Abstract
Developmental changes in the coordinative organization of masticatory muscles were examined longitudinally in four children over 49 experimental sessions spanning the age range of 12–48 mo. Electromyographic (EMG) records were obtained for right and left masseter muscles, right and left temporalis muscles, and the anterior belly of the digastric. Two independent analytic processes were employed, one that relied on identification of onset and offset of muscle activation and a second that used pairwise cross-correlational techniques. The results of these two analyses, which were found to be consistent with each other, demonstrated that the basic chewing pattern of reciprocally activated antagonistic muscle groups is established by 12 mo of age. Nevertheless, chewing efficiency appears to be improved through a variety of changes in the chewing pattern throughout early development. Coupling of activity among the jaw elevator muscles was shown to strengthen with maturation, and the synchrony of onset and offset of these muscles also increased. Coactivation of antagonistic muscles decreased significantly with development. This decrease in antagonistic coactivation and increase in synchrony among jaw elevators, and a parallel decrease in EMG burst duration, were taken as evidence of increased chewing efficiency. No significant differences in the frequency of chewing were found across the ages studied. Additional considerations include the appropriateness of this coordinative infrastructure for other developing oromotor skills, such as speech production. It is suggested that the relatively fixed coordinative framework for chewing exhibited by these children would not be suitable for adaptation to speech movements, which have been shown to rely on a much more variable and adjustable coordinative organization.
INTRODUCTION
In addition to providing a theoretical framework for the motor control processes underlying chewing, an understanding of the ontogeny of masticatory control is essential to our understanding of the neurophysiological basis of such varied behaviors as speech and sucking. In fact, early emerging behaviors have often been viewed as providing the infrastructure from which speech motor coordination emerges (Grillner 1982; Ling 1976; Mysak 1980; Thelen 1991). It is therefore surprising that, despite the role of masticatory motor control in these various proposed mechanisms of orofacial development, the development of chewing in humans has been largely neglected.
The relatively few quantitative studies of the ontogeny of human mastication (Ahlgren 1966; Alvarado Larrinaga et al. 1989; Gisel 1988; Gisel 1991; Ishikawa et al. 1988; Møller 1966; Pancherz 1980) share the common finding of distinct patterns of muscle activation for children and adults. These studies have relied primarily on cross-sectional experimental designs to study ontogenetic changes in mastication, even though large idiosyncratic differences in masticatory patterns (Ahlgren 1966; Luschei and Goldberg 1981; Møller 1966) suggest the need for longitudinal studies. More detailed descriptions of the development of chewing have been generated for pigs (Herring 1985; Huang et al. 1994), monkeys (Luschei and Goodwin 1974; McNamara 1974), rats (Westneat and Hall 1992), dogs (Iinuma et al. 1991), and hamsters (Lakars and Herring 1980).
A pronounced shift in oromotor behavior occurs with the transition from sucking to chewing, which in humans typically occurs between 5 and 8 mo of age (Sheppard and Mysak 1984). Mastication is shaped by such developmental factors as musculoskeletal growth, neural maturation, peripheral afferent input, and motor learning. Herring (1985) has speculated that the earliest stages of oral motor development are “dominated by the progressive formation of neuromuscular and CNS connections”, whereas later changes are represented as secondary to musculoskeletal growth. For example, the reorganization of central motor pathways has been observed to coincide with the early shift from sucking to chewing (Iriki et al. 1988), whereas more gradual changes in muscle activation patterns through development have been correlated with ontogenetic changes in skull/mandibular size and geometry (Herring 1985; Lakars and Herring 1980; Nakata 1981).
Movement of the mandible may appear deceptively simple. Careful observation reveals characteristic asymmetry in movement paths and wide cycle-to-cycle variations. In fact, the jaw muscles form a complex coordinative network that permits the mandible to meet a variety of task demands posed by chewing, sucking, and speech (Moore et al. 1988). Muscles primarily associated with mandibular elevation include the masseter, temporalis, and medial pterygoid (Ahlgren 1966; Luschei and Goldberg 1981; Møller 1966), whereas mandibular depression is associated with activation of the digastric, lateral pterygoid, and suprahyoid group (Luschei and Goldberg 1981; Møller 1966). One of the earliest accounts of coordination of these structures for chewing was Sherrington’s chained reflex hypothesis (Sherrington 1917), with more recent models relying on brain-stem-level central pattern generators (CPGs; Dellow and Lund 1971; Lund 1991). The action of the masticatory CPG in producing rhythmic jaw motion is well established in a number of animal models (Dellow and Lund 1971; Nozaki et al. 1986).
A large number of descriptive studies has sought to quantify muscle activation patterns characteristic of chewing by humans (Ahlgren 1966; Alvarado Larrinaga et al. 1989; Garrett and Kapur 1986; Horio and Kawamura 1989; Josell et al. 1984; Luschei and Goldberg 1981; McCarroll et al. 1989; Møller 1966; Moore 1993; Moore et al. 1988; Pancherz 1980; Schwartz et al. 1984; Steiner et al. 1974; Vitti and Basmajian 1975, 1977). Despite large intersubject variability (Ahlgren 1966; Dellow and Lund 1971; Møller 1966), a consistent pattern of muscle activation for chewing has emerged. Most prominent in the chewing cycle is the rigid, reciprocal pattern of alternating activity between antagonist muscles (e.g., anterior digastric with masseter) (Ahlgren 1966; Møller 1966; Moore 1993; Moore et al. 1988; Vitti and Basmajian 1977). During the opening phase of mastication the jaw depressors are active, and activity in the jaw elevators is inhibited (Møller 1966; Vitti and Basmajian 1975), yielding a relatively rapid jaw opening phase (Josell et al. 1984). During the closing phase, activation of the jaw elevators is not observed until after the mandible has begun to displace upward (Ahlgren 1966), suggesting that the initial closure is driven by elastic recoil of the elevating muscles. Activity of the digastric gradually decreases while jaw elevator activity gradually increases. This use of antagonistic forces provides stability and relatively finer control during jaw closure. During the final stage of closure, a cessation of activity in the jaw elevators has been reported (Josell et al. 1984), possibly protecting the teeth from excessive grinding and closing forces.
More detailed analyses of chewing by humans have revealed its complex coordinative organization. Homologous muscle pairs (e.g., right and left masseter) are generally coactivated (Ahlgren 1966; Møller 1966; Moore 1993; Vitti and Basmajian 1977), although levels of activation within these pairs are unequal, with the working side exhibiting greater activity (Ahlgren 1966; Vitti and Basmajian 1975). Ipsilateral synergistic pairs (e.g., right masseter and right temporalis) are similarly coactive during chewing (Ahlgren 1966; Møller 1966; Moore 1993; Vitti and Basmajian 1977). Several studies have described asymmetric activity in contra-lateral synergists (e.g., right masseter and left temporalis) (Luschei and Goldberg 1981; Møller 1966; Moore 1993). Luschei and Goldberg (1981) reported, for example, that activity in the working-side temporalis begins earlier than that of the balancing-side masseter.
Table 1 summarizes the ontogenic characteristics of chewing across several species. These findings of decreasing variability and increasing speed and efficiency suggest that chewing undergoes progressive stabilization. Although the coordinative development in humans has not been investigated, limited inferences from other species may be possible. The present investigation was designed to describe early development of chewing. Specifically, developmental changes in muscle activation patterns have been described, especially with respect to intrinsic coordinative linkages and gross behavioral changes, including chewing rate and rate stability.
