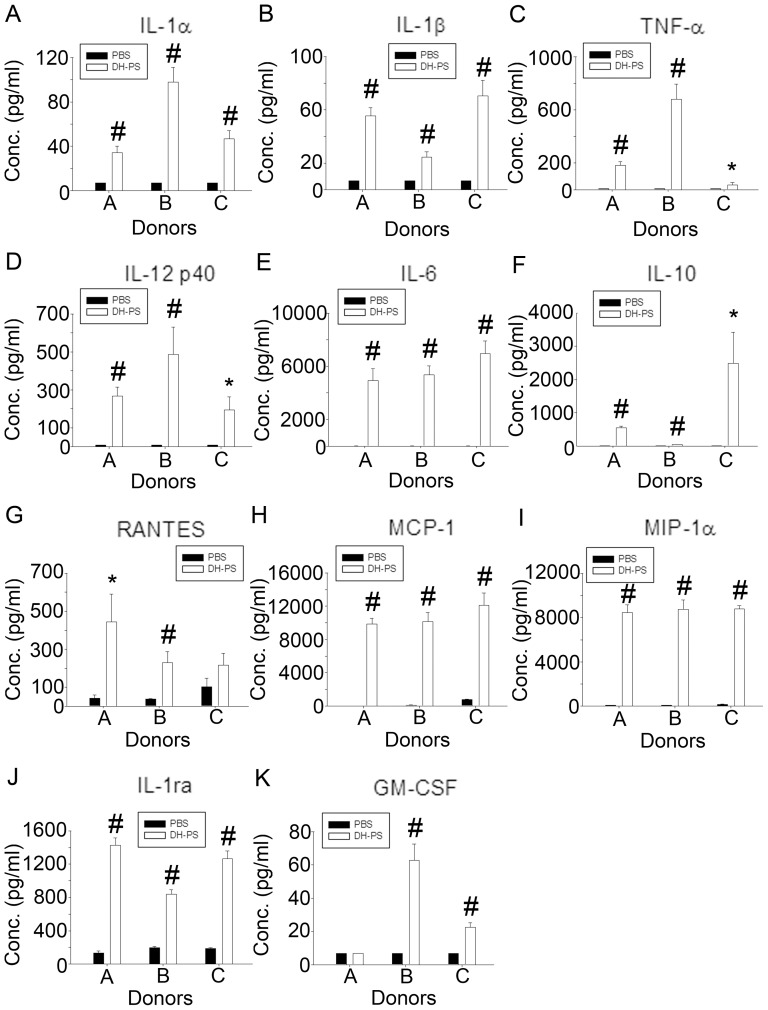
We next investigated the effects of DH-PS on primary human immune cells. Human peripheral blood mononuclear cells (PBMC) were isolated from three healthy donors and cultured with DH-PS (50 μg/ml) or PBS as control for 18 hrs. Supernatants were collected for the measurements of cytokines, chemokines and IL-1ra. As shown in Figure 3, TH1 (IL-12 p40), TH2 (IL-6, IL-10), inflammatory cytokines (IL-1α, IL-1β TNF-α) and chemokines (MIP-1α) were induced in PBMC by DH-PS. A growth factor GM-CSF was also augmented by DH-PS. Consistent with mouse data, IL-1ra was induced by DH-PS in human PBMC. Since PBMC contained a mixture of immune cells, we next focused on the DH-PS-induced effects on CD14+ cells which were reported to play a key role in rheumatoid arthritis. CD14+ cells were isolated from PBMC and cultured with DH-PS (50 μg/ml) or PBS as control for 18 hrs. The supernatants were collected for the measurements of cytokines, chemokines and IL-1ra. As shown in Figure 4, there were significant increases in IL-1α, IL-1β and IL-1ra along with IL-12 p40, IL-6, TNF-α, IL-10, GM-CSF, RANTES, MCP-1 and MIP-1α. Moreover, DH-PS boosted IL-1ra production from basal levels of (135–196) to 1427, 837 and 1264 (pg/ml) and induced IL-1β from 7 to 55, 24 and 70 (pg/ml) in CD14+ cells of 3 healthy donors. Thus the calculated IL-1ra/IL-1β ratios were 25.9, 34.8 and 18.1, suggesting that the IL-1ra induced by DH-PS might over-ride IL-1β activities. These data indicated that DH-PS induced Th1, Th2, inflammatory cytokines, chemokines and an anti-inflammatory molecule, IL-1ra, with an overall anti-IL-1β activity and the pattern of cytokines and chemokines induced by DH-PS was similar to the results in mice.
Figure 3. DH-PS elicited the productions of cytokines and chemokines in human peripheral blood mononuclear cells (PBMC).
Human PBMCs isolated from three healthy donors were cultured (2×106 cells/ml) with DH-PS (50 μg/ml) or PBS as control for 18 hrs. Supernatants were harvested for the measurements of cytokines and chemokines. Y-axis represented the mean concentrations (Conc.) of cytokines/chemokines with error bars showing the standard deviation of triplicate. Statistically significant difference: * compared with PBS-treated group, p<0.05. # compared with PBS-treated group, p<0.005.
Figure 4. DH-PS elicited the productions of cytokines and chemokines in human CD14+cells.
Human CD14+cells isolated from three healthy donors were cultured (1×106 cells/ml) with DH-PS (50 μg/ml) or PBS as control for 18 hrs. Supernatants were harvested for the measurements of cytokines and chemokines. Y-axis represented the mean concentrations (Conc.) of cytokines/chemokines with error bars showing the standard deviation of triplicate. Statistically significant difference: * compared with PBS-treated group, p<0.05. # compared with PBS-treated group, p<0.005.
Dose-dependency of IL-1ra induction by DH-PS in mice
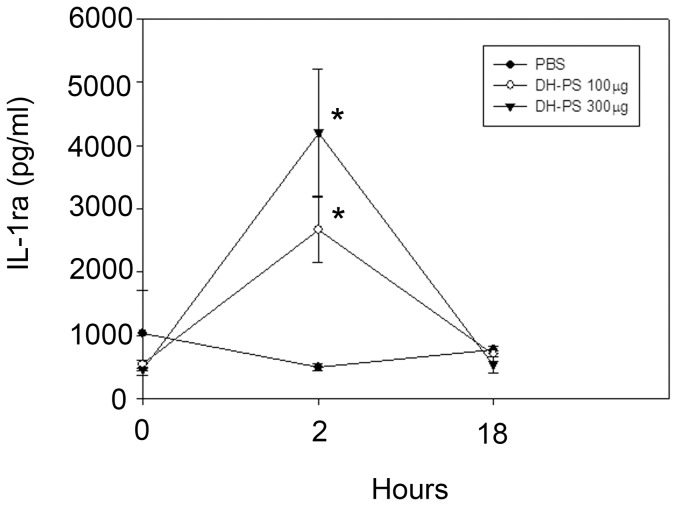
As a potent antagonist of the biological functions of IL-1, IL-1ra has been in clinical use for the treatment of IL-1-induced pathogenic conditions including rheumatoid arthritis. To further delineate the kinetics and dose effects of DH-PS on IL-1ra production, BALB/c mice (n = 3 for each group) were injected intraperitoneally with DH-PS (100 or 300 μg/mouse) or PBS. Sera were collected at 0 (before injection), 2 and 18 hours for the measurements of IL-1ra. As shown in Figure 5, IL-1ra was significantly increased in a dose-dependent manner at 2 hrs after the administration of DH-PS, as compared to PBS group, and declined rapidly to basal level at 18 hrs.
