Abstract
Objective
Abdominal obesity has been associated with increased risk of Barrett’s oesophagus (BE) but the underlying mechanism is unclear. We examined the association between visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) and the risk of BE.
Design
A case-control study among eligible patients scheduled for elective oesophagastroduodenoscopy (EGD) and in a sample of patients eligible for screening colonoscopy recruited at the primary care clinic. All cases with definitive BE and a random sample of controls without BE were invited to undergo standardised mid-abdomen non-contrast computerised axial tomography images, which were analysed by semiautomated image segmentation software. The effect of VAT and SAT surface areas and their ratio (VAT to SAT) on BE were analysed in logistic regression models.
Results
A total of 173 BE cases, 343 colonoscopy controls and 172 endoscopy controls underwent study EGD and CT scan. Participants with BE were more than twice as likely to be in the highest tertile of VAT to SAT ratio (OR: 2.42 (1.51 to 3.88) and adjusted OR 1.47 (0.88 to 2.45)) than colonoscopy controls, especially for those long (≥3 cm) segment BE (3.42 (1.67 to 7.01) and adjusted OR 1.93 (0.92 to 4.09)) and for white men (adjusted OR 2.12 (1.15 to 3.90)). Adjustment for gastroesophageal reflux disease (GERD) symptoms and proton pump inhibitors (PPI) use attenuated this association, but there was a significant increase in BE risk even in the absence of GERD or PPI use.
Conclusions
Large amount of visceral abdominal fat relative to subcutaneous fat is associated with a significant increase in the risk of BE. GERD may mediate some but not all of this association.
INTRODUCTION
Barrett’s oesophagus (BE) is commonly diagnosed among individuals undergoing oesophagastroduodenoscopy (EGD). Because BE is a precursor lesion for oesophageal adenocarcinoma, screening for BE and surveillance for oesophageal neoplasia (dysplasia and cancer) among patients with BE are commonly advocated and practiced.1 Assessment of BE risk factors may allow for better understanding of disease pathophysiology, and prevention including targeted screening and surveillance strategies. Established risk factors for BE include older age, male sex, Caucasian race and GERD symptoms, of which only the latter is potentially modifiable by treatment.2 Additional research and validation of BE risk factors are therefore warranted.
Obesity has been shown to be a modest risk factor for GERD symptoms and oesophageal adenocarcinoma but its association with BE is less clear.3,4 Part of the inconsistency among published studies may be related to measuring the relevant aspects of obesity. Body mass index (BMI), the commonly used indicator of body fat, was not associated with BE risk in most studies.5,6 Abdominal obesity on the other hand may have an important role in GERD pathogenesis. Large waist circumference has been associated with an increased intragastric pressure and frequency of transient relaxations of the lower oesophageal sphincter as well as formation of hiatus hernia and increased oesophageal acid exposure.4 Few but not all studies have indicated a modestly strong association between a large tape-measured waist circumference and the risk of BE.7 In addition, high waist to hip ratio (WHR) has been associated with increasing risk of BE.8
However, waist circumference and WHR are non-specific measures of abdominal fat, which has a visceral and a subcutaneous component. It is possible that the relative distribution of abdominal fat in these two compartments has a varied effect on the risk of BE.9 Visceral abdominal fat in addition to exerting a mechanical effect on the stomach and oesophagus secrets multiple pro-inflammatory cytokines, and is strongly associated with insulin resistance10,11 thus facilitating the development of BE by additional mechanisms. Subcutaneous fat may contribute to the mechanical effect of abdominal fat but is metabolically inert. Despite the biological plausibility of a role of visceral abdominal obesity in BE development, the hypothesis that the visceral adipose tissue (VAT) to subcutaneous adipose tissue (SAT) ratio may increase the risk of BE has not been examined. In addition, the use of CT imaging is necessary to measure the amount of fat in each compartment.
Two small studies have evaluated the effect of visceral abdominal fat measured by imaging on the risk of BE.9,12 For example, we previously reported in a retrospective study of only 36 BE patients and 93 controls who underwent abdominal CT scan for clinical reasons that surface area of visceral abdominal fat was significantly greater in patients with BE than those without BE.13 These studies provided preliminary evidence to the possible role of VAT but were limited by small sample size and selection bias. We therefore conducted a large case-control study in which we performed a standardised mid-abdominal CT image and measured surface areas of visceral and subcutaneous abdominal fat using two different control groups to minimise selection bias.
METHODS
Study population and design
We performed a case-control study at the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, USA. Our choice of controls was driven by the desire to ensure that controls should be chosen to represent the base population from which the cases arise in terms of the exposure (abdominal obesity). Since abdominal obesity among endoscopy controls may differ from that in the base population, using controls referred to endoscopy for clinical reasons may introduce selection bias. Therefore, in addition to endoscopy controls, controls without BE came from individuals attending primary care clinics who were eligible for a screening colonoscopy (and agreed to undergo study EGD).
We recruited subjects between 15 February 2008 and 31 July 2011 from consecutive patients identified at one of seven primary care facilities in the MEDVAMC system who were eligible for a routine screening colonoscopy and agreed to also undergo an EGD at the same time as their colonoscopy. Our secondary recruitment source was consecutive eligible patients undergoing an elective EGD. The eligibility criteria were: (1) age between 50 and 80 years (and 40–80 years for the elective EGD group); (2) no previous gastro-oesophageal surgery; (3) no previous gastro-oesophageal cancer; (4) no active lung, liver, colon, breast or stomach cancer; (5) no anticoagulants; (6) no significant liver disease indicated by platelet count below 70 000, ascites or known gastro-oesophageal varices; and (7) no history of major stroke or mental condition.