TABLE 1.
Developmental changes in EMG patterns associated with chewing
| Results | References | Species |
|---|---|---|
| EMG burst duration decreases |
Huang et al. 1994 Westneat and Hall 1992 |
Pig Rat |
| EMG burst duration variability decreases | Huang et al. 1994 | Pig |
| Overlap of EMG activity between antagonistic pairs decreases | Westneat and Hall 1992 | Rat |
| EMG burst activity becomes greater on working side | Huang et al. 1994 | Pig |
| Amplitude of EMG activity from masseter increases relative to that of temporalis |
Herring 1977 Iinuma et al. 1991 McNamara 1974 Pancherz 1980 |
Pig Dog Monkey Human |
| Number of chewing cycles per bolus decreases and becomes less variable |
Gisel 1988 Huang et al. 1994 Schwaab et al. 1986 |
Human Pig Human |
EMG, electromyogram.
METHODS
Subjects
Four children (3 girls, 1 boy) were studied from 12 to 48 mo of age. Experimental observations were made at 3-mo intervals to yield a total of 13 sessions per child. Of the 52 possible experimental sessions, 49 were successfully completed, with three missed sessions because of illness or scheduling problems. According to parental report, subjects had achieved gross motor, fine motor, cognitive, and language milestones within the expected age range for each. Each child was free of neurological deficit, and, when tolerated by the child, passed otoscopic and tympanometric screenings.
Experimental protocol
Children were seated in a high chair and secured with a lap strap and a sliding tray. The child’s caregiver and an experimenter were seated beside the subject throughout data collection. The assistant monitored the child’s activities and provided an on-line verbal description of all behaviors, including their context. The foods chewed were selected and supplied by the parent and consisted only of solids that the children enjoyed as part of their typical diets. Chewing of a wide variety of foods was observed, including: fresh fruit (grapes, apricot, bananas, and apples), candy (jelly beans and Gummy Bears), potato chips, Cheetos, raisins, cereal, crackers, cookies, cheese, french fries, pretzels, and tofu. One unfortunate consequence of working with these very young children was that it was rarely possible to identify the working side during chewing. Subjects were observed to chew centrally (i.e., incisal bite), laterally (i.e., molar bite), and bilaterally (i.e., simultaneous left and right molar bite), frequently moving the bolus from one side to the other. Observations of working- versus nonworking-side activity were therefore precluded.
Electromyography and data recording
Data were recorded continuously for 45 min during each experimental session. Electromyographic (EMG) signals were obtained with the use of miniature surface Ag/AgCl electrodes (InVivo Metric) applied over the main belly of each of the muscles studied. Targeted muscles included primary mandibular muscles of mastication, including 1) right masseter, 2) left masseter, 3) right temporalis, 4) left temporalis, and 5) the anterior belly of the digastric (ABD), which was recorded bilaterally with the use of a single electrode pair. Electrodes were spaced ~0.5 cm apart. Placement of the masseter electrodes was based on palpation of the main mass of the muscle anterior and superior to the angle of the mandible. Temporalis electrodes were placed just superior to the zygomatic arch, which was identified by palpation. For both the masseter and temporalis sites, the electrodes within each pair were aligned parallel to the orientation of the muscle fibers of the targeted muscle. The digastric recording site was immediately posterior to the mental symphysis. The small size of this muscle and the presence of substantial tissue overlying the muscle more posterior to the chin precluded unilateral recording of digastric with the use of surface electrodes. Accordingly, the electrode pair was situated with one electrode over each belly (i.e., the right and left portions) of the digastric. Interelectrode distance was ~0.5 cm. A reference electrode was located ~2 cm superior to the nasion. EMG signals were amplified (Teac XR-510; gain ranged from 10,000 to 100,000; band-pass: 3–3,000 Hz) and recorded with the use of a 14-channel instrumentation frequency-modulated recorder frequency response: DC-1,250 Hz; signal-to-noise (S/N) ratio > 50 dB.
A significant challenge posed by these experimental conditions was appropriate placement of the electrodes and maintenance of secure electrical contact. Isolation of activity from the temporalis muscles was simplified by the anatomic configuration of this muscle; the recording site used was relatively distant from other potentially interfering muscles. Similarly, masseter is the most superficial muscle at the recording site used, such that recorded signals are most likely to reflect masseter activity rather than that of deeper or more distant muscles. Digastric presents the most serious concern. Even though this muscle is most superficial at the recording site, it is very small and is proximal to several larger muscles, including mylohyoid and platysma. These issues were addressed by the present methods in several ways. All EMG signals were monitored constantly for movement artifact and for the presence of extraneous activity, either of which was sufficient to reject the recorded period from further analyses. In addition, concern for contamination by some of the most proximal muscles, mylohyoid in particular, is minimized by the recognition that activity in these muscles is biomechanically consistent with mandibular depression and is appropriately studied in the current context of muscles of mastication. Thus, even though the digastric was the targeted muscle, correlated activity in other mandibular depressors may also be present in the recorded signals, giving rise to signals with more complex origin than those of the mandibular elevators.
An obvious concern in using surface EMG recording in children was cross talk among recording channels. This concern was addressed in several ways in this study, which is one of a series of investigations of these children (e.g., Moore and Ruark 1996). First, the distance between electrode pairs was large compared with distance within electrode pairs (e.g., 0.5-cm interelectrode distance for ABD electrode pairs, which were ~5 cm from the nearest masseter pair) somewhat reducing volume conduction effects. Second, Moore and Ruark (1996) studied these subjects at 15 mo, explicitly quantifying cross talk. With the use of coherence analysis these investigators found no evidence of cross talk. Finally, none of the present results is consistent with the effects of cross talk. Overlap of activity, illustrated in Fig. 3, never was complete; onsets and offsets rarely, if ever, coincided across channels. Moreover, the complementary cross-correlational analysis showed increased coupling with age, which is the opposite of what would be expected if cross talk were occurring with closely spaced electrode pairs in children at the younger ages.
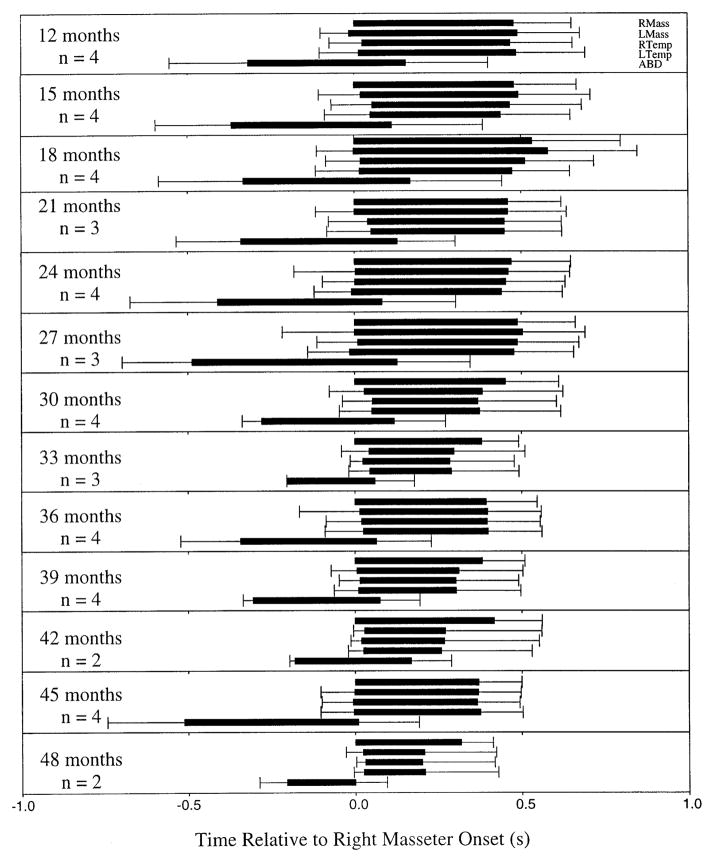
FIG. 3.