Figure 5. DH-PS induced IL-1ra production in vivo.
BALB/c mice (n = 3 for each group) were injected intraperitoneally with DH-PS (100 or 300 μg/mouse) or PBS only. Sera collected at 0 (before injection), 2 and 18 hours were used for the measurements of IL-1ra by ELISA assay. Results were presented as mean concentrations with error bars showing the standard deviation of three mice. Statistically significant difference: * compared with PBS-treated group, p<0.05.
DH-PS induced IL-1ra secretion in human monocytes but not neutrophils
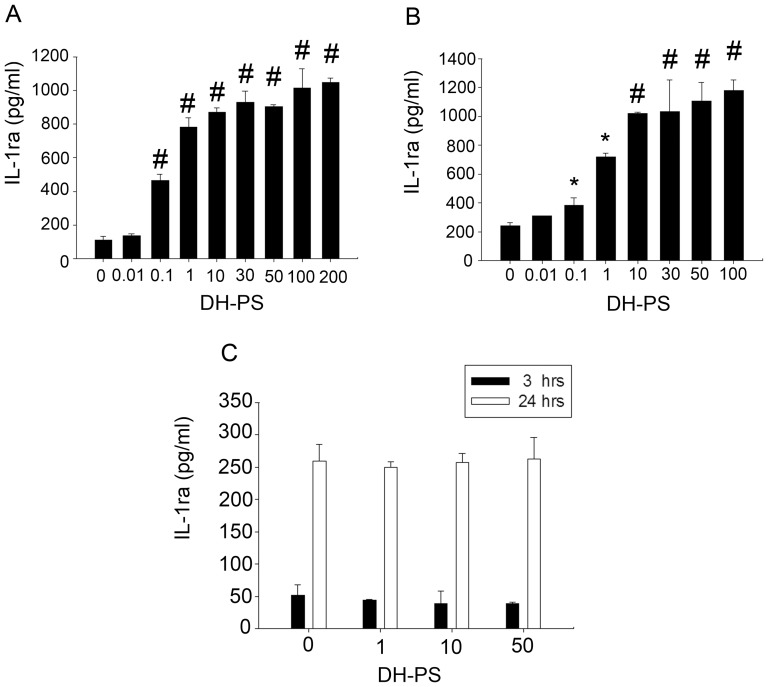
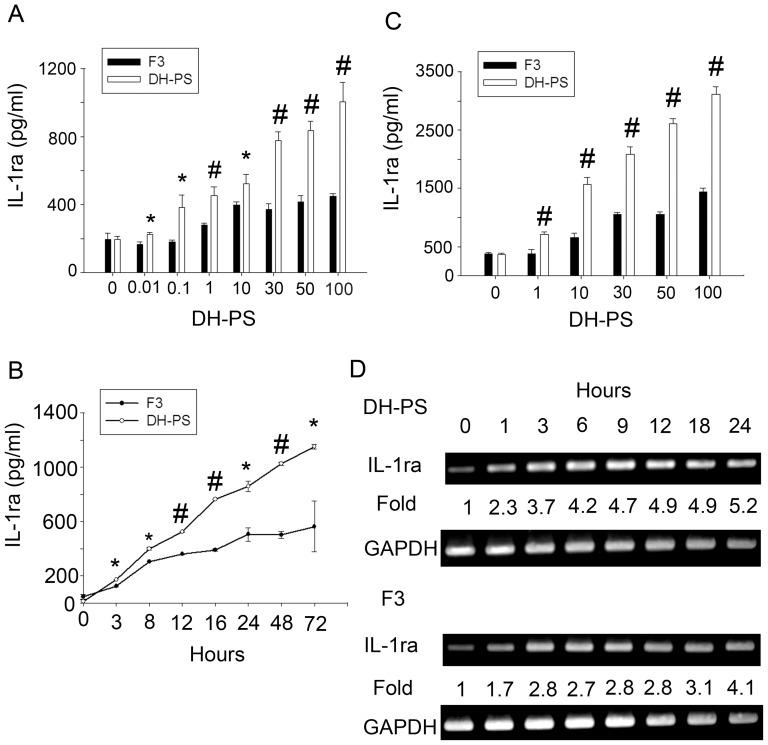
Next, we investigated the effects of DH-PS on IL-1ra secretion in human primary immune cells. At the concentrations ranging from 0 (use of PBS as control) to 200 μg/ml, DH-PS induced dose-dependent increases of IL-1ra production in PBMC, reaching the maximal level of ∼10 fold rise at 100 μg/ml (Fig.6A). Since monocytes and neutrophils were two types of immune cells reported to produce IL-1ra upon stimulations [24], we further investigated the responses of the two types of cells stimulated by DH-PS. CD14+ cells were isolated from PBMC and cultured with increasing concentrations of DH-PS ranging from 0 (use of PBS as control) to 100 μg/ml for 18 hrs. As shown in Figure 6B, DH-PS induced CD14+ cells to produce IL-1ra dose-dependently, reaching the maximal level of ∼6 fold increases at 100 μg/ml. However, neutrophils showed no significant production of IL-1ra with the stimulation of DH-PS for 3 or 24 hrs (Fig.6C). Taken together, DH-PS stimulated IL-1ra production in human CD14+ cells but not neutrophils.
Figure 6. DH-PS induced IL-1ra production in human PBMC, monocytes, but not neutrophils.
(A) Human PBMCs were cultured (2×106 cells/ml) with increasing concentrations of DH-PS for 18 hrs and the supernatants were harvested for IL-1ra measurements. (B) Human monocytes (CD14+ cells) were cultured (1×106 cells/ml) with increasing concentrations of DH-PS for 18 hrs and the supernatants were harvested for IL-1ra measurements. (C) Neutrophils were cultured (1×105 cells/ml) with the increasing concentrations of DH-PS for 3 or 24 hours and supernatants were harvested for IL-1ra measurements. Y-axis represented the mean concentration of IL-1ra (pg/ml). X-axis represented the concentration of DH-PS (μg/ml). Concentration 0 represented the use of PBS only as vehicle control. Results were presented as mean values with error bars showing the standard deviation of triplicate. Statistically significant difference: * compared with PBS-treated group, p<0.05. # compared with PBS-treated group, p<0.005.