This research was approved by the Institutional Review Boards for the MEDVAMC and Baylor College of Medicine.
Data collection
Study participants completed a computer assisted survey before the study EGD. The survey ascertained information regarding lifetime use of alcohol and smoking, frequency and severity of GERD symptoms, and use of H2 receptor antagonists, proton pump inhibitors (PPI), non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin. Research assistants measured participants’ body weight using a digital upright scale (Health o meter Professional). Height in inches was obtained using a study designated stadiometer. A flexible tape was used to measure waist and hip circumference.
All study participants underwent a study EGD with systematic recording of suspected BE based on the Prague circumferential and maximum length (CFM) classification14 and targeted biopsies from these areas using Jumbo biopsy forceps. Definitive BE was considered in the presence of small intestinal epithelium with goblet cells in the histopathological examination of biopsy samples obtained from suspected BE areas. Patients with no suspected or definitive BE served as potential controls.
Study CT scan
We invited all BE cases and a sample of controls, oversampling for colonoscopy controls, to undergo a mid-abdominal CT scan (1:1:2 ratio). Within the two control groups we created a random ordering of remaining eligible patients using the Ranuni function in SAS and chose the appropriate number of needed patients starting from the beginning of the list.
CT scanning was performed using a Gemini GXL scanner at 120 Kv exposures and a slice thickness of 6 mm. Three single cut non-contrast images were taken at L4–L5 level, and were reviewed by a study radiologist (MR) blinded to the case/control status who chose the most representative image for analysis. The images were analysed by using semiautomated image segmentation software implemented in the Analyse software system 10.0 (Mayo Clinic Foundation, Biomedical Imaging Resource, Rochester, Minnesota, USA).15–20 The threshold tool of the software was set between −150 and −50 Hounsfield Units which is the range for adipose tissues on CT images. VAT was defined as the intra-abdominal fat confined within the rectus sheath. The SPLINE tool was used to demarcate the VAT by drawing a line around the spine and intra-abdominal muscles (Rectus Abdominis, Transverse Abdominis, Quadratus Lumborum and Psoas). The fat around the paraspinous muscle, intervertebral bodies and intramuscular fat was excluded from the VAT and SAT calculations.21,22 The remaining fat interposed between the muscle and the subcutaneous tissue was selected and calculated as SAT. VAT and SAT surface areas were calculated and reported in cm2 (figure 1). A single investigator (AH) blinded to the case/control status performed the VAT and SAT calculations. To further examine intraobserver variation, the same investigator remeasured a 30% random sample of images, as well as the top and the bottom 10th percentile areas while blinded to the initial measurements. There was a high correlation (Rho=0.99, p<0.0001 for both SAT and VAT) between the first and the second measurements.
Figure 1.
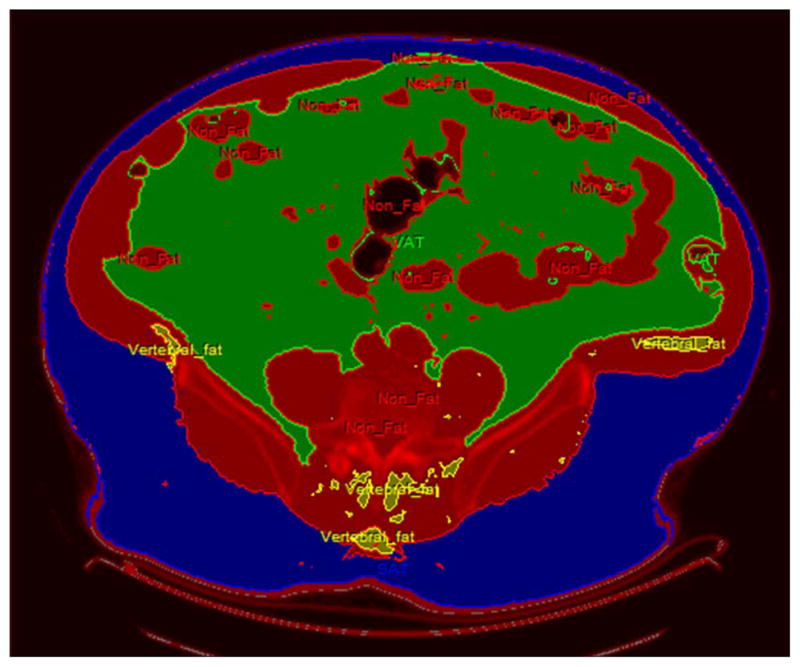
Procedure for measurement of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) CT scan at L4–L5 level. Thresholding was used and tissue with attenuation of −150 to −50 Hounsfield Units was designated as FAT and rest as NON-FAT (RED). Using semiautomated segmentation VAT (GREEN) and SAT (BLUE) were delineated. Para vertebral and intramuscular fat (YELLOW) was selected and not included in the analysis.
Data analysis
We compared cases with definitive BE to controls without BE. We also performed an analysis for long segment BE (≥3 cm) compared with no BE. The main exposure variables were surface areas of VAT, SAT and VAT to SAT ratio examined as tertiles as well as continuous variables. With 173 cases and 343 colonoscopy controls we had 81% and 82% power to detect an unadjusted OR of 2.0 for the 3rd and 2nd tertiles of VAT/SAT ratio, respectively, compared with the 1st tertile. We also examined the association between abdominal fat measures and the presence and duration of GERD symptoms. Given the possible racial differences in abdominal fat we also performed a sensitivity analysis limited to Caucasian male subjects only. We also conducted age-stratified analyses in an attempt to examine the association between abdominal fat distribution at a time that is closest to the time of BE onset. In all analyses, patients with endoscopic BE only (not confirmed with histopathology) were excluded because of their unclear case/control status.