Horizontal bars: EMG activity from (top to bottom in each group of bars) RMass, LMass, RTemp, LTemp, and ABD averaged across all repetitions and all subjects. Error bars: average within-subject SD. EMG onsets and offsets were calculated relative to onset of RMass.
In addition to the five frequency-modulated recorder channels dedicated to EMG signals, two amplitude-modulated channels on the same recorder carried audio information. The first of these two audio tracks, using a high-quality wireless lapel microphone worn by the child, recorded continuous commentary by the experimenter seated with the child. This commentary included description of each of the child’s actions and the food consumed, verbal marks of the onset of chewing or swallowing, and notation of the occurrence of extraneous movement. Speech and babbling productions by the child were also recorded on this channel. The second audio track was recorded on the edge track of the recorder and included ongoing descriptions of experimental conditions, including changes in EMG gain and additional subject description information.
Digitization
Subsequent to digitization and further signal processing, acceptable periods of EMG activity associated with chewing were identified. Activity associated with nonchewing oromotor behaviors, such as sucking, speech, or babbling, or nonnutritive chewing and movement artifacts were rejected. With the use of the audio description provided by the two experimenters, as well as visual inspection of the signals themselves, continuous intervals of EMG activity associated with chewing were digitized with the use of a commercially available hardware/software system (Dataq Instruments; sample rate: 1,000 samples/s). This resulted in a cumulative data file that contained ~100 s of continuous chewing or ~60 acceptable chew cycles per session.
Data analyses
Two independent techniques were implemented to quantify the detailed, as well as the general, coordinative organization exhibited by these EMG signals. Custom routines written for Matlab, a commercially available signal processing package (v0.4.2c; The Math-works, 1993), were developed 1) to measure the time of EMG onset and offset and 2) to compute cross-correlation functions across EMG records (Moore 1993). This combination of analyses was intended to yield a complete representation of the coordinative organization of chewing, as well as to provide a comparative evaluation of these analytic techniques. Event marking analysis followed more traditional approaches to EMG pattern description. Its weaknesses were that all measures depend on the identification of single points and consequently neglected the complete waveform. This focus made this technique vulnerable to significant bias and measurement error. Cross-correlational analyses overcame these weaknesses by analyzing the entire available signal without the need for identification of single points, but obscured detail in the time domain. Together, these methods revealed distinct, but related, characteristics of the chewing cycle.
Event marking
Event marking provided a detailed description of timing of muscle onsets and offsets during chewing. Preliminary measurements demonstrated a tradeoff between automatic detection and computer-assisted detection methods. Whereas automatic detection is susceptible to false positives in signals such as those in the present data set, computer-assisted measures are susceptible to subjective biases that may be imposed by an observer. Better reliability was obtained with the use of operationally defined criteria for onsets and offsets with computer enhancement of those points before judgment by an experimenter.
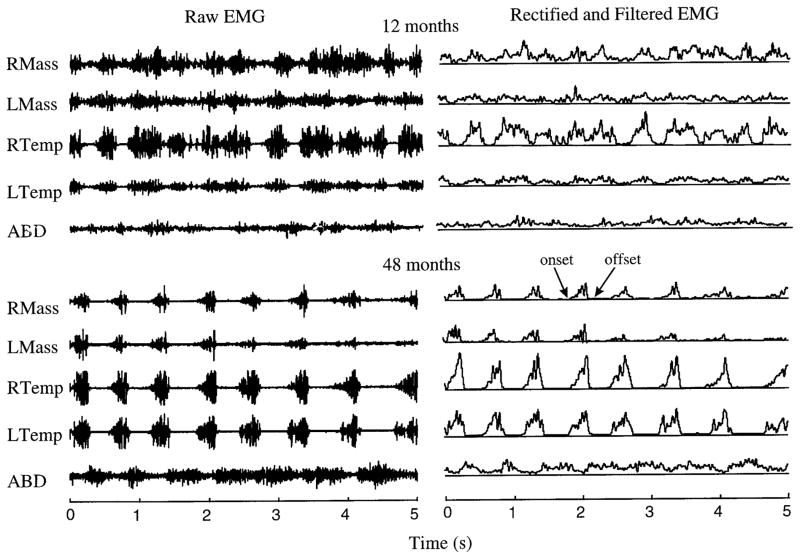
Computer-assisted identification of onset of offset of muscle activity relied on a custom algorithm written for Matlab. Initially, rectified and digitally filtered (8-pole Butterworth filter, low-pass cutoff = 30 Hz) displays of the raw EMG records were computed. An investigator positioned cursors around a portion of the signal that could unambiguously be identified as exhibiting the lowest level of muscle activity seen at each of the five recording sites (i.e., muscle activity at rest). The algorithm computed the SD for the marked portion for each channel and subsequently used this value to plot a horizontal line across the floor of the rectified and filtered EMG. This reference line was used to aid in the determination of onset and offset of EMG activity. Burst onsets and offsets were identified by placing a cursor at the point at which activity exceeded this threshold. The time coordinate of the selected event was automatically exported to an external file that included coding for the event time, event type (onset or offset), and EMG channel, as well as subject and session identifiers. Figure 1 illustrates the raw (left) and processed (right) EMG and the identified onset and offset of EMG activity (shown at bottom right for right masseter), for a single subject at 12 mo (top) and 48 mo (bottom).
FIG. 1.
Electromyographic (EMG) activity during chewing by the same subject at 12 and 48 mo of age. Left: raw EMG activity. Right: rectified and filtered signals. Modulation of the EMG signals at 12 mo is much more poorly defined than in the later samples. RMass, right masseter; LMass, left masseter; RTemp, right temporalis; LTemp, left temporalis; ABD, anterior belly of digastric.
An important aspect of this approach was the option for the experimenter to decline to measure an event, designating it as unmeasurable. The cumulative number of unmeasurable bursts provided an indirect indication of overall EMG clarity for each record, with a greater number suggesting poorer EMG burst definition.
The cumulative data file was subjected to subsequent analysis to derive additional measures of EMG activation patterns. The duration of each burst, as well as the time of occurrence in the overall chewing cycle (i.e., relative to onset of right masseter activity), was computed. These values were subjected to statistical analysis to evaluate developmental changes in burst characteristics.
Measurement reliability
Three experimenters completed the event marking analysis. Interjudge reliability was assessed by analysis of five of the same EMG records, repeated by each of the three experimenters. These five records were chosen pseudorandomly across subjects to represent the entire age span of the data set (i.e., random selections from among the samples at 12, 18, 27, 36, and 45 mo). The total number of chewing cycles measured by these three experimenters for the reliability analysis was ~900 (3 experimenters × 5 records × 60 cycles of chewing per record). The average discrepancy among the onset and offset points measured was 40 ± 10 (SD) ms. Relative to the average burst duration (510 ± 160 ms, mean ± SD), interjudge measurement error was estimated to be 7.8%, which was judged to be acceptable for the present level of analysis.