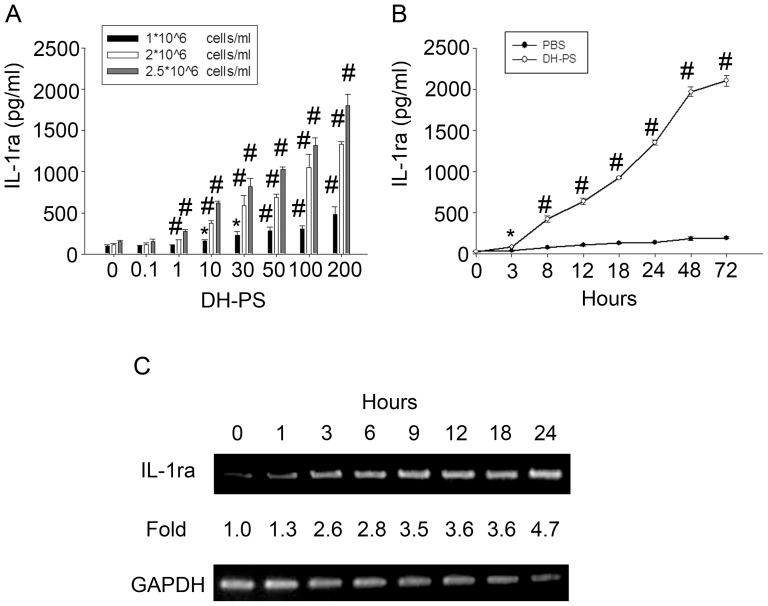
Next, we used a human monocytic cell line, THP-1, as our model for more detailed studies. THP-1 cells were seeded at different densities and cultured with increasing concentrations of DH-PS for 18 hr. As shown in Figure 7A, the production of IL-1ra was dose-dependent starting from 1 μg/ml. The kinetics of IL-1ra induced by DH-PS was examined in THP-1 cells cultured at 2×106 cells/ml with DH-PS (100 μg/ml) or PBS as control. The supernatants were collected for IL-1ra measurement at various time points. As shown in Figure 7B, DH-PS induced 2.2 fold increases of IL-1ra production at 3 hrs and the induction steadily increased to 72 hrs. The kinetics of IL-1ra induced by DH-PS at the RNA level was also examined by RT-PCR. As shown in Figure 7C, IL-1ra mRNA became detectable at 1 hr and appeared to reach the plateau at 12 hrs. In addition to the induction of IL-1ra in THP-1 cells, there was a dose-dependent increase in cell number upon incubation with increasing concentrations of DH-PS (up to 1.5 folds), suggesting that DH-PS might directly promote cell expansions besides the stimulation of cytokine secretions (Fig.S2).
Figure 7. DH-PS dose-dependently induced IL-1ra production in human monocytic cell line THP-1 cells.
(A) THP-1 cells were cultured with increasing concentrations of DH-PS at different cell densities for 18 hrs and supernatants were harvested for IL-1ra measurements. X-axis represented the concentration of DH-PS (μg/ml). Concentration 0 represented the use of PBS only as vehicle control. (B) THP-1 cells were cultured (2×106 cells/ml) with DH-PS (100 μg/ml) or PBS and supernatants were collected in indicated time points for IL-1ra measurements. Results were presented as mean values with error bars showing the standard deviation of triplicate. Statistically significant difference: * compared with PBS-treated group, p<0.05. # compared with PBS-treated group, p<0.005. (C) THP-1 cells were cultured (2×106 cells/ml) with DH-PS (100 μg/ml) and cells were collected in indicated time points for the assessment of mRNA expression of IL-1ra by RT-PCR.
Intracellular signaling of DH-PS-induced IL-1ra production in monocytes
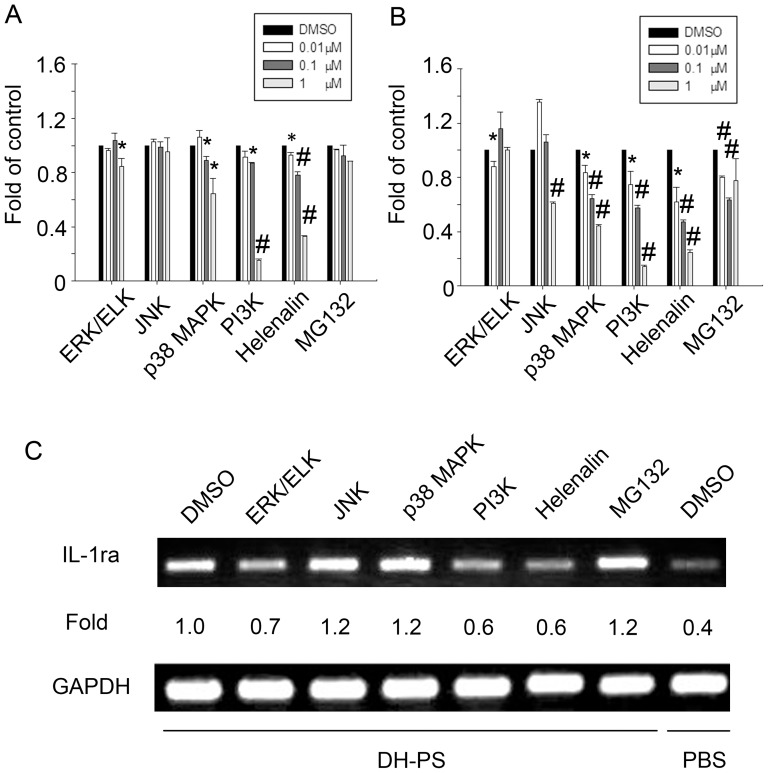
To investigate DH-PS-induced intracellular signaling mediating IL-1ra expression, we utilized inhibitors for various kinases including ERK/ELK (PD98059), JNK (SP600125), p38 MAPK (SB203580), PI3K (Ly294002) and NFκB inhibitors (Helenalin and MG132) at concentrations showing no toxicity to cells (Fig.S1). THP-1 cells (Fig.8A) or human CD14+ cells (Fig.8B) were pretreated with these inhibitors at increasing concentrations or DMSO as control for 60 minutes and cultured with DH-PS (100 μg/ml) for another 18 hrs. In both THP-1 and human CD14+ cells, the inhibitors of ERK/ELK, JNK, p38 MAPK, PI3K and NFκB diminished the secretion of IL-1ra in a dose-dependent manner. In order to determine whether these signaling molecules regulated the IL-1ra production at transcriptional level, THP-1 cells were pretreated with the inhibitors at indicated concentrations or DMSO as control for 60 minutes and cultured with DH-PS (100 μg/ml) for another 12 hrs. The mRNA expression level of IL-1ra was examined by RT-PCR. The results showed that inhibitors of ERK/ELK, PI3K and NFκB (Helenalin) dampened IL-1ra mRNA expression (Fig.8C), which was consistent with the results of IL-1ra production in protein level (Fig.8A). On the other hand, the inhibitors of JNK and p38 MAPK had no significant effects on mRNA expression.
Figure 8. DH-PS induced IL-1ra production through MAPK, PI3K and NF-κB.