We adjusted for potential confounders including age, sex, race, tobacco smoking, alcohol use, Helicobacter pylori status and NSAID or aspirin use. We subsequently adjusted for presence and duration of GERD symptoms and H2 receptor antagonists/PPI medication use as possible explanatory variable for any association observed with VAT, SAT or VAT/SAT and BE. We also explored the role of GERD by conducting stratified analyses based on the absence or presence of GERD symptoms or PPI use.
GERD was defined as the sum of the duration of heartburn or regurgitation symptoms at least weekly and categorised as none, <5 years, 5–<10 years and ≥10 years. H pylori positive status was defined based on organisms that were seen on histopathology of any gastric biopsy, previously positive biopsy or serum, or any H pylori treatment received.
χ2 Tests were used for categorical variables and Wilcoxon’s test for continuous variables. For tertiles, we also used Kendall τ test for trends. Logistic regression models were used for multivariable adjustment and only variables with p<0.1 were retained in the model. Parameter estimates and SEs from the model were used to calculate ORs and their accompanying 95% CIs.
RESULTS
We recruited 235 cases with definitive BE and invited all of them for study CT scan. We also invited for study CT scan a randomly identified group endoscopic and colonoscopy controls in 1 (case):1 (endoscopic control):2 (colonoscopy control) ratio. A total of 176 cases with definitive BE, 344 colonoscopy controls and 173 endoscopy controls underwent study CT scan with participation rates of 74.9%, 74.3% and 73.2%, respectively. Accurate measurement of abdominal fat distribution from the CT scans was not obtained for five patients because of movement during the procedure: three definitive BE cases and one from each control group. There were no significant differences in BMI between cases or controls who underwent CT scan and their counterparts who did not have a CT scan. For example, the mean BMI for cases with CT scan was 30.4 (SD 5.4) compared with mean BMI 29.6 (5.5) in cases without CT scan.
Of the 173 cases with definitive BE and accurately measured abdominal fat distribution, most were newly diagnosed or incident cases and only 37 were previously diagnosed BE. Similarly, most of the BE cases were identified from patients electively referred to endoscopy and only 37 were diagnosed from the colonoscopy group.
There were no significant differences in age, NSAID use, alcohol drinking or BMI among the three groups (table 1). Given the underlying veteran population, most subjects were men; however, there were significantly more men in the BE case than the two control groups (98.3% vs 97.4% and 93.1%, respectively). As expected, there was a significantly higher proportion of Caucasians in the BE group than the colonoscopy or endoscopy control groups (88.1% vs 56.7% and 67.1%, respectively). Similarly, longer duration of GERD symptoms (≥10 years) was associated with a higher BE risk (46.7% vs 16.8% and 38.9%) (table 1). Consistent with known associations of BE, cases were more likely to report tobacco smoking and less likely to be infected with H pylori compared with controls.
Table 1.
Demographic and clinical characteristics of BE cases and the two control groups
| BE cases (n=173) | Colonoscopy controls (n=343) | Endoscopy controls (n=172) | |||
|---|---|---|---|---|---|
| n (%) | n (%) | p Value | n (%) | p Value | |
| Age, mean SD | 63.1 (7.2) | 62.9 (6.9) | 0.91 | 63.1 (7.3) | 0.88 |
| Sex, men | 170 (98) | 334 (97) | 0.53 | 160 (93) | 0.02 |
| Race | <0.001 | <0.001 | |||
| White | 152 (88) | 194 (57) | 115 (67) | ||
| African American | 18 (10) | 142 (41) | 52 (30) | ||
| Other | 3 (2) | 7 (2) | 5 (3) | ||
| GERD duration | |||||
| No GERD | 61 (36) | 245 (74) | <0.001 | 68 (41) | 0.55 |
| <5 years | 17 (10) | 14 (4) | 20 (12) | ||
| 5–<10 years | 12 (7) | 18 (5) | 14 (8) | ||
| ≥10 years | 79 (47) | 56 (17) | 65 (34) | ||
| PPI use | 119 (72) | 72 (22) | <0.001 | 98 (60) | 0.01 |
| NSAID intake | 84 (50) | 171 (52) | 0.85 | 80 (49) | 0.74 |
| Tobacco smoking | |||||
| <1 pack-year | 35 (22) | 97 (29) | 0.06 | 57 (35) | 0.02 |
| 1–30 pack-years | 57 (36) | 125 (38) | 57 (35) | ||
| ≥30 pack-years | 68 (42) | 107 (33) | 50 (30) | ||
| Alcohol drinking | |||||
| Never | 12 (7) | 23 (7) | 0.46 | 12 (7) | 0.69 |
| Former | 85 (52) | 189 (57) | 77 (47) | ||
| Current | 68 (41) | 118 (36) | 75 (46) | ||
| BMI | |||||
| Mean | 30.3 (5.0) | 31.4 (6.1) | 0.05 | 30.5 (7.1) | 0.76 |
| <25 | 24 (14) | 42 (12) | 0.79 | 36 (21) | 0.17 |
| 25–30 | 63 (36) | 121 (35) | 52 (30) | ||
| >30 | 86 (50) | 180 (53) | 84 (49) | ||
| Helicobacter pylori | 32 (19) | 120 (36) | <0.001 | 51 (30) | 0.02 |
BE, Barrett’s oesophagus; BMI, Body mass index; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitors.