Cross-correlation and autocorrelation analysis
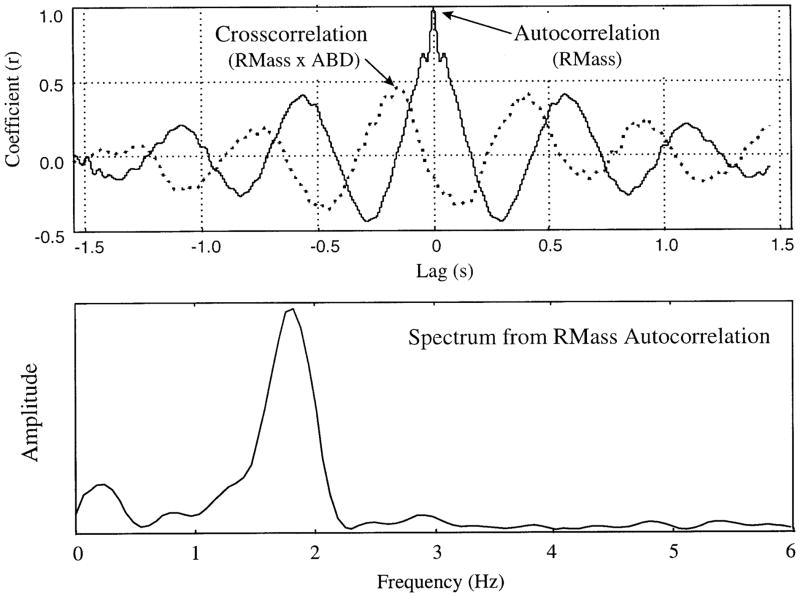
Cross-correlational analysis was applied to the full-wave-rectified and low-pass filtered (8-pole Butterworth filter, low-pass cutoff = 30 Hz) signals. Cross-correlations, including autocorrelations for each channel, were performed on 4- to 10-s sections of continuous chewing in a pairwise manner for each emg record. This procedure yielded 15 functions per computation (i.e., 1 autocorrelation for each of the 5 EMG channels, and 10 pairwise cross-correlations for each channel with each of the remaining channels). Approximately 10–18 cross-correlation matrices were computed for each experimental session, depending on the length and number of occurrences of uninterrupted chewing. A single cross-correlation function is presented in Fig. 2. This function, which is derived from cross-correlation of right masseter with digastric, was one of 15 obtained for the analysis of one interval of chewing for a 48- mo-old subject. The peak coefficient (~0.48 in the example in Fig. 2) and associated lag (about −0.2 s) were extracted from each cross-correlation function. A peak picking algorithm was employed to extract the peak coefficient value. This algorithm searched for a local maximum in a 100-point window centered on a point an experimenter identified by cursor placement. All peak coefficients were converted to absolute values and transformed with the use of the Fisher’s z transform to allow for further statistical treatment.
FIG. 2.
Top: cross-correlation (RMass × ABD) and autocorrelation (RMass) functions of chewing data collected at 48 mo. Peak coefficient and associated lag were extracted from cross-correlation function for subsequent analysis. Bottom: chewing rate was calculated by identifying the dominant peak in the spectrum of the autocorrelation.
Chewing frequency was also extracted from the results of the cross-correlational analyses. Fast fourier transform functions were computed for each of the autocorrelation functions. The peak in this function yielded the predominant frequency over the entire interval studied. The advantages of this method, as opposed to measuring chewing period cycle by cycle, included the automatic execution of the algorithm, as well as the inclusion of a larger amount of data in each estimate. Figure 2, top, shows the autocorrelation function from the right masseter (solid line); Fig. 2, bottom, illustrates the corresponding frequency spectrum with its peak at ~1.8 Hz.
The accuracy of the algorithms for these analyses was tested with the use of a synthetic signal composed of 10-Hz pulse trains that varied in both phase and level of additive random noise. The results of the testing indicated that the algorithm performed with perfect accuracy for S/N ratios of 4 dB or better.
Statistical treatment
Statistical testing of developmental effects was completed for burst duration, chewing frequency, peak cross-correlation coefficient, and lag to peak cross-correlation coefficient. Regression analyses were completed to describe and test developmental trends for these variables as well.
RESULTS
EMG data were obtained from all five of the targeted recording sites in 49 experimental sessions, during which the developmental changes in the coordinative organization of chewing were examined. Completion of preliminary analyses yielded a data set that included EMG onset and offset times for each muscle throughout each chewing interval observed, the peak cross-correlation coefficient obtained for each interval studied, the lags associated with each peak coefficient, and the dominant frequency of the autocorrelation function for each muscle (i.e., chewing rate). From the onset and offset measures, additional descriptors were derived, including burst duration and relative times of onset and offset with respect to onset of right masseter activity. These measures were subjected to regression analyses to evaluate developmental changes. Regression analyses (factor × age) were completed for the following eight measures: chewing rate, burst duration, frequency of occurrence of unmeasurable bursts, overlap of antagonistic activity, peak coefficients between synergistic pairs, peak coefficients between antagonistic pairs, lag to the peak coefficient between antagonistic pairs, and lag to the peak coefficient between synergistic pairs. A preliminary examination of the raw data revealed distinct developmental changes in the patterns of activity between synergistic and antagonistic muscle pairs. This observation combined with previous findings (Moore 1993) motivated separate analyses of the cross-correlational data from synergists and antagonists.
Activation patterns
The average chewing cycle is shown in Fig. 3 for each of the age groups studied. This figure illustrates the muscle activation patterns exhibited for a cycle of chewing, combined across subjects for each age group. Each horizontal bar represents the duration of muscle activity, derived from the average onsets and offsets across all subjects, aligned to reflect time relative to onset of right masseter activity. From top to bottom, each group of bars includes right masseter, left masseter, right temporalis, left temporalis, and ABD. Figure 3 reveals several developmental patterns that emerged during this 36-mo period of investigation. Most significantly, chewing was characterized by reciprocal activity among antagonistic muscles across all age levels. Starting at ~30 mo, several changes in the chewing pattern were apparent at a finer level of comparison. The onset of activity among the jaw elevating muscles appeared to become more synchronous, although there did not appear to be a consistent order of onset for these muscles when they were asynchronously activated. The activity in right masseter tended to be slightly more prolonged and to begin earlier than in its synergists (i.e., from 30 to 48 mo, 5 of these 7 groups exhibited relatively earlier and longer burst durations in right masseter than in the other jaw elevating muscles). These findings are similar to those reported for chewing by adults, in which masseter was seen to be the first jaw elevating muscle to be activated (Steiner et al. 1974), but contradict the findings of Ahlgren (1966), in which temporalis was the first elevator activated for most subjects.