(A) THP-1 cells (2×106 cells/ml) were pretreated with the indicated concentrations of inhibitors for various kinases including ERK/ELK, JNK, p38 MAPK, PI3K and NFκB (0.01, 0.1 and 1 μM, except for ERK and PI3K: 0.1, 1 and 10 μM) or DMSO (0.1%) as control for 60 minutes and cultured with DH-PS (100 μg/ml) for another 18 hrs (B) Human CD14+ cells were pretreated with the indicated concentrations of inhibitors or DMSO as control like (A) and cultured with DH-PS (100 μg/ml) for another 18 hrs. Supernatants were harvested for IL-1ra measurements. Results were presented as fold of control (Y-axis) derived from the mean values of IL-1ra concentrations of inhibitor-treated groups divided by DMSO control group and error bars showed the standard deviation of triplicate. Statistically significant difference (Mean concentrations of IL-1ra were used for the comparisons): * compared with DMSO-treated group, p<0.05. # compared with DMSO-treated group, p<0.005. (C) THP-1 cells (2×106 cells/ml) were pretreated with inhibitors for ERK/ELK (10 μM), JNK (1 μM), p38 MAPK (1 μM), PI3K (10 μM) and NFκB (1 μM) or DMSO (0.1%) as control and cultured with DH-PS (100 μg/ml) for another 12 hrs. Cells were collected for the assessment of mRNA expression of IL-1ra.
DH-PS induced larger amounts of IL-1ra than F3 (G. lucidum)
Ganoderma lucidum is a Chinese herbal medicine that has been used for centries to treat a variety of diseases including inflammation and cancer [28]. F3, the polysaccharide extract of Ganoderma lucidum has been reported to possess immune-modulating functions and induced IL-1ra in mice [27]. Therefore, we examined the induction of IL-1ra by DH-PS or F3 in human CD14+ cells and THP-1 cells and the kinetics of IL-1ra mRNA expression in THP-1 cells. Human CD14+ cells (Fig.9A) and THP-1 cells (Fig.9C) were cultured with increasing concentrations of DH-PS or F3 for 18 hrs. The kinetics of IL-1ra secretion in CD14+ cells upon the treatments of DH-PS and F3 (100 μg/ml) was also measured (Fig.9B). As shown in Figure 9A and 9C, both DH-PS and F3 induced dose-dependent productions of IL-1ra, but the maximal level induced by DH-PS was 2.2 folds of that by F3 in both CD14+ and THP-1 cells. As to the kinetics of IL-1ra induction in CD14+ cells, DH-PS elicited faster and larger amounts of IL-1ra than F3 with 1.4, 1.7 and 2.0 folds at 3, 24 and 48 hrs, respectively, reaching 2.1 folds at 72 hrs (Fig.9B). We also assessed the kinetics of IL-1ra mRNA expression in THP-1 cells cultured with DH-PS or F3. As shown in Figure 9D, DH-PS induced higher IL-1ra mRNA expression than F3, which was consistent with the ELISA data. On the other hand, F3 induced larger amounts of IL-1β (366 pg/ml, ∼52 fold increases of PBS control) (Fig.S3) than DH-PS in human CD14+ cells (55, 24, 70 pg/ml for 3 healthy donors respectively, Fig.4). Taken together, DH-PS-induced IL-1ra rise could over-ride IL1β-induced activity far more effectively than F3.
Figure 9. DH-PS induced more IL-1ra production than F3 in human CD14+ cells and THP-1 cells.
(A) Human CD14+ cells isolated from one healthy donor were cultured (2×106 cells/ml) with increasing concentrations of DH-PS or F3 for 18 hours and supernatants were collected for the measurements of IL-1ra. (B) Human CD14+ cells were cultured with DH-PS (100 μg/ml) or F3 (100 μg/ml) and supernatants were collected at the indicated time points for IL-1ra measurements. (C) THP-1 cells were cultured (2×106 cells/ml) with increasing concentrations of DH-PS or F3 for 18 hours and supernatants were collected for the measurements of IL-1ra. In A and C, X-axis represented the concentration of DH-PS (μg/ml). Concentration 0 represented the use of PBS only as vehicle control. Results were presented as mean concentrations of IL-1ra with error bars showing the standard deviation of triplicate. Statistically significant difference: * compared with F3-treated group, p<0.05. # compared with F3-treated group, p<0.005. (D) THP-1 cells were cultured with DH-PS (100 μg/ml) or F3 (100 μg/ml) and cells were collected at the indicated time points for the assessment of mRNA expression of IL-1ra.
Discussion
Dendrobium huoshanense (DH) is a versatile and valuable Chinese herbal medicine that has been used for a long period of time in China. The main bioactive molecules are polysaccharides and alkaloids. Since it is highly valuable but rare, strategies have been developed to enhance its growth and increases in polysaccharides production [29]. Using crude extracts of polysaccharides isolated from DH, we previously showed the in vitro induction of G-CSF and a few other cytokines in mouse splenocytes [30]. Our present study further extended the investigation to cover a comprehensive panel of cytokines and chemokines induced by DH-PS in immune cells of human in vitro and mouse in vivo. Our results clearly showed that the administration of DH-PS in mice modulated immune functions through modest activation and/or expansion of various immune cells including NK cells/activated NK cells, NKT cells/activated NKT cells, regulatory T cells, B cells/activated B cells, CD4+ T cells/activated CD4+ T cells and CD8+ T cells/activated CD8+ T cells. This was accompanied by the production of Th1 (IL-12 p40), Th2 cytokines (IL-6 and IL-10), chemokines (KC, MCP-1, MIP-1β, RANTES) and inflammatory cytokines (TNF-α). We also provided the first evidence that many cytokines and chemokines were induced by DH-PS in human PBMC (IL-1α, IL-1β, IL-12 p40, IL-6, IL-10, TNF-α, MIP-1α GM-CSF and IL-1ra) and monocytes (IL-1α, IL-1β, IL-12 p40, IL-6, IL-10, TNF-α, RANTES, MCP-1, MIP-1α, GM-CSF and IL-1ra). Notably, both IL-6 and IL-10 which have been shown to stimulate the production of IL-1ra were also induced by DH-PS. These findings were reminiscent of the potent immune-modulating effects induced by Ganoderma lucidum (Reishi), another popular Chinese herbal medicine. It was reported that polysaccharides isolated from Ganoderma lucidum (Reishi) enhanced the proliferation of Con A-stimulated mouse splenocytes [31]. We also showed that F3 stimulated the expansions of several types of immune cells including regulatory T cells [32]. But unlike DH-PS, F3 appeared to induce fewer regulatory T cells than DH-PS and larger amounts of IFN-r and IL-12 p70 which were below the detection limit upon the treatment with DH-PS, suggesting that DH-PS might exert greater anti-inflammatory activities. Taken together, DH-PS might exert its immune modulations not only by directly stimulating cytokine secretions but also through promoting the expansions and/or activations of immune cells.