The median VAT surface area was significantly larger in the BE group by approximately 43 cm2 compared with the colonoscopy control group (p=0.006), and 33 cm2 than the endoscopy control group (p=0.06). Similarly, a larger proportion of BE cases (38.7%) had the highest tertile of VAT surface area than colonoscopy (29.5%) or endoscopy (35.5%) controls. There were no significant differences in SAT surface area among the three groups, albeit there was a non-significant increase in SAT among colonoscopy controls compared with the other two groups. Overall, SAT comprised a larger proportion (61.5%) of abdominal fat surface area than VAT (38.5%) measured on CT scan.
The median VAT to SAT ratio was significantly greater in BE than the colonoscopy control group (p=0.007). The association between BE and VAT/SAT compared with colonoscopy control group was greater in magnitude than that observed for VAT only. The highest tertile of VAT/SAT was associated with more than a twofold increase of having BE than the lowest tertile (OR=2.42 (1.51 to 3.88)). Adjustment for age, gender, race, H pylori, smoking, alcohol use and NSAID use led to a diminished association for the second tertile 1. 63 (0.98 to 2.68) and the highest tertile (OR=1.47 (0.88 to 2.45)) compared with the lowest tertile. The main reasons for the attenuated adjusted OR were race and age. Although the median VAT to SAT ratio was also greater in BE cases than endoscopy controls, the difference was not statistically significant (p=0.12). The highest two tertiles were more common in cases than endoscopy controls, reached significance for the second tertile only and became non-significant in the adjusted models (table 2).
Table 2.
The distribution of abdominal fat measured by CT scan among BE cases and the two control groups
| BE cases (n=173) | Colonoscopy controls (n=343) | Unadjusted OR | Adjusted* OR | Endoscopy controls (n=172) | Unadjusted OR | Adjusted* OR | |
|---|---|---|---|---|---|---|---|
| VAT | |||||||
| Median | 206.9 | 163.7 | 172.8 | ||||
| 1st tertile (<142.92) | 41 (23) | 128 (37) | 1.00 | 1.00 | 60 (35) | 1.00 | 1.00 |
| 2nd tertile (142.923–<226.03) | 65 (38) | 114 (33) | 1.78 (1.12 to 2.84) | 1.40 (0.85 to 2.30) | 51 (30) | 1.87 (1.09 to 3.20) | 1.58 (0.89 to 2.79) |
| 3rd tertile (≥226.03) | 67 (39) | 101 (30) | 2.07 (1.30 to 3.31) | 1.30 (0.79 to 2.15) | 61 (35) | 1.61 (0.95 to 2.72) | 1.05 (0.59 to 1.85) |
| p Trend | 0.002 | 0.11 | |||||
| SAT | |||||||
| Median | 288.1 | 311.7 | 286.4 | ||||
| 1st tertile (<246.91) | 55 (32) | 112 (33) | 1.00 | 1.00 | 62 (36) | 1.00 | 1.00 |
| 2nd tertile (246.91–<360.18) | 73 (42) | 106 (31) | 1.40 (0.90 to 2.18) | 1.72 (1.01 to 2.92) | 51 (30) | 1.61 (0.97 to 2.69) | 1.54 (0.97 to 2.46) |
| 3rd tertile (≥360.18) | 45 (26) | 125 (36) | 0.73 (0.46 to 1.17) | 1.04 (0.59 to 1.84) | 59 (34) | 0.86 (0.51 to 1.46) | 0.80 (0.48 to 1.30) |
| p Trend | 0.19 | 0.67 | |||||
| VAT to SAT ratio | |||||||
| Median | 0.65 | 0.55 | 0.61 | ||||
| 1st tertile (<0.47) | 39 (22) | 134 (39) | 1.00 | 1.00 | 56 (33) | 1.00 | 1.00 |
| 2nd tertile (0.47–<0.76) | 65 (38) | 111 (32) | 2.01 (1.26 to 3.22) | 1.63 (0.98 to 2.68) | 54 (31) | 1.73 (1.00 to 2.98) | 1.00 (0.53 to 1.92) |
| 3rd tertile (≥0.76) | 69 (40) | 98 (29) | 2.42 (1.51 to 3.88) | 1.47 (0.88 to 2.45) | 62 (36) | 1.60 (0.94 to 2.73) | 0.84 (0.44 to 1.60) |
| p Trend | <0.001 | 0.12 | |||||
Adjusted for age, gender, race, NSAID, Helicobacter pylori infection, smoking and alcohol.
BE, Barrett’s oesophagus; NSAID, non-steroidal anti-inflammatory drug; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Considering that GERD symptoms may be one of the mediating variables between abdominal obesity and BE, we examined the association among VAT, SAT, VAT/SAT and the presence and duration of self-reported GERD and found that VAT and VAT/SAT but not SAT is associated with both the presence as well as longer duration of GERD symptoms. There was a progressive increase in the proportions of patients with the highest two levels of VAT and VAT/SAT among patients with longer durations of GERD symptoms (Kendall’s tau p=0.007 and p<0.001, respectively ). The additional adjustment for GERD symptoms and PPI use attenuated the associations between BE and VAT/SAT, and rendered them statistically non-significant (OR=1.41 (0.81 to 2.46); data not shown). We further stratified the analyses by the presence or absence of GERD symptoms/PPI use (table 3), and given the relatively small number of observations in each stratum we examined VAT and VAT/SAT as binary variables. The associations between BE and each of VAT and VAT/SAT were statistically significant among 31 cases, 219 controls and 41 endoscopy controls with no GERD symptoms.
Table 3.