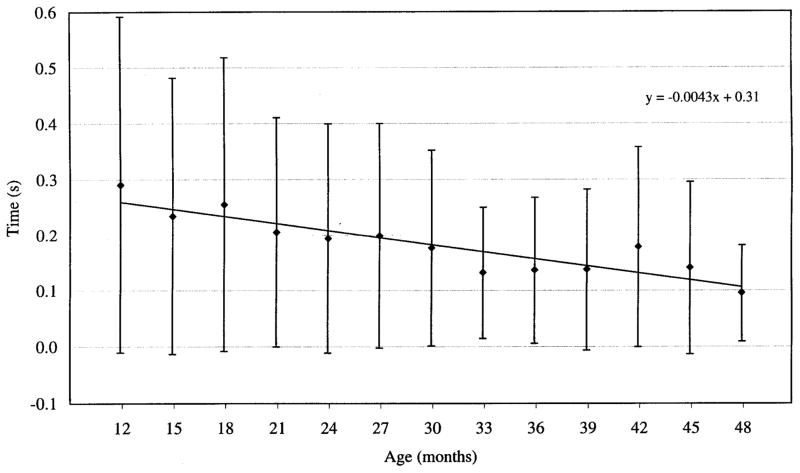
Another measure of interest was the temporal overlap of activity in antagonistic muscles. This value was calculated as the difference between the offset time of the jaw depressor (ABD) and the onset of the first jaw closer to be activated. Figure 4 illustrates this overlap across age groups. The result of a regression analysis (overlap × age) showed that the overlap declined at a rate of ~4 ms/month (β = −0.004, t = −12.86, P < 0.001). The mean overlap decreased from 290 ± 300 (SD) ms at 12 mo to 100 ± 90 (SD) ms at 48 mo. The decrease in variability seen in this figure further suggests that overlap of antagonistic became more stabilized with age.
FIG. 4.
Duration of overlap between antagonistic muscle pairs averaged across all repetitions, all antagonistic muscle pairs, and all subjects. Duration of overlap was found to decrease with age, as did variability of overlap.
Burst duration
A decrease in burst duration with age is supported by observation of Fig. 3, which illustrates the trend toward shorter horizontal bars toward the bottom. This trend was supported statistically. The duration of burst activity combined across all muscles decreased at a rate of ~3 ms/month (β = −0.003, t = −4.95, P < 0.001). Across all muscles and subjects, burst durations ranged from 299 to 773 ms. The average burst duration at 12 mo was 440 ± 65 (SD) ms; at 48 mo it was 350 ± 10 (SD) ms.
Synchrony and coupling of muscle activation by age
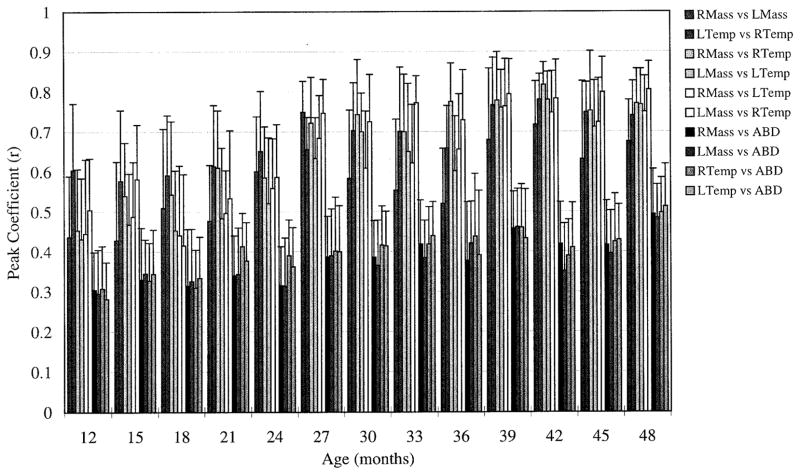
Coupling among synergistic muscles as well as antagonistic muscles increased in strength with age. This increase can be seen as a general increase in values observed across Fig. 5, and was measured and evaluated as an increase in Fisher’s z values, the transform of peak cross-correlation coefficients. The increase was significant for both synergists (β = 0.009, t = 14.12, P < 0.001) and antagonists (β = 0.004, t = 19.87, P < 0.001). Figure 5 displays the peak coefficient values (averaged across all subjects) obtained at each age. Peak coefficient values ranged between 0.42 and 0.82 for synergist and between 0.29 and 0.50 for antagonist. These values are slightly lower than those reported by Moore et al. (1988) for zero lag coefficients, but comparable with those reported by Moore (1993) for peak cross-correlation coefficients. The decrease in within-pair variability from left to right in Fig. 5 suggests that stability of synergistic coupling tended to increase with age.
FIG. 5.
Vertical bars: peak coefficients obtained from pairwise cross-correlations, averaged across all repetitions and all subjects. Error bars: average within-subject SD. For each age, from left to right, 1st 6 bars represent synergistic muscle pairs; the last 4 bars represent antagonistic pairs. Greater values were interpreted as representing increases in coupling of activation. Increases in coupling were observed in both synergistic and antagonistic muscles pairs.
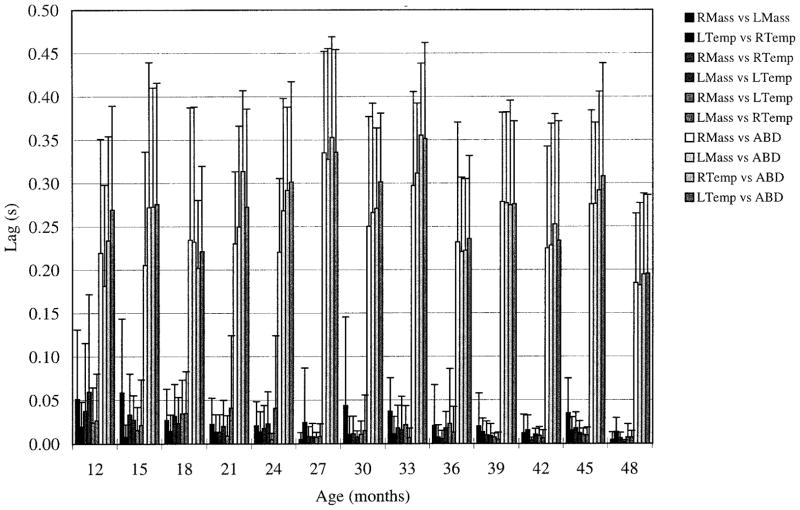
Synchrony of activity among synergists increased slightly with age, although there was no parallel change in the relative timing of activity among antagonists. Figure 6 displays the lag values associated with peak cross-correlation coefficients combined across all subjects for each age. The gradual decrease seen in asynchrony among synergists (the shorter grouping of bars at left of each age group) was statistically significant (β = −0.001, t = −0.16, P < 0.001). Lags ranged from 2 to 59 ms among synergists, and from 182 to 356 ms for antagonists. Variability of lags also decreased with age, most notably among synergists.
FIG. 6.
Vertical bar height corresponds to absolute lag value averaged across all repetitions and subjects for a given muscle pair. Error bars: average within-subject SD. For each age, from left to right, 1st 6 bars represent synergistic muscle pairs; the last 4 are antagonistic muscle pairs. Increase in synchrony among synergists with age was statistically significant.
Chewing rate
Chewing rate did not change significantly with age, although variability of rate did decrease. Average chewing rate and variability for all subjects for each age group are shown in Fig. 7. Across all subjects and all age groups, chewing rate ranged from 0.88 to 2.11 Hz. Although the maximum chewing rate observed in the present study (2.11 Hz) was slightly higher than those reported previously, these values were in good agreement with those reported in adults by Møller (1966) (1.46–1.73 Hz) and Steiner et al. (1974) (0.6–1.8 Hz) and in children by Ahlgren (1966) (1.73 Hz), Schwaab et al. (1986) (0.62–1.25 Hz), and Gisel (1988) (0.71–1.25 Hz). It is likely that some of the discrepancies among these chewing rates are related to methodologic differences, including analytic techniques, as well as differences in the material that was chewed.
FIG. 7.