Another important anti-inflammatory molecule found to be induced by DH-PS was IL-1ra which was an acute phase protein [33] and often elevated in the peripheral blood of patients with sepsis [34], chronic rheumatic diseases [35], [36], [37] and following surgical trauma [38], [39]. The beneficial effects of IL-1ra on inflammatory disorders had been demonstrated in many experimental animal models of disease by administration of recombinant IL-1ra [26], [40], [41]. In fact, recombinant IL-1ra had been in clinical use for sepsis syndrome [42], [43] and rheumatoid arthritis. Many molecules including cytokines (IL-6 and IL-10, for example) and β-glucans [26] which were the backbone components of the main bioactive polysaccharides of G. lucidum were reported to induce IL-1ra production. Here, we demonstrated for the first time that DH-PS induced the production of IL-1ra both in vivo and in vitro. The rapid induction of IL-1ra by DH-PS in both mouse and human suggested a direct stimulation by DH-PS. Many types of immune cells were known to secrete IL-1ra including macrophages, mast cells, neutrophils and monocytes, and our results showed that DH-PS stimulated the production of IL-1ra in monocytes but not neutrophils. We also found that unlike in vitro cell experiments, the level of IL-1ra in sera declined rapidly at 18 hrs after the treatment of DH-PS. This is consistent with the known short biological half-life of IL-1ra in vivo with rapid renal clearance and excretion in the urine [44]. In addition, DH-PS promoted THP-1 cell expansions dose-dependently, suggesting that DH-PS might also stimulate the proliferation of cells. Since certain glycans were the ligands for Toll-Like Receptors (TLRs) which were important for the activation of monocytes and involved in the transcription of IL-1ra [25], [45], it will be interesting to delineate whether and which TLRs are the receptors for DH-PS in the future.
There were several intracellular signaling mechanism reported to be involved in the regulation of IL-1ra expression. For examples, NF-κB and C/EBP had been shown to be involved in the expression of IL-1ra gene in hepatocytes [33], and MAPK (ERK1/ERK2) was associated with the IL-1ra induction stimulated by LPS [46], [47]. Serine/threonine phosphatases also participated in IL-1ra production when monocytes were contacted by stimulated T cells [48]. In addition, STAT6 was involved in IL-4 mediated IL-1ra production [49] and PI3K was reported to be essential for the IFN-β-mediated IL-1ra production [50]. Our results suggested that ERK/ELK, p38 MAPK, PI3K and NF-κB were involved in DH-PS mediated production of IL-1ra since their specific inhibitors decreased the expression of IL-1ra mRNA and the IL-1ra production in protein level. Our results suggested several possible signaling pathways involved in DH-PS-induced IL-1ra secretion.
Ganoderma lucidum (Reishi) has been known for its benefits in human health with the possession of anti-tumor and immune-modulating activities [51], [52], [53], [54]. The polysaccharides isolated from Reishi were composed of a branched (1->6)-β-D-glucan moiety. The main structure of polysaccharides isolated from DH-PS had been confirmed as acetylated glucomannan [30] which is different from Reishi. The effects of Reishi on the immune system had been attributed to cytokine inductions [55]. We compared the cytokine profiles of human monocytes cultured with DH-PS or F3 and found that both DH-PS and F3 induced several cytokines and chemokines including IL-1β, TNF-α, GM-CSF, IL-12 p40, RANTES, MCP-1, MIP-1α, IL-6 and IL-10. However, IL-12 p70 and IFN-γ were induced only by F3, suggesting a more TH1 bias activity of F3 compared with DH-PS since IL-12 p70 and IFN-γ had been reported to contribute to the differentiation of type 1 T helper cell [56]. The ability of F3 to induce the production of IL-1ra has also been reported [27]. However, we found that DH-PS induced higher IL-1ra and lower IL-1β than F3 in human CD14+ cells and THP-1 cells Collectively, our findings of lower levels of IL-12 p70, IFN-γ, IL-1β, larger amounts of IL-1ra and more regulatory T cells induced by DH-PS suggested that DH-PS might possess better anti-inflammatory activities than F3. Thus, DH-PS might be more potent than F3 for alleviation of inflammatory disorders. Since the main structure of DH-PS had been reported as acetylated glucomannan, it will be worthwhile to further identify the specific glycan moieties responsible for its anti-inflammatory effects and its therapeutic potential in certain immune disease models.
Materials and Methods
Ethics statement
Normal human blood was obtained from Taipei Blood Center with the approval of the Human Subject Research Ethics committee of both Academia Sinica and the Taiwan Blood Services Foundation. All participants provided written informed consent to Taipei Blood Services Foundation. All animal studies were performed under the approved protocol #TMIZ00JY2005158 by Institutional Animal Care and Utilization Committee of Academia Sinica.
Preparations for crude polysaccharide extracts from Dendrobium huoshanense (DH-PS)
The plant material of D. huoshanense was obtained from Yuen-Foong-Yu Biotech Co. Taiwan [30]. Non-lignified primary mucilage polysaccharides were collected from ground leaves and stems at 4°C by dd-H2O extraction and the extracts were filtrated to remove the insoluble parts. The dd-H2O extractions (DH-PS) were dried for storage and resuspended in PBS before animal or cell experiments.
Cell culture and reagents
Human peripheral blood mononuclear cells (PBMC) were isolated from healthy donors by Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) according to manufacturer's suggestions. CD14+ cells were further purified from PBMC by anti-CD14 microbeads and magnetically activated cell sorting (MACS) system (Miltenyi Biotech, Auburn, CA) according to manufacturer's instruction. PBMC and purified CD14+ cells (purity >90%) were cultured in RPMI 1640 medium (Sigma-Aldrich, USA) supplemented with 10% heat-inactivated Fetal bovine serum (FBS) (Sigma-Aldrich, USA) and penicillin/streptomycin (100 units/ml) (Invitrogen, CA, USA). For isolation of neutrophils from healthy donors, leukocytes (including neutrophils and PBMC) were separated from red blood cells (RBC) by differential sedimentation using 1.5% dextran in PBS. Neutrophils were separated from PBMC by Ficoll-Paque PLUS gradient method and further separated from the remaining RBC in the pellet by hypotonic lysis method (purity>90%) [57]. THP-1 cells (ATCC number: TIB-202) was maintained in RPMI 1640 supplemented with 10% FBS and penicillin/streptomycin (100 units/ml). For experiments of cell signaling pathway, inhibitors including PD98059, SP600125, SB203580, LY294002, Helenalin and MG132 were purchased from Calbiochem (Merck Millipore, Germany) and dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA) for cell experiments.
RNA isolation and RT-PCR
Total RNA was isolated by Trizol (Invitrogen, CA, USA) according to manufacturer's protocol. RNA (1 μg) was reverse-transcribed to cDNA by ThermoScript RT-PCR system (Invitrogen, CA, USA). PCR was performed using Go Taq green master mix (Promega, USA). Primers for IL-1ra: forward primer: GGCCTCCGCAGTCACCTAATCACTCT, reverse primer: TACTACTCG TCCTCCTGGAAGTAGAA. The PCR conditions were as follows: 94°C for 1 minute followed by 35 cycles of 94°C for 1 minute, 58°C for 30 seconds, 72°C for 2 minutes and a final step of 72°C for 10 minutes.