The distribution of abdominal fat measured by CT scan among BE cases, colonoscopy control group and endoscopy control groups all stratified by the absence or presence of GERD symptoms or PPI use
| GERD and PPI negative patients | BE Cases (n=31) % | Colonoscopy controls (n=219) % | Unadjusted OR | Adjusted* OR | Endoscopy controls (n=41) % | Unadjusted OR | Adjusted* OR |
|---|---|---|---|---|---|---|---|
| VAT | |||||||
| Below median | 13 (42) | 122 (56) | 1.00 | 1.00 | 29 (71) | 1.00 | 1.00 |
| Median and above | 18 (58) | 97 (44) | 1.74 (0.81–3.73) | 1.24 (0.56–2.75) | 12 (29) | 3.35 (1.26–8.92) | 2.30 (0.79–6.72) |
| p Trend | 0.16 | 0.01 | |||||
| VAT/SAT ratio | |||||||
| Below median | 12 (39) | 121 (55) | 1.00 | 1.00 | 23 (56) | 1.00 | 1.00 |
| Median and above | 19 (61) | 98 (45) | 1.96 (0.91–4.22) | 1.35 (0.61–3.03) | 18 (44) | 2.02 (0.78–5.23) | 1.15 (0.36–3.53) |
| p Trend | 0.09 | 0.14 | |||||
| GERD or PPI positive patients | BE Cases (n=136) % | Colonoscopy controls (n=115) % | Unadjusted OR | Adjusted* OR | Endoscopy controls (n=127) % | Unadjusted OR | Adjusted* OR |
|---|---|---|---|---|---|---|---|
| VAT | |||||||
| Below median | 49 (36) | 62 (54) | 1.00 | 1.00 | 60 (47) | 1.00 | 1.00 |
| Median and above | 87 (64) | 53 (46) | 2.08 (1.25–3.45) | 1.68 (0.98–2.88) | 67 (53) | 1.59 (0.97–2.61) | 1.28 (0.75–2.18) |
| p Trend | <0.01 | 0.06 | |||||
| VAT/SAT ratio | |||||||
| Below median | 57 (42) | 59 (51) | 1.00 | 1.00 | 56 (44) | 1.00 | 1.00 |
| Median and above | 79 (58) | 56 (49) | 1.46 (0.89–2.41) | 1.00 (0.57–1.74) | 71 (56) | 1.09 (0.67–1.78) | 0.67 (0.39–1.78) |
| p Trend | 0.22 | 0.72 | |||||
Adjusted for age, gender, NSAID, Helicobacter pylori infection, smoking and alcohol.
BE, Barrett’s oesophagus; PPI, propton pump inhibitors; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
In the comparison between BE cases with ≥3 cm versus colonoscopy control, an even larger proportion of BE cases was in the highest tertile of VAT than controls (41.5% vs 29.7%) than that observed for the overall BE or for short segment BE (table 4). The risk of BE was significantly elevated in the unadjusted analysis, but attenuated when adjusted for age, gender, race, H pylori, smoking, alcohol use and NSAID use, and further attenuated by adjusting for GERD symptoms and PPI use. The median VAT to SAT ratio in cases with BE ≥3 cm segment was significantly greater than the colonoscopy controls (0.68 vs 0.64; p=0.006). The two higher tertiles of VAT to SAT ratio were significantly associated with BE≥3 cm (39% vs 36% p=0.006, and 44% vs 38% p=0.001, respectively), although this association was reduced in the adjusted analyses (table 4), (p=0.04 and 0.05) and with further adjustment for GERD symptoms and PPI use (p=0.08 and p=0.18; data not shown). The associations between each of VAT and VAT/SAT with BE were larger in magnitude for BE≥3 cm than those for BE <3 cm; for example, the ORs for the third VAT tertiles were 3.06 and 1.66 for long and short BE, respectively, and the ORs for the third VAT/SAT tertiles were 3.42 and 1.98 for long and short BE, respectively.
Table 4.
The distribution of abdominal fat measured by CT scan among BE cases and the colonoscopy control group
| BE<3 cm (n=104) | BE≥3 cm (n=69) | |||||
|---|---|---|---|---|---|---|
| n (%) | Unadjusted OR for BE<3 cm vs no BE | Adjusted* OR for BE<3 cm (no GERD or PPI) | n (%) | Unadjusted OR for BE≥3 cm vs no BE | Adjusted* OR for BE≥3 cm (no GERD or PPI) | |
| VAT (cm2) | ||||||
| Median | 171.1 | 182.7 | ||||
| 1st tertile (<142.9) | 29 (28) | 1.0 | 1.0 | 12 (17) | 1.0 | 1.0 |
| 2nd tertile (142.9–<226.0) | 37 (36) | 1.43 (0.83 to 2.48) | 1.30 (0.71 to 2.39) | 28 (41) | 2.62 (1.27 to 5.39) | 1.98 (0.93 to 4.21) |
| 3rd tertile (≥226.0) | 38 (36) | 1.66 (0.96 to 2.88) | 1.20 (0.65 to 2.22) | 29 (42) | 3.06 (1.49 to 6.30) | 1.76 (0.83 to 3.74) |
| p Trend | 0.07 | 0.001 | ||||
| SAT (cm2) | ||||||
| Median | 269.0 | 314.6 | ||||
| 1st tertile (<246.9) | 31 (30) | 1.0 | 1.0 | 24 (35) | 1.0 | 1.0 |
| 2nd tertile (246.9–<360.2) | 51 (49) | 1.74 (1.03 to 2.92) | 1.99 (1.12 to 3.51) | 22 (32) | 0.97 (0.51 to 1.83) | 0.96 (0.48 to 1.92) |
| 3rd tertile (≥360.2) | 22 (21) | 0.64 (0.35 to 1.16) | 0.71 (0.37 to 1.36) | 23 (33) | 0.86 (0.46 to 1.61) | 0.94 (0.48 to 1.82) |
| p Trend | 0.13 | 0.63 | ||||
| VAT to SAT ratio | ||||||
| Median | 0.64 | 0.68 | ||||
| 1st tertile (<0.47) | 27 (26) | 1.0 | 1.0 | 12 (17) | 1.0 | 1.0 |
| 2nd tertile (0.47–<0.76) | 38 (36) | 1.70 (0.98 to 2.96) | 1.63 (0.98 to 2.68) | 27 (39) | 2.72 (1.32 to 5.61) | 2.19 (1.03 to 4.68) |
| 3rd tertile (≥0.76) | 39 (38) | 1.98 (1.13 to 3.44) | 1.47 (0.88 to 2.45) | 30 (44) | 3.42 (1.67 to 7.01) | 1.93 (0.92 to 4.09) |
| p Trend | 0.01 | <0.001 | ||||
BE cases categorised by length of BE segment as <3 and ≥3 cm.