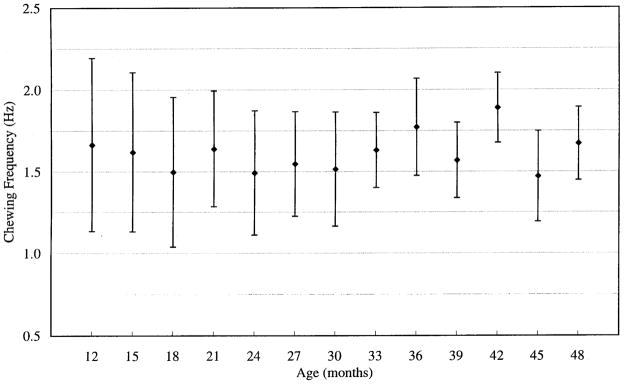
Chewing rate averaged across all repetitions and all subjects within each age level. Variability of chewing rate decreased with age, although chewing frequency itself did not vary substantially across ages.
Unmeasurable bursts
One indication of stability in muscle activation patterns overall was the frequency of occurrence of muscle activity that was so poorly defined that clear onsets and offsets could not be reliably identified. The number of these “unmeasurable” bursts decreased markedly with age (β = −1.41, t = −3.50, P < 0.001). Across all the data, the number of unmeasurable bursts in a single experimental session ranged from 0 to 214. Figure 2 illustrates the qualitatively poorer definition observed in the EMG records obtained from children at the age of 12 mo with respect to those at 48 mo. Generally, muscle activation patterns observed in the younger subjects were more poorly defined, and exhibited poorer S/N ratios and more frequent occurrences of apparently random activity. It is likely that these attributes contributed to the difficulty with which the onset and offset of EMG activity could be identified by experimenters. Although this gross level of analysis merits only casual interpretation, this result mirrors the ubiquitous developmental trend of increasingly well-defined neural organization with learning of other skilled motor behaviors.
DISCUSSION
The present results may be interpreted to suggest that the general coordinative organization of chewing is well established by 12 mo of age but continues to be refined during early development. As in more general observations of motor skill acquisition (Bruner 1973), development of chewing appears to be characterized by decreased variability and increased motor efficiency. In the second year of life, EMG signals typically exhibited poorer S/N ratios, as well as poorer pattern definition (see Fig. 1) with respect to later observations. Perhaps surprisingly, there appeared to be no stepwise shifts in coordinative organization either within or across subjects. Abrupt changes in EMG patterning might have been expected in these young children, given the occurrence of such dramatic changes as eruption of new teeth, changes in diet, and rapid mandibular growth.
Developmental changes in the chewing pattern
Figure 3 illustrates several of the distinct changes in the coordinative organization of chewing observed during this developmental period. In children of age ≥30 mo, the masseter was often (i.e., in 5 of 7 age levels) seen to be the first jaw elevating muscle to be activated in a chewing cycle and the last to be inactivated. This finding parallels observations across species that burst amplitude of masseter increases with maturation relatively more than that in temporalis (Herring 1977; Iinuma et al. 1991; McNamara 1974; Pancherz 1980). Other distinct developmental changes in coordinative organization included the increasing synchrony of onset and offset of activity of other jaw elevators, and the offset of the ABD, which tended to terminate earlier relative to the onset of the elevators with age, thereby decreasing overlap of antagonistic activity.
Although the reciprocal activation of mandibular muscles was already quite evident at 12 mo of age, inspection of Fig. 5 suggests that coupling among jaw elevators strengthened throughout the 3-yr period observed. In older children jaw depressor activity terminated earlier, precluding cocontraction of antagonistic muscles and the consequential increase in chewing efficiency (see Fig. 3). Moreover, the absence of antagonistic cocontraction in 48-mo-old subjects suggests that the basic coordinative organization for chewing is well established by that age, with future modifications yielding only incremental motor skill development. The level of coordinative stability exhibited by these children is further defined by the finding that the average rate of chewing did not change significantly with age. This result was consistent with earlier studies of humans (Gisel 1988; Schwaab et al. 1986).
Evidence of increased chewing efficiency
The impression created by the present results combined is one of increasing coordinative efficiency; in later recordings mandibular muscles appeared to be more effectively recruited in generating occlusal force over a shorter period of the chewing cycle. Several independent, statistically significant observations contribute to this conclusion: an increase in synchrony and coupling strength among synergists, a decrease in antagonistic cocontraction (e.g., compare Fig. 3, top and bottom), and a decrease in burst duration. The anticipated result of these adjustments is that more force could be brought to bear on the food bolus over a shorter period of time with less energy expended.
The observed developmental increases in synchrony and the strength of coupling of synergistic muscle activity were further supported by a significant decline in the lag to the peak cross-correlation coefficient of these pairs (see Fig. 6) and a significant increase in peak coefficient (see Fig. 5), respectively. Increased synchrony among synergists may be assumed to augment the rate at which force is developed, and would be expected to improve the application of force across the occlusal surfaces of the teeth. Increased coupling among synergists supports the assertion that the coordinative organization of chewing becomes more regular and stereotypic through maturation.
The developmental decreases in duration and variability of EMG bursts were anticipated from known developmental patterns of other species (i.e., pigs: Huang et al. 1994; rats: Westneat and Hall 1992). This decrease in burst duration coupled with the lack of change in chewing frequency suggests that, at later ages, children produce the same rate of movement with shorter bursts of activity, again pointing to increased chewing efficiency with age. The present findings parallel those of Gisel (1988) and Schwaab et al. (1986), which demonstrated that, in humans, the number of chewing cycles required to break down a food bolus decreases with age. Undoubtedly, some portion of this increase in chewing efficiency is related to the emergence of teeth, as well as the child’s increased capacity to generate masticatory force, consequential to musculoskeletal growth. The present findings, however, suggest that it is likely that maturation of motor control mechanisms also underlies increasing masticatory efficiency.
Evidence of stabilities
The stabilities observed in the present results may be interpreted as evidence of the robust nature of the underlying control structures. This conclusion is especially remarkable given the magnitude of the ongoing anatomic and physiological changes in orofacial morphology (e.g., changes in neural connectivity and neural growth; mandibular dimensions and geometry, Bosma 1985; muscle fiber composition, Nakata 1981; Takarada et al. 1990; dentition; and differential development of the mandible with respect to the rest of the skull). The fundamental coordinative organization (i.e., the basic reciprocal pattern of mandibular muscle activation repeating at a rate of ~1.7 chewing cycles/s) appeared to be resistant to a range of potential influences, including changes in musculoskeletal mass and dentition as well as changes in diet. Whereas the influence of peripheral and extrinsic factors (e.g., consistency and position of bolus) are surely significant, the essential organizational properties of mastication appear to dominate these early masticatory efforts by the child. Similar to chew rate, the characteristic reciprocal pattern of activation for chewing appeared very early in these children and was maintained throughout the observational period. Thus, consistent with the known properties of the masticatory CPG in other species, it appears that the basic coordinative elements of mastication were present in our earliest observations. Support for the presence of central patterning mechanisms very early in development can be drawn from observations of neonatal rats, in which the locomotor CPG exhibits oscillatory properties and can be entrained by rhythmic afferent input (Sqalli-Houssaini et al. 1993). The present results similarly supported the early establishment of rudimentary control structures for chewing.
