Abstract
Objectives
Necrotizing enterocolitis (NEC) is complex disease thought to occur as a result of an immaturity of the gastrointestinal tract of preterm infants. Intestinal dysfunction induced by total parental nutrition (TPN) may increase the risk for NEC upon introduction of enteral feeding. We hypothesized that the intestinal trophic and anti-inflammatory actions previously ascribed to the gut hormone, glucagon-like peptide-2 (GLP-2), would reduce the incidence of NEC when given in combination with TPN in preterm piglets.
Methods
Preterm, newborn piglets were nourished by TPN and infused continuously with either human GLP-2 (100 μg · kg−1 · day−1) or control saline for 2 days (n = 12/group). On day 3, TPN was discontinued and pigs were given orogastric formula feeding every 3 hours, and continued GLP-2 or control treatment until the onset of clinical signs of NEC for an additional 96 hours and tissue was collected for molecular and histological endpoints.
Results
GLP-2 treatment delayed the onset of NEC but was unable to prevent a high NEC incidence (~70%) and severity that occurred in both groups. GLP-2–treated pigs had less histological injury and increased proximal intestinal weight and mucosal villus height, but not crypt depth or Ki-67–positive cells. Inflammatory markers of intestinal myeloperoxidase were unchanged and serum amyloid A levels were higher in GLP-2–treated pigs.
Conclusions
GLP-2 did not prevent NEC and a proinflammatory response despite some reduction in mucosal injury and increased trophic effect.
Keywords: inflammation, neonate, serum amyloid A
As improvements in neonatal care have allowed for the survival of more preterm infants, the incidence of necrotizing enterocolitis (NEC) has risen (1). Preterm infants enter the world with immature organ systems, including the gastrointestinal tract (GIT). The GIT is not fully matured until the end of the third trimester, so prematurity represents a significant risk factor for the development of NEC (1). NEC is a complex disease whose etiology is not completely understood; however, it is thought to occur as a result of a combination of factors dominated by an immature innate immunity, bacterial colonization, maldigestion of enteral feeds, as well as genetic predisposition, dysmotility, and poor barrier function (2). The incidence of NEC is inversely proportional to birth weight, with approximately 12% of very-low-birth-weight infants (500–750 g) developing NEC (1); however, despite advances in prevention and treatment, significant proportions of neonates who develop NEC do not survive or require surgery to remove large portions of the bowel.
Recently, many studies have begun to identify biomarkers associated with disease progression. One such study found several plasma proteins elevated in human infants with NEC compared with non-NEC control infants, including transthyretin and serum amyloid A (SAA). The identification and validation of biomarkers may assist physicians in determining a definitive NEC diagnosis, which is notoriously difficult to predict and diagnose (2). Furthermore, identification of markers of NEC severity in animal models provides justification to test these markers in human patients.
A common standard of clinical care used to circumvent enteral feeding intolerance and prevent NEC is to nourish preterm neonates with total parental nutrition (TPN) before the introduction of enteral feeds. TPN allows for normal weight gain and somatic growth, and a recent report from a multicenter study of NICU admissions indicated that 69% of neonates received parenteral nutrition for up to 14 days (3–5). Our studies in neonatal pigs also have shown that administration of TPN induces mucosal atrophy, reduces barrier function, and reduces digestive and absorptive