Adjusted for age, gender, race, NSAID, Helicobacter pylori infection, smoking and alcohol.
BE, Barrett’s oesophagus; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitors; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
We found the age specific estimates of association between BE and VAT/SAT to be generally similar to each other with a trend of higher estimates for younger ages. The ORs for the highest tertile (vs lowest) for <55-years-old and ≥55-years-old were 2.75 (95% CI 0.77 to 9.80) and 2.45 (95% CI 1.47 to 4.09), respectively (data not shown).
Given the known racial and sex differences in fat distribution and the strong confounding effect of the race variable in our study, we performed similar analyses that were limited to Caucasian men using 149 BE cases and 177 colonoscopy controls (table 5). The unadjusted associations between BE and VAT/SAT were similar in direction and magnitude to those observed for the overall group. However, unlike the analyses in the overall group, significant associations (>twofold increase in BE risk) persisted even with adjustment for age, NSAID, H pylori infection, smoking and alcohol (table 5), as well as the additional adjustment for GERD symptoms and PPI use (OR 2.27, 95% CI 1.09 to 4.72, and 1.96, 95% CI 0.97 to 3.97, for the 2nd and 3rd tertiles, respectively); data not shown.
Table 5.
The distribution of abdominal fat measured by CT scan among white men only (n=326) including BE cases and colonoscopy control group
| BE (n=149) n (%) | Controls (n=177) n (%) | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | |
|---|---|---|---|---|
| VAT (cm2) | ||||
| Median | 207.4 | 193.9 | 0.12 | |
| 1st tertile (<142.9) | 29 (20) | 47 (27) | 1.00 | 1.00 |
| 2nd tertile (142.9–<226.0) | 57 (38) | 59 (33) | 1.57 (0.87 to 2.82) | 1.81 (0.97 to 3.35) |
| 3rd tertile (≥226.0) | 63 (42) | 71 (40) | 1.44 (0.81 to 2.55) | 1.53 (0.85 to 2.78) |
| p Trend | 0.33 | |||
| SAT (cm2) | ||||
| Median | 287.4 | 305.4 | 0.37 | |
| 1st tertile (<246.9) | 51 (34) | 58 (33) | 1.00 | 1.00 |
| 2nd tertile (246.9–<360.2) | 64 (43) | 54 (30) | 1.35 (0.80 to 2.27) | 1.38 (0.81 to 2.37) |
| 3rd tertile (≥360.2) | 34 (23) | 65 (37) | 0.60 (0.34 to 1.04) | 0.62 (0.35 to 1.11) |
| p Trend | 0.08 | |||
| VAT to SAT ratio | ||||
| Median | 0.71 | 0.66 | 0.12 | |
| 1st tertile (<0.47) | 24 (16) | 53 (30) | 1.00 | 1.00 |
| 2nd tertile (0.47–<0.76) | 58 (39) | 54 (31) | 2.37 (1.29 to 4.36) | 2.38 (1.26 to 4.50) |
| 3rd tertile (≥0.76) | 67 (45) | 70 (39) | 2.11 (1.18 to 3.80) | 2.12 (1.15 to 3.90) |
| p Trend | 0.04 | |||
Adjusted for age, NSAID, Helicobacter pylori infection, smoking and alcohol.
BE, Barrett’s oesophagus; NSAID, non-steroidal anti-inflammatory drug; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
DISCUSSION
We found that visceral abdominal obesity measured as the ratio of VAT to SAT surface areas on mid-abdomen CT scan imaging was associated with a significantly increased risk of having BE. The association between BE and abdominal obesity was specific to visceral but not subcutaneous fat. In particular, the VAT/SAT BE association was strong among white men and for BE segment ≥3 cm. In white men, the odds of having BE was almost twofold higher in the group with the highest tertile of VAT/SAT than those in the lowest tertile. The VAT or VAT/SAT BE association was stronger in the comparison between BE cases and colonoscopy controls than that seen with endoscopy controls and this was mostly explained by the high prevalence of GERD in endoscopy controls.
Our study was uniquely designed to address the association between obesity and BE. We used an additional control group that consisted of patients who were eligible (not already scheduled) for screening colonoscopy identified in primary care clinics and recruited specifically for the study. Our a priori rationale is that endoscopy controls (ie, those who were referred to EGD for clinical reasons and did not have BE) are likely overmatched to BE cases in terms of GERD symptoms and possibly other BE risk factors. In general, the 2nd tertiles of VAT or VAT/SAT had at least as strong an association (compared with 3rd tertile) with BE in most of the analyses. This is possibly due to the fact that this was a relatively obese (including the 2nd tertile) study population.