Animal experiments and flow cytometric analysis
Mice (Six-week-old female BALB/c, from National Laboratory Animal Center, Taiwan) were injected intraperitoneally with DH-PS (100 μg or 300 μg) in PBS or PBS only as control and sacrificed at 72 hrs for the harvest of splenocytes. For flow cytometric analysis, cells were resuspended in PBS (1×106 cells/ml) containing 2% FBS and 0.1% sodium azide (Sigma-Aldrich, USA) and stained with cell surface markers including NK-1.1+CD3− (NK cells), NK-1.1+CD3−CD69+ (activated NK cells), NK-1.1+CD3+ (NKT cells), NK-1.1+CD3+CD69+ (activated NKT cells), Ly6G+ (granulocytes), CD11b+ (monocytes and macrophages), CD11c+ (dendritic cells), CD11C+CD80+CD86+ (activated dendritic cells), B220+CD23+ (B cells), B220+CD23+CD69+ (activated B cells), CD3+CD4+ (CD4+ T cells), CD3+CD4+CD69+ (activated CD4+ T cells), CD3+CD8+ (CD8+ T cells) and CD3+CD8+CD69+ (activated CD8+ T cells). For the staining of regulatory T cells (CD4+CD25+FOXP3+), cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, USA) in PBS for 30 minutes at room temperature and permeabilized with 1% triton x-100 (Sigma-Aldrich, USA) in PBS for another 30 minutes before staining with characteristic markers for flow cytometric analysis. After washing cells with PBS, cells were analyzed on FACS Calibur (Becton Dickinson, San Jose, CA) with CellQuest software. Antibodies included PE-conjugated NK-1.1, Ly6G, CD3, CD25, CD86, FITC-conjugated CD3, CD4, CD69, CD80 and APC-conjugated CD11b, CD11c, Foxp3. All antibodies were purchased from BD bioscience, USA.
Measurements of cytokines, chemokines and IL-1ra
To determine whether DH-PS changed the profiles of the secretions of cytokines and/or chemokines in vivo, mouse sera (obtained from facial vein blood sampling, Lancet) were collected at 0 (before the injection), 2 and 18 hrs after the intraperitoneal injection of DH-PS or PBS. Cytokines and chemokines were quantified by the Beadlyte mouse 21-Plex Cytokine Detection system (Millipore, Temecula, CA). For the detection of IL-1ra, sera were quantified by Mouse IL-1ra/IL-1F3 Quantikine ELISA Kit (R&D system, USA). To determine whether DH-PS changed the profiles of the secretions of cytokines and/or chemokines in human cells, cell culture supernatants were collected at 18 hrs after DH-PS or PBS treatment. Cytokines and chemokines were quantified by the Beadlyte human 22-Plex Cytokine Detection system (Millipore, Temecula, CA). For the detection of IL-1ra, supernatants were quantified by Human IL-1ra/IL-1F3 Quantikine ELISA Kit (R&D system, USA). Beadlyte human 22-Plex Cytokine Detection system included the measurements of IL-3, IL-1α, IL-1β, IL-2, IL-12 p40, IL-12 p70, GM-CSF, IP-10, MCP-1, MIP-1α, RANTES, TNF-α, IFN-γ, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, IL-15 and Eotaxin. Beadlyte mouse 21-Plex Cytokine Detection system included the measurements of IL-3, IL-1α, IL-1β, IL-2, IL-12 p40, IL-12 p70, GM-CSF, KC, MCP-1, MIP-1β, RANTES, TNF-α, IFN-γ, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, VEGF and IL-17.
Validation of viability or the proliferation of THP-1 cells
Cell viability and proliferation assays were determined by adding MTS reagents to cell culture medium at 1∶5 (v/v) (Promega, USA) and incubating for another 1-4 hr as the manufacturer's suggestions. The results were observed through the detection of the absorbance at 490 nm by spectrophotometer (Molecular Devices, USA).
Statistical analysis
The Student's t test was utilized to analyze the results of cytokine and chemokine productions, cell numbers of subpopulations of splenocytes, IL-1ra measurements, assays of the inhibitor-treated cell viability and cell proliferation. For the determination of statistical significance of results in Figure 8, S1 and S2, the mean concentrations of IL1ra (pg/ml) (Fig.8) and mean values of absorbance at 490 nm (Fig.S1, S2) were used to statistical analysis before being converted to fold of control. P value was considered to be significant at < 0.05. Data were expressed as the mean values ± standard deviation (S.D).
Supporting Information
Kinase inhibitors were not toxic to THP-1 cells in indicated concentrations. Cells were cultured (2×106 cells/ml) with inhibitors for ERK/ELK (PD98059, 10 μM), JNK (SP600125, 1 μM), p38 MAPK (SB203580, 1 μM), PI3K (Ly294002, 10 μM), NFκB (Helenalin and MG132, 1 μM) or DMSO (0.1%) as control for 18 hrs. Viability was determined by MTS assay. Results were presented as fold of control (Y-axis) derived from the mean values of absorbance at 490 nm of inhibitor-treated groups divided by DMSO control group and error bars showed the standard deviation of triplicate.
(TIF)
DH-PS promoted the proliferation of THP-1 cells. THP-1 cells were cultured (2×106 cells/ml) with increasing concentrations of DH-PS or PBS (Concentration 0) for 18 hrs and the proliferation rate was determined by MTS assay. X-axis represented the concentration of DH-PS (μg/ml). Results were presented as fold of control derived from the mean values of absorbance at 490 nm of DH-PS-treated groups divided by PBS control group and error bars showed the standard deviation of triplicate. Statistically significant difference (Mean values of absorbance were used for the comparisons): * compared with PBS-treated group, p<0.05.
(TIF)
F3 elicited the productions of cytokines and chemokines in human CD14+ cells. Human CD14+ cells isolated from one healthy donor were cultured with F3 (50 μg/ml) or PBS for 18 hrs and supernatants were collected for the measurements of cytokines and chemokines. Y-axis represented the mean concentrations (Conc.) of cytokines/chemokines with error bars showing the standard deviation of triplicate. Statistically significant difference: * compared with PBS-treated group, p<0.05. # compared with PBS-treated group, p<0.005.
(TIF)
Acknowledgments
We would like to thank the excellent technical assistance, service and advice provided by flow cytometry facility and Luminex at the division of Medical biology, Genomic Research Center, Academia Sinica.