The role of afferent input in the development of chewing has been studied with respect to the influence of the emergence of dentition and periodontal sensation. There is evidence that periodontal sensation is critical for the development of rhythmic chewing in dogs (Iinuma et al. 1991), although the relationship between the emergence of teeth and the transition from sucking to chewing is not evident across species (Huang et al. 1994). In guinea pigs, which are born with complete permanent dentition, the transition from sucking to chewing is similar to that of species exhibiting postnatal eruption of teeth (Iriki et al. 1988). Differences such as these highlight the need for a more thorough understanding of the morphogensis of the transition from sucking to chewing in humans. Without the benefit of data from earlier development, the emergence of this pattern cannot yet be evaluated.
Methodologic considerations
There are a number of methodologic factors that influenced these results and require further attention. One of the most obvious factors was the difference in quality of the EMG signals obtained earlier versus later in the sampling period. EMGs obtained from younger children were characterized by more poorly defined patterns of activation, which made measurement of onsets and offsets difficult or, frequently, impossible. Rejecting these unmeasurable intervals from the analysis probably biased the outcome to suggest that early chewing is better formed than it actually is. On a more pragmatic level, although the longitudinal approach used provided a more complete description of the progression within an individual, the small number of subjects precluded statistical assessment of intersubject variability and post hoc analysis of individual ages or EMG sites. With respect to the calculations themselves, the autocorrelation and cross-correlation techniques used are sensitive to S/N ratios (see Moore and Ruark 1996), such that signals with greater S/N ratio will yield higher peak correlation values. It is possible that some of the developmental increases in peak coefficient values were because of improved S/N ratios in older children, not increased coordinative coupling as suggested. Without a complete description of the signal composition (including shared and isolated noise sources, intrinsic and extrinsic noise) the extent of this problem cannot be estimated. Finally, even though all the foods chewed in the present study were solids, it is likely that some of the variation observed in chew rate was related to subtle differences in food texture. Previous investigations have demonstrated changes in chewing rate with differences in food texture in humans (Gisel 1988; Schwaab et al. 1986; Steiner et al. 1974) as well as animals (Luschei and Goodwin 1974). Harder foods elicit faster chewing rates than softer foods, although the effect is actually rather small. What is perhaps more significant with respect to the present findings is that, even though it was not possible to control the consistency of the food chewed by each child, the results across children and across sessions were remarkably similar.
Potential role of mastication in the development of speech
A core concern motivating the present investigation, although not studied directly, was the role of the coordinative organization for chewing as a precursor to other, later-emerging oromotor behaviors, especially speech development (Moore and Ruark 1996). Although the notion of the coordinative framework for chewing providing the basis from which speech motor coordination emerges has been strongly promoted for its intuitive appeal, very little is known about the coordination organization of chewing itself. Whether or not the coordinative framework for chewing is appropriate for speech is unknown. One motivation of the present investigation was to provide this description in a form that makes it accessible to comparable measurements of speech coordination. Most obviously, speech and chewing share peripheral structures (Lund et al. 1982), but the potentially more significant overlap is in the underlying control structures. Grillner (1982) has suggested that vocalization represents the sum of “fractionations” of CPGs, including parts of the respiratory and masticatory generators. Recent observations of speech development have supported the representation of very early speech movements as rhythmic elevation and depression of the mandible with superimposed vocalization (Davis and MacNeilage 1995), although no evidence exists to suggest that the coordinative patterns of chewing necessarily precede those essential to speech production. In fact, Moore et al. (1988) have demonstrated in adults that the coordinative strategies for mandibular control during speech are quite different from those employed during chewing. Similarly, toddlers have failed to exhibit gross similarities in speech and mastication (Moore and Ruark 1996). The present findings extend previous comparisons, revealing that the coordinative organization for chewing did not exhibit characteristics similar to those observed for speech production, either by adults or children.
Summary
The challenge that remains is to identify and isolate the developmental changes in chewing related to coordinative development from those dictated by neural and musculoskeletal growth and from those that are responsive to changing external factors, such as diet. Evidence from earlier studies suggests that in some species the masticatory motor control system is established before the appearance of chewing (Herring 1985; Iriki et al. 1988) and that the earliest changes in chewing behavior are driven by morphological factors. The present findings of a significant increase in synchrony among synergists and a significant decrease in the overlap among antagonists support the speculation that developmental changes in the chewing pattern stem from refinement of the extant coordinative organization rather than from the potential effects of a wide variety of external factors. Similarly, the observed increase in the role of the masseter over time (i.e., relatively long duration, earlier onset) among the synergists has been reported in animal studies (Herring 1985; Huang et al. 1994) and has been associated with developmental changes in muscle orientation. These changes are significant, but are relatively slight in comparison with their relationship to the developmental target (i.e., mature mastication); even the earliest acquired records exhibited characteristics that were remarkably similar to adult muscle activation patterns. This observations renders the toddler’s task as one of refinement and adjustment, rather than one of complete learning or generalization of earlier established skills.
Acknowledgments
This work was supported by National Institute of Deafness and Other Communications Disorders Grants R29 DC-00822 and T3 DC-00033 by the University of Washington, Seattle, WA.
References
- Ahlgren J. Mechanism of mastication: a quantitative cinematographic and electromyographic study of mastication movements in children, with special reference to occlusion of the teeth. Acta Odontol Scand. 1966;24:5–109. [Google Scholar]
- Alvarado Larrinaga G, Takarada T, Nishida F, Nishino M. Muscle action potential and masticatory rhythm of anterior temporal and masseter muscles in children and adults. Shoni Shikagaku Zasshi. 1989;27:895–906. [PubMed] [Google Scholar]
- Bosma JF. Postnatal ontogeny of performances of the pharynx, larynx, and mouth. Am Rev Respir Dis. 10;131(Suppl):15–1985. doi: 10.1164/arrd.1985.131.S5.S10. [DOI] [PubMed] [Google Scholar]
- Bruner JS. Organization of early skilled action. Child Dev. 1973;44:1–11. [PubMed] [Google Scholar]
- Byrd KE. Loci and characteristics of EMG silent periods during masticatory mandibular movements in rats. J Dent Res. 1988;67:1284–1288. doi: 10.1177/00220345880670100801. [DOI] [PubMed] [Google Scholar]
- Davis BL, MacNeilage PF. The articulatory basis of babbling. J Speech Hear Res. 1995;38:1199–1211. doi: 10.1044/jshr.3806.1199. [DOI] [PubMed] [Google Scholar]