We examined the mediating role of GERD symptoms. Our analyses suggest that GERD symptoms only partly explained the visceral abdominal obesity effect on BE in white men. It is possible that acid or bile reflux is still present (or absent) in the absence (or absence) of symptoms. It is likely in this study that GERD is misclassified non-differentially and thus would have no effect on the observed associations; however, it is also possible misclassified (absent) GERD symptoms are more common in the BE case group, and then the explanatory effect of the GERD variable would be even greater than what we described. Furthermore, given that the relevant exposure period to obesity in the life of a patient with BE is uncertain, and that the onset of both BE and visceral obesity cannot be examined in this study, one cannot completely exclude GERD as the main mechanism of obesity-related BE. Nevertheless, additional mechanisms for the association between abdominal obesity and increased BE risk need to be considered. Visceral abdominal obesity has been associated with increased risk of several disorders such as diabetes, ischaemic heart disease and malignancies including colorectal cancer.23,24 Visceral abdominal fat is metabolically active and has been associated with low serum levels of potentially protective adipokines (eg, adiponectin) and high pro-inflammatory cytokines (eg, interleukin-1β, interleukin-6 and tumour necrosis factor-α).25 These cytokines may potentially increase the inflammation and hence the malignant transformation in patients with BE.26 These actions are additive to the known mechanical factors related to increased intragastric pressure, increased hiatus formation and size and increased frequency of transient relaxations of the lower oesophageal sphincter.
This is the first large study to evaluate the distribution of abdominal fat compartments between BE cases and appropriate controls. Mid-abdomen CT scan was applied in a standardised fashion for the study purposes and was not driven by diagnostic work up. The detailed measurement of the different components of abdominal fat has given us greater insight into the possible mechanisms of BE pathogenesis in relation to obesity. Measurement of VAT surface area in CT scan cuts taken at the level of intervertebral disc between L4 and L5 has been shown in previous studies to be a highly accurate and reproducible measure of VAT volume (correlation coefficient=0.9) obtained from 3-D image reconstruction of multiple CT or MRI cuts.27–31 The advantage of the three cuts that the small amount of radiation (2.0 rads) and the amount of time necessary is short (10 min). Our study confirmed very high interobserver and intraobserver agreement in these measurements reported by previous studies.7,26 Last, the a priori use of VAT to SAT ratio was planned to account for the individual variability in VAT and SAT and for possibility that both VAT and SAT may increase the risk of BE.
Abdominal obesity explains some of the epidemiological features of BE and oesophageal adenocarcinoma. The distribution of body fat tends to be more abdominal than truncal in high-risk groups for BE including Caucasians (compared with African Americans) and men (compared with women). A population-based study reported an association between abdominal diameter and GERD symptoms in white populations, but not in African Americans or Asians.32 In our study, race was the strongest confounder of the association between BE and VAT, and therefore we conducted additional stratified analysis limited to white men (table 4). VAT/SAT was significantly higher in Caucasian than African Americans (p<0.001), and this ratio was a significant predictor of BE only in Caucasians. However in our study, the number of African Americans (18 cases and 129 controls) was small and differences could have been missed. These findings, combined with the increased prevalence of abdominal obesity in male subjects,8,33 suggest that increased obesity may disproportionately increase GERD in white subjects and in male subjects. Two other recent studies have shown abdominal obesity measured as waist circumference to be a risk factor of BE independent of BMI, while any association between BMI and BE was explained away by adjustment of abdominal diameter.7,8 These studies indicate that abdominal fat is the key factor in the pathogenesis of BE and may also explain the remarkable disproportionate increase in BE (and EA) risk among white subjects.
The study has a few limitations. The case-control design does not allow for examining the temporal association between abdominal obesity and BE and thus causal inferences are limited. This is further complicated by the unclear time of onset in virtually all patients with BE. However, the association between VAT/SAT and BE persisted in relatively younger group (<55 years) when one expects a relatively recent onset of BE. Last, most participants in the VA study were men and therefore the generalisability of our findings to other groups is unknown. However, we believe that these limitations are outweighed by the use of CT scan as an objective, highly reproducible and well-validated measure of abdominal fat, the large and well-defined groups of cases and controls, and the low possibility of recall and interviewer bias because of the systematic identification and adjustment for multiple possible confounders before the case and control status was defined.
In summary, our study showed that CT scan measured visceral abdominal fat is a significant independent risk factor for BE. The mechanisms for this association need to be further examined.
Significance of this study.
What is already known on this subject?
The obesity epidemic has paralleled the increase in oesophageal adenocarcinoma.
Abdominal, but not general obesity, has been associated with increased risk of Barrett’s oesophagus.
Abdominal fat in comprised of two functionally distinct types of fat: visceral and subcutaneous.
The effect of the distribution of visceral and subcutaneous abdominal fat on the risk of Barretts oesophagus is unknown.
What are the new findings?
In this large scale case-control study, standardised abdominal CT scans were used to estimate the amount and distribution of abdominal fat.
The amount of visceral, but not subcutaneous, abdominal fat was associated with a significant increase in the risk of Barretts oesophagus.
The association was partly explained by presence of GERD symptoms but also seen among those without GERD.
How might it impact on clinical practice in the foreseeable future?
These important findings point toward humoral mechanisms of obesity-related increased Barretts oesophagus risk.