Funding Statement
This work is supported by Academia Sinica and National Science Council grants for A.L. Yu (NSC 101-2325-B-001-025). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zha X-Q, Luo J-P, Jiang S-T (2007) Induction of Immunomodulating Cytokines by Polysaccharides from Dendrobium huoshanense. Pharmaceutical Biology 45: 71–76. [Google Scholar]
- 2. Meng LZ, Lv GP, Hu DJ, Cheong KL, Xie J, et al. (2013) Effects of polysaccharides from different species of Dendrobium (Shihu) on macrophage function. Molecules 18: 5779–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Berg WB (2001) Anti-cytokine therapy in chronic destructive arthritis. Arthritis Res 3: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brennan FM (2001) A follow-up to “Anti-cytokine therapy in chronic destructive arthritis” by Wim B van den Berg. Arthritis Res 3: 211–213 discussion 214–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kupper TS, Chua AO, Flood P, McGuire J, Gubler U (1987) Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J Clin Invest 80: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stylianou E, O'Neill LA, Rawlinson L, Edbrooke MR, Woo P, et al. (1992) Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J Biol Chem 267: 15836–15841. [PubMed] [Google Scholar]
- 7. Seckinger P, Williamson K, Balavoine JF, Mach B, Mazzei G, et al. (1987) A urine inhibitor of interleukin 1 activity affects both interleukin 1 alpha and 1 beta but not tumor necrosis factor alpha. J Immunol 139: 1541–1545. [PubMed] [Google Scholar]
- 8. Seckinger P, Lowenthal JW, Williamson K, Dayer JM, MacDonald HR (1987) A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol 139: 1546–1549. [PubMed] [Google Scholar]
- 9. Seckinger P, Dayer JM (1987) Interleukin-1 inhibitors. Ann Inst Pasteur Immunol 138: 486–488. [DOI] [PubMed] [Google Scholar]
- 10. Balavoine JF, de Rochemonteix B, Williamson K, Seckinger P, Cruchaud A, et al. (1986) Prostaglandin E2 and collagenase production by fibroblasts and synovial cells is regulated by urine-derived human interleukin 1 and inhibitor(s). J Clin Invest 78: 1120–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dripps DJ, Brandhuber BJ, Thompson RC, Eisenberg SP (1991) Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem 266: 10331–10336. [PubMed] [Google Scholar]
- 12.Thompson RC, Dripps DJ, Eisenberg SP (1991) IL-1ra: properties and uses of an interleukin-1 receptor antagonist. Agents Actions Suppl 35: 41–49. [PubMed]
- 13. Arend WP, Malyak M, Guthridge CJ, Gabay C (1998) Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol 16: 27–55. [DOI] [PubMed] [Google Scholar]
- 14. Irikura VM, Lagraoui M, Hirsh D (2002) The epistatic interrelationships of IL-1, IL-1 receptor antagonist, and the type I IL-1 receptor. J Immunol 169: 393–398. [DOI] [PubMed] [Google Scholar]
- 15. Fischer E, Van Zee KJ, Marano MA, Rock CS, Kenney JS, et al. (1992) Interleukin-1 receptor antagonist circulates in experimental inflammation and in human disease. Blood 79: 2196–2200. [PubMed] [Google Scholar]
- 16. Dinarello CA (1991) Interleukin-1 and interleukin-1 antagonism. Blood 77: 1627–1652. [PubMed] [Google Scholar]
- 17. Dinarello CA, Thompson RC (1991) Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today 12: 404–410. [DOI] [PubMed] [Google Scholar]
- 18. Alexander HR, Doherty GM, Venzon DJ, Merino MJ, Fraker DL, et al. (1992) Recombinant interleukin-1 receptor antagonist (IL-1ra): effective therapy against gram-negative sepsis in rats. Surgery 112: 188–193 discussion 193–184. [PubMed] [Google Scholar]
- 19. McCarthy PL Jr, Abhyankar S, Neben S, Newman G, Sieff C, et al. (1991) Inhibition of interleukin-1 by an interleukin-1 receptor antagonist prevents graft-versus-host disease. Blood 78: 1915–1918. [PubMed] [Google Scholar]
- 20. Bendele AM, Chlipala ES, Scherrer J, Frazier J, Sennello G, et al. (2000) Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum 43: 2648–2659. [DOI] [PubMed] [Google Scholar]
- 21. Joosten LA, Helsen MM, van de Loo FA, van den Berg WB (1996) Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum 39: 797–809. [DOI] [PubMed] [Google Scholar]
- 22. So A, De Smedt T, Revaz S, Tschopp J (2007) A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther 9: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagaman DD, Okayama Y, D'Ambrosio C, Prussin C, Gilfillan AM, et al. (2001) Secretion of interleukin-1 receptor antagonist from human mast cells after immunoglobulin E-mediated activation and after segmental antigen challenge. Am J Respir Cell Mol Biol 25: 685–691. [DOI] [PubMed] [Google Scholar]
- 24. Malyak M, Smith MF Jr, Abel AA, Hance KR, Arend WP (1998) The differential production of three forms of IL-1 receptor antagonist by human neutrophils and monocytes. J Immunol 161: 2004–2010. [PubMed] [Google Scholar]
- 25. Darragh J, Ananieva O, Courtney A, Elcombe S, Arthur JS (2010) MSK1 regulates the transcription of IL-1ra in response to TLR activation in macrophages. Biochem J 425: 595–602. [DOI] [PubMed] [Google Scholar]
- 26. Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87: 2095–2147. [PubMed] [Google Scholar]
- 27. Hua KF, Hsu HY, Chao LK, Chen ST, Yang WB, et al. (2007) Ganoderma lucidum polysaccharides enhance CD14 endocytosis of LPS and promote TLR4 signal transduction of cytokine expression. J Cell Physiol 212: 537–550. [DOI] [PubMed] [Google Scholar]
- 28. Chen X, Hu ZP, Yang XX, Huang M, Gao Y, et al. (2006) Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int Immunopharmacol 6: 499–508. [DOI] [PubMed] [Google Scholar]
- 29. Wei M, Jiang ST, Luo JP (2007) Enhancement of growth and polysaccharide production in suspension cultures of protocorm-like bodies from Dendrobium huoshanense by the addition of putrescine. Biotechnol Lett 29: 495–499. [DOI] [PubMed] [Google Scholar]
- 30. Hsieh YS, Chien C, Liao SK, Liao SF, Hung WT, et al. (2008) Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg Med Chem 16: 6054–6068. [DOI] [PubMed] [Google Scholar]
- 31. Wang YY, Khoo KH, Chen ST, Lin CC, Wong CH, et al. (2002) Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg Med Chem 10: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 32. Lai CY, Hung JT, Lin HH, Yu AL, Chen SH, et al. (2010) Immunomodulatory and adjuvant activities of a polysaccharide extract of Ganoderma lucidum in vivo and in vitro. Vaccine 28: 4945–4954. [DOI] [PubMed] [Google Scholar]
- 33. Gabay C, Smith MF, Eidlen D, Arend WP (1997) Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest 99: 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pruitt JH, Welborn MB, Edwards PD, Harward TR, Seeger JW, et al. (1996) Increased soluble interleukin-1 type II receptor concentrations in postoperative patients and in patients with sepsis syndrome. Blood 87: 3282–3288. [PubMed] [Google Scholar]