- Dellow PG, Lund JP. Evidence for central timing of rhythmical mastication. J Physiol Lond. 1971;215:1–13. doi: 10.1113/jphysiol.1971.sp009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett NR, Kapur KK. Replicability electromyographic recordings of the masseter muscle during mastication. J Prosthet Dent. 1986;55:352–356. doi: 10.1016/0022-3913(86)90119-8. [DOI] [PubMed] [Google Scholar]
- Gisel EG. Chewing cycles in 2- to 8-year-old normal children: a developmental profile. Am J Occup Ther. 1988;42:40–46. doi: 10.5014/ajot.42.1.40. [DOI] [PubMed] [Google Scholar]
- Gisel EG. Effect of food texture on the development of chewing of children between six months and two years of age. Dev Med Child Neurol. 1991;33:69–79. doi: 10.1111/j.1469-8749.1991.tb14786.x. [DOI] [PubMed] [Google Scholar]
- Grillner S. Possible analogies in the control of innate motor acts and the production of sound in speech. In: Grillner S, Lindblom P, Lubker J, Persson A, editors. Speech Motor Control. New York: Pergamon; 1982. pp. 217–230. [Google Scholar]
- Herring SW. Mastication and maturity: a longitudinal study in pigs. J Dent Res. 1977;56:1377–1382. doi: 10.1177/00220345770560111701. [DOI] [PubMed] [Google Scholar]
- Herring SW. The ontogeny of mammalian mastication. Am Zool. 1985;25:339–349. [Google Scholar]
- Horio T, Kawamura Y. Effects of texture of food on chewing patterns in the human subject. J Oral Rehabil. 1989;16:177–183. doi: 10.1111/j.1365-2842.1989.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang G, Herring SW. Age changes in mastication in the pig. Comp Biochem Physiol. 1994;107:647–654. doi: 10.1016/0300-9629(94)90364-6. [DOI] [PubMed] [Google Scholar]
- Iinuma M, Yoshida S, Funakoshi M. Development of masticatory muscles and oral behavior from suckling to chewing in dogs. Comp Biochem Physiol A Comp Physiol. 1991;100:789–794. doi: 10.1016/0300-9629(91)90293-l. [DOI] [PubMed] [Google Scholar]
- Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: corticobulbar projection is reorganized during conversion from sucking to chewing. Brain Res Dev Brain Res. 1988;44:189–196. doi: 10.1016/0165-3806(88)90217-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa C, Okazaki Y, Suzuki Y, Mukoyama K, Hirota KY, Sasa R. The eating functional development of children. 1. The present state of things in one year and six months old children. Shoni Shikagaku Zasshi. 1988;26:30–40. [PubMed] [Google Scholar]
- Josell SD, Gay T, Yaeger JA. Relationship between stage of dental development and electromyographic silent periods during chewing. J Prosthet Dent. 1984;52:593–597. doi: 10.1016/0022-3913(84)90354-8. [DOI] [PubMed] [Google Scholar]
- Lakars TC, Herring SW. Ontogeny of oral function in hamsters (Mesocricetus auratus) J Morphol. 1980;165:237–254. doi: 10.1002/jmor.1051650303. [DOI] [PubMed] [Google Scholar]
- Ling D. Speech and the Hearing-Impaired Child: Theory and Practice. Washington, DC: Alexander Graham Bell Assoc. for the Deaf; 1976. [Google Scholar]
- Lund JP. Mastication and its control by the brain stem. Crit Rev Oral Biol Med. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- Lund JP, Appenteng K, Seguin JJ. Analogies and common features in the speech and masticatory control systems. In: Grillner S, Lindblom P, Lubker J, Persson A, editors. Speech Motor Control. New York: Pergamon; 1982. pp. 231–245. [Google Scholar]
- Luschei ES, Goldberg LJ. Handbook of Physiology. The Nervous System. Motor Control. II. Bethesda, MD: Am. Physiol. Soc; 1981. Neural mechanisms of mandibular control: mastication and voluntary biting; pp. 1237–1274. sect. 1. [Google Scholar]
- Luschei ES, Goodwin GM. Patterns of mandibular movement and jaw muscle activity during mastication in the monkey. J Neurophysiol. 1974;37:954–966. doi: 10.1152/jn.1974.37.5.954. [DOI] [PubMed] [Google Scholar]
- McCarroll RS, Naeije M, Hansson TL. Balance in masticatory muscle activity during natural chewing and submaximal clenching. J Oral Rehabil. 1989;16:441–446. doi: 10.1111/j.1365-2842.1989.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Mc Namara JA., Jr An electromyographic study of mastication in the rhesus monkey (Macaca mulatta) Arch Oral Biol. 1974;19:821–823. doi: 10.1016/0003-9969(74)90172-1. [DOI] [PubMed] [Google Scholar]
- Møller E. The chewing apparatus: an electromyographic study of the action of the muscles of mastication and its correlation to facial morphology. Acta Physiol Scand. 1966;69 [PubMed] [Google Scholar]
- Moore CA. Symmetry of mandibular muscle activity as an index of coordinative strategy. J Speech Hear Res. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Ruark JL. Does speech emerge from earlier appearing oral motor behaviors? J Speech Hear Res. 1996;39:1034–1047. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel RL. Task-specific organization of activity in human jaw muscles. J Speech Hear Res. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Mysak ED. Neurospeech Therapy for the Cerebral Palsied. New York: Teachers College Press; 1980. [Google Scholar]
- Nakata S. Relationship between the development and growth of cranial bones and masticatory muscles in postnatal mice. J Dent Res. 1981;60:1440–1450. doi: 10.1177/00220345810600080801. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Iriki A, Nakamura Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol. 1986;55:806–825. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- Pancherz H. Temporal and masseter muscle activity in children and adults with normal occlusion. An electromyographic investigation. Acta Odontol Scand. 1980;38:343–348. doi: 10.3109/00016358009033603. [DOI] [PubMed] [Google Scholar]
- Schwaab LM, Niman CW, Gisel EG. Comparison of chewing cycles in 2-, 3-, 4-, and 5-year-old normal children. Am J Occup Ther. 1986;40:40–43. doi: 10.5014/ajot.40.1.40. [DOI] [PubMed] [Google Scholar]
- Schwartz JL, Niman CW, Gisel EG. Chewing cycles in 4-and 5-year-old normal children: an index of eating efficacy. Am J Occup Ther. 1984;38:171–175. doi: 10.5014/ajot.38.3.171. [DOI] [PubMed] [Google Scholar]
- Sheppard JJ, Mysak ED. Ontogeny of infantile oral reflexes and emerging chewing. Child Dev. 1984;55:831–843. [PubMed] [Google Scholar]
- Sherrington CS. Reflexes elicitable in the cat from pinna, vibrissae, and jaws. J Physiol Lond. 1917;51:404–431. doi: 10.1113/jphysiol.1917.sp001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR, Clarac F. Oscillatory properties of the central pattern generator for locomotion in neonatal rats. J Neurophysiol. 1993;70:803–813. doi: 10.1152/jn.1993.70.2.803. [DOI] [PubMed] [Google Scholar]
- Steiner JE, Michman J, Litman A. Time sequence of the activity of the temporal and masseter muscles in healthy young human adults during habitual chewing of different test foods. Arch Oral Biol. 1974;19:29–34. doi: 10.1016/0003-9969(74)90221-0. [DOI] [PubMed] [Google Scholar]
- Takarada T, Larrinaga GA, Nishida F, Nishino M. Frequency analyses of EMG power spectra of anterior temporal and masseter muscles in children and adults. Dent Jpn. 1990;27:119–125. [PubMed] [Google Scholar]
- Thelen E. Motor aspects of emergent speech: a dynamic approach. In: Krasnegor NA, Rumbaugh DM, Schiefelbusch RL, Studdert-Kennedy M, editors. Biological and Behavioral Determinants of Language Development. Hillsdale, NJ: Erlbaum; 1991. pp. 339–362. [Google Scholar]
- Vitti M, Basmajian JV. Muscles of mastication in small children: an electromyographic study. Am J Orthod. 1975;68:412–419. doi: 10.1016/0002-9416(75)90182-7. [DOI] [PubMed] [Google Scholar]
- Vitti M, Basmajian JV. Integrated actions of masticatory muscles: simultaneous EMG from eight intramuscular electrodes. Anat Rec. 1977;187:173–189. doi: 10.1002/ar.1091870205. [DOI] [PubMed] [Google Scholar]
- Westneat MW, Hall WG. Ontogeny of feeding motor patterns in infant rats: an electromyographic analysis of suckling and chewing. Behav Neurosci. 1992;106:539–554. doi: 10.1037//0735-7044.106.3.539. [DOI] [PubMed] [Google Scholar]