Targeting such mechanisms may have important implications in understanding the pathogenesis of Barrett’s as well as its prevention.
Acknowledgments
Funding This work is funded in part by NIH grant NCI R01 116845, the Houston VA HSR&D Center of Excellence (HFP90-020), and the Texas Digestive Disease Center NIH DK58338. Dr El-Serag is also supported by NIDDK K24-04-107.
Footnotes
Competing interests None.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors HBE-S: funding, conception, design, analysis, interpretation results, manuscript writing, editing, decision to publish. AH: conception, data collection; editing manuscript, decision to publish. JG: analysis, editing manuscript, decision to publish. PR, AA, SF, M Velez and JRK: data collection, editing manuscript, decision to publish. M Vela, YS and MBR: analysis, editing manuscript, decision to publish. NSA, RC and BA: conception, analysis, editing manuscript, decision to publish. DYG: analysis, conception, design, editing manuscript, decision to publish.
References
- 1.Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361:2548–56. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 2.Winberg H, Lindblad M, Lagergren J, et al. Risk factors and chemoprevention in Barrett’s esophagus—an update. Scand J Gastroenterol. 2012;47:397–406. doi: 10.3109/00365521.2012.667145. [DOI] [PubMed] [Google Scholar]
- 3.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag H. Role of obesity in GORD-related disorders. Gut. 2008;57:281–4. doi: 10.1136/gut.2007.127878. [DOI] [PubMed] [Google Scholar]
- 5.Kamat P, Wen S, Morris J, et al. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:655–62. doi: 10.1016/j.athoracsur.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am J Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 7.Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut. 2007;56:756–62. doi: 10.1136/gut.2006.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403–11. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Nelsen EM, Kirihara Y, Takahashi N, et al. Distribution of body fat and its influence on esophageal inflammation and dysplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2012;10:728–34. doi: 10.1016/j.cgh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu P, Poirier P, Pibarot P, et al. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–84. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 11.Yudkin JS. Inflammation, obesity, and the metabolic syndrome. Horm Metab Res. 2007;39:707–9. doi: 10.1055/s-2007-985898. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Kvapil P, Hacken-Bitar J, et al. Abdominal obesity and the risk of Barrett’s esophagus. Am J Gastroenterol. 2005;100:2151–6. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 13.Anand O, Wani S, Sharma P. When and how to grade Barrett’s columnar metaplasia: the Prague system. Best Pract Res Clin Gastroenterol. 2008;22:661–9. doi: 10.1016/j.bpg.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Bonekamp S, Ghosh P, Crawford S, et al. Quantitative comparison and evaluation of software packages for assessment of abdominal adipose tissue distribution by magnetic resonance imaging. Int J Obes (Lond) 2008;32:100–11. doi: 10.1038/sj.ijo.0803696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortimore IL, Marshall I, Wraith PK, et al. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–3. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 16.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–80. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 18.Siegel MJ, Hildebolt CF, Bae KT, et al. Total and intraabdominal fat distribution in preadolescents and adolescents: measurement with MR imaging. Radiology. 2007;242:846–56. doi: 10.1148/radiol.2423060111. [DOI] [PubMed] [Google Scholar]
- 19.Koska J, Stefan N, Permana PA, et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87:295–302. doi: 10.1093/ajcn/87.2.295. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama T, Yoneda M, Inamori M, et al. Visceral obesity and the risk of Barrett’s esophagus in Japanese patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2009;9:56. doi: 10.1186/1471-230X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam SY, Choi IJ, Ryu KH, et al. Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women. Gastroenterology. 2010;139:1902–11. doi: 10.1053/j.gastro.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–5. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 23.Kaess BM, Pedley A, Massaro JM, et al. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55:2622–30. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito T, Murata M, Otani T, et al. Association of subcutaneous and visceral fat mass with serum concentrations of adipokines in subjects with type 2 diabetes mellitus. Endocr J. 2012;59:39–45. doi: 10.1507/endocrj.ej11-0132. [DOI] [PubMed] [Google Scholar]
- 25.Picardo SL, Maher SG, O’Sullivan JN, et al. Barrett’s to oesophageal cancer sequence: a model of inflammatory-driven upper gastrointestinal cancer. Dig Surg. 2012;29:251–60. doi: 10.1159/000341498. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner RN, Heymsfield SB, Roche AF, et al. Abdominal composition quantified by computed tomography. Am J Clin Nutr. 1988;48:936–45. doi: 10.1093/ajcn/48.4.936. [DOI] [PubMed] [Google Scholar]
- 27.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes. 1986;10:53–67. [PubMed] [Google Scholar]
- 28.Kvist H, Chowdhury B, Grangard U, et al. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 29.Kvist H, Chowdhury B, Sjostrom L, et al. Adipose tissue volume determination in males by computed tomography and 40K. Int J Obes. 1988;12:249–66. [PubMed] [Google Scholar]
- 30.Seidell JC, Oosterlee A, Deurenberg P, et al. Abdominal fat depots measured with computed tomography: effects of degree of obesity, sex, and age. Eur J Clin Nutr. 1988;42:805–15. [PubMed] [Google Scholar]
- 31.Thaete FL, Colberg SR, Burke T, et al. Reproducibility of computed tomography measurement of visceral adipose tissue area. Int J Obes Relat Metab Disord. 1995;19:464–7. [PubMed] [Google Scholar]
- 32.Zhao G, Ford ES, Li C, et al. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: National Health and Nutrition Examination Survey 2005–2006. BMC Psychiatry. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi B, Liang Y, He T, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Rev. 2012;13:287–96. doi: 10.1111/j.1467-789X.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