- 35. Prieur AM, Kaufmann MT, Griscelli C, Dayer JM (1987) Specific interleukin-1 inhibitor in serum and urine of children with systemic juvenile chronic arthritis. Lancet 2: 1240–1242. [DOI] [PubMed] [Google Scholar]
- 36. Gabay C, Gay-Croisier F, Roux-Lombard P, Meyer O, Maineti C, et al. (1994) Elevated serum levels of interleukin-1 receptor antagonist in polymyositis/dermatomyositis. A biologic marker of disease activity with a possible role in the lack of acute-phase protein response. Arthritis Rheum 37: 1744–1751. [DOI] [PubMed] [Google Scholar]
- 37. De Benedetti F, Pignatti P, Massa M, Sartirana P, Ravelli A, et al. (1995) Circulating levels of interleukin 1 beta and of interleukin 1 receptor antagonist in systemic juvenile chronic arthritis. Clin Exp Rheumatol 13: 779–784. [PubMed] [Google Scholar]
- 38. EM ON, Puri P, Reen DJ (1993) Early induction of IL-1 receptor antagonist (IL-1Ra) in infants and children undergoing surgery. Clin Exp Immunol 93: 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grzelak I, Olszewski WL, Zaleska M, Durlik M, Lagiewska B, et al. (1996) Blood cytokine levels rise even after minor surgical trauma. J Clin Immunol 16: 159–164. [DOI] [PubMed] [Google Scholar]
- 40. Arend WP (1993) Interleukin-1 receptor antagonist. Adv Immunol 54: 167–227. [DOI] [PubMed] [Google Scholar]
- 41. Lennard AC (1995) Interleukin-1 receptor antagonist. Crit Rev Immunol 15: 77–105. [DOI] [PubMed] [Google Scholar]
- 42. Fisher CJ Jr, Slotman GJ, Opal SM, Pribble JP, Bone RC, et al. (1994) Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med 22: 12–21. [DOI] [PubMed] [Google Scholar]
- 43. Fisher CJ Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, et al. (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. Jama 271: 1836–1843. [PubMed] [Google Scholar]
- 44. Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, et al. (1992) Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine 4: 353–360. [DOI] [PubMed] [Google Scholar]
- 45. Flo TH, Halaas O, Lien E, Ryan L, Teti G, et al. (2000) Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol 164: 2064–2069. [DOI] [PubMed] [Google Scholar]
- 46. Guthridge CJ, Eidlen D, Arend WP, Gutierrez-Hartmann A, Smith MF Jr (1997) Lipopolysaccharide and Raf-1 kinase regulate secretory interleukin-1 receptor antagonist gene expression by mutually antagonistic mechanisms. Mol Cell Biol 17: 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dreyer MG, Juge-Aubry CE, Gabay C, Lang U, Rohner-Jeanrenaud F, et al. (2003) Leptin activates the promoter of the interleukin-1 receptor antagonist through p42/44 mitogen-activated protein kinase and a composite nuclear factor kappa B/PU.1 binding site. Biochem J 370: 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vey E, Dayer JM, Burger D (1997) Direct contact with stimulated T cells induces the expression of IL-1beta and IL-1 receptor antagonist in human monocytes. Involvement of serine/threonine phosphatases in differential regulation. Cytokine 9: 480–487. [DOI] [PubMed] [Google Scholar]
- 49. Ohmori Y, Smith MF Jr, Hamilton TA (1996) IL-4-induced expression of the IL-1 receptor antagonist gene is mediated by STAT6. J Immunol 157: 2058–2065. [PubMed] [Google Scholar]
- 50. Molnarfi N, Hyka-Nouspikel N, Gruaz L, Dayer JM, Burger D (2005) The production of IL-1 receptor antagonist in IFN-beta-stimulated human monocytes depends on the activation of phosphatidylinositol 3-kinase but not of STAT1. J Immunol 174: 2974–2980. [DOI] [PubMed] [Google Scholar]
- 51. Miyazaki T, Nishijima M (1981) Studies on fungal polysaccharides. XXVII. Structural examination of a water-soluble, antitumor polysaccharide of Ganoderma lucidum. Chem Pharm Bull (Tokyo) 29: 3611–3616. [DOI] [PubMed] [Google Scholar]
- 52. Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, et al. (1997) The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer 70: 699–705. [DOI] [PubMed] [Google Scholar]
- 53. Gao Y, Gao H, Chan E, Tang W, Xu A, et al. (2005) Antitumor activity and underlying mechanisms of ganopoly, the refined polysaccharides extracted from Ganoderma lucidum, in mice. Immunol Invest 34: 171–198. [PubMed] [Google Scholar]
- 54. Liao SF, Liang CH, Ho MY, Hsu TL, Tsai TI, et al. (2013) Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc Natl Acad Sci U S A 110: 13809–13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hsu HY, Hua KF, Lin CC, Lin CH, Hsu J, et al. (2004) Extract of Reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. J Immunol 173: 5989–5999. [DOI] [PubMed] [Google Scholar]
- 56. Anthony RM, Rutitzky LI, Urban JF Jr, Stadecker MJ, Gause WC (2007) Protective immune mechanisms in helminth infection. Nat Rev Immunol 7: 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hsu MJ, Lee SS, Lee ST, Lin WW (2003) Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum. Br J Pharmacol 139: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kinase inhibitors were not toxic to THP-1 cells in indicated concentrations. Cells were cultured (2×106 cells/ml) with inhibitors for ERK/ELK (PD98059, 10 μM), JNK (SP600125, 1 μM), p38 MAPK (SB203580, 1 μM), PI3K (Ly294002, 10 μM), NFκB (Helenalin and MG132, 1 μM) or DMSO (0.1%) as control for 18 hrs. Viability was determined by MTS assay. Results were presented as fold of control (Y-axis) derived from the mean values of absorbance at 490 nm of inhibitor-treated groups divided by DMSO control group and error bars showed the standard deviation of triplicate.
(TIF)
DH-PS promoted the proliferation of THP-1 cells. THP-1 cells were cultured (2×106 cells/ml) with increasing concentrations of DH-PS or PBS (Concentration 0) for 18 hrs and the proliferation rate was determined by MTS assay. X-axis represented the concentration of DH-PS (μg/ml). Results were presented as fold of control derived from the mean values of absorbance at 490 nm of DH-PS-treated groups divided by PBS control group and error bars showed the standard deviation of triplicate. Statistically significant difference (Mean values of absorbance were used for the comparisons): * compared with PBS-treated group, p<0.05.
(TIF)
F3 elicited the productions of cytokines and chemokines in human CD14+ cells. Human CD14+ cells isolated from one healthy donor were cultured with F3 (50 μg/ml) or PBS for 18 hrs and supernatants were collected for the measurements of cytokines and chemokines. Y-axis represented the mean concentrations (Conc.) of cytokines/chemokines with error bars showing the standard deviation of triplicate. Statistically significant difference: * compared with PBS-treated group, p<0.05. # compared with PBS-treated group, p<0.005.
(TIF)