Abstract
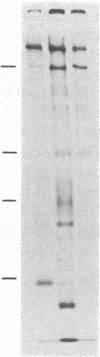
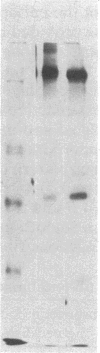
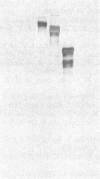
Fibronectins isolated from the conditioned medium produced by cultures of undifferentiated (monolayer) and differentiated (nodular) swine vascular smooth muscle cells are similar but not identical. In general, the nodular-cell fibronectin has a smaller molecular mass than monolayer-cell fibronectin and appears to lack the COOH-terminal interchain disulfide linkage. We studied the incorporation of cellular and plasma fibronectins into the cell layer. Smooth muscle cells bound 2.5 times more monolayer-cell fibronectin than nodular-cell fibronectin. Polypeptide fragments of human plasma fibronectin were used as a model system to investigate fibronectin incorporation into the cell layer. Only intact molecules were incorporated into the cell layer and subsequently organized into fibers. Polypeptide fragments of molecular mass 205 kDa and 185 kDa were not incorporated even though they retained the collagen-, cell-, and heparin-binding regions. Incorporation appears to require an activity associated with either the NH2-terminal or COOH-terminal domains. We propose that fibronectin activity is lost during differentiation of smooth muscle cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Vaheri A., Krieg T., Timpl R. Biosynthesis of two subunits of type IV procollagen and of other basement membrane proteins by a human tumor cell line. Eur J Biochem. 1980 Aug;109(1):247–255. doi: 10.1111/j.1432-1033.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Baumann H., Eldredge D. Dexamethasone increases the synthesis and secretion of a partially active fibronectin in rat hepatoma cells. J Cell Biol. 1982 Oct;95(1):29–40. doi: 10.1083/jcb.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdwell C. R., Gospodarowicz D., Nicolson G. L. Identification, localization, and role of fibronectin in cultured bovine endothelial cells. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3273–3277. doi: 10.1073/pnas.75.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Millis A. J., Fritz K. E. Fibronectin inhibits morphological changes in cultures of vascular smooth muscle cells. J Cell Physiol. 1982 Aug;112(2):284–290. doi: 10.1002/jcp.1041120219. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Millis A. J., Mann D., Fritz K. E. Structural alterations in fibronectin correlated with morphological changes in smooth muscle cells. Dev Biol. 1983 Jun;97(2):391–397. doi: 10.1016/0012-1606(83)90095-7. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar S., Millis A. J. Fibronectin from aged fibroblasts is defective in promoting cellular adhesion. J Cell Physiol. 1980 Apr;103(1):47–54. doi: 10.1002/jcp.1041030108. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Sorrentino J. A., Millis A. J. Interaction of fibronectin with collagen: age-specific defect in the biological activity of human fibroblast fibronectin. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4747–4751. doi: 10.1073/pnas.80.15.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B. Alteration in cell surface LETS protein during myogenesis. Cell. 1977 Mar;10(3):393–400. doi: 10.1016/0092-8674(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Choi M. G., Hynes R. O. Biosynthesis and processing of fibronectin in NIL.8 hamster cells. J Biol Chem. 1979 Dec 10;254(23):12050–12055. [PubMed] [Google Scholar]
- Ehrismann R., Chiquet M., Turner D. C. Mode of action of fibronectin in promoting chicken myoblast attachment. Mr = 60,000 gelatin-binding fragment binds native fibronectin. J Biol Chem. 1981 Apr 25;256(8):4056–4062. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G. Immunocytochemical localization of fibronectin (LETS proteins) on the surface of L6 myoblasts: light and electron microscopic studies. Cell. 1978 Feb;13(2):263–271. doi: 10.1016/0092-8674(78)90195-2. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S. Human vascular smooth muscle in culture. Growth and ultrastructure. Lab Invest. 1975 Jul;33(1):16–27. [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R., Pennypacker J. P., Kleinman H. K., Pratt R. M., Yamada K. M. Enhanced cellular fibronectin accumulation in chondrocytes treated with vitamin A. Cell. 1979 Aug;17(4):821–826. doi: 10.1016/0092-8674(79)90322-2. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem. 1983 Mar 10;258(5):3332–3340. [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., Ruoslahti E. Concomitant loss of cell surface fibronectin and laminin from transformed rat kidney cells. J Cell Biol. 1981 Feb;88(2):352–357. doi: 10.1083/jcb.88.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Yamada K. M. Isolation of an actin-binding fragment of fibronectin. Biochem J. 1981 Feb 1;193(2):615–620. doi: 10.1042/bj1930615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Martin G. R., Fishman P. H. Ganglioside inhibition of fibronectin-mediated cell adhesion to collagen. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3367–3371. doi: 10.1073/pnas.76.7.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983 Aug;97(2):466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Johnson R. B. In vitro formation of disulfide-bonded fibronectin multimers. J Biol Chem. 1983 May 25;258(10):6595–6601. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Oh E., Pierschbacher M., Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981 May;78(5):3218–3221. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Engvall E., Cothran W. C., Butler W. T. Alignment of biologically active domains in the fibronectin molecule. J Biol Chem. 1981 Jul 25;256(14):7277–7281. [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984 Mar 25;259(6):3993–4007. [PubMed] [Google Scholar]
- Sekiguchi K., Fukuda M., Hakomori S. Domain structure of hamster plasma fibronectin. Isolation and characterization of four functionally distinct domains and their unequal distribution between two subunit polypeptides. J Biol Chem. 1981 Jun 25;256(12):6452–6462. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Vartio T., Hovi T., Vaheri A. Human macrophages synthesize and secrete a major 95,000-dalton gelatin-binding protein distinct from fibronectin. J Biol Chem. 1982 Aug 10;257(15):8862–8866. [PubMed] [Google Scholar]
- Vartio T., Vaheri A. A gelatin-binding 70,000-dalton glycoprotein synthesized distinctly from fibronectin by normal and malignant adherent cells. J Biol Chem. 1981 Dec 25;256(24):13085–13090. [PubMed] [Google Scholar]
- Wagner D. D., Hynes R. O. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. J Biol Chem. 1979 Jul 25;254(14):6746–6754. [PubMed] [Google Scholar]
- Warburton M. J., Head L. P., Rudland P. S. Redistribution of fibronectin and cytoskeletal proteins during the differentiation of rat mammary tumor cells in vitro. Exp Cell Res. 1981 Mar;132(1):57–66. doi: 10.1016/0014-4827(81)90082-3. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Leivo I., Vaheri A. Expression of the cell surface-associated glycoprotein, fibronectin, in the early mouse embryo. Dev Biol. 1979 Mar;69(1):247–257. doi: 10.1016/0012-1606(79)90289-6. [DOI] [PubMed] [Google Scholar]
- West C. M., Lanza R., Rosenbloom J., Lowe M., Holtzer H., Avdalovic N. Fibronectin alters the phenotypic properties of cultured chick embryo chondroblasts. Cell. 1979 Jul;17(3):491–501. doi: 10.1016/0092-8674(79)90257-5. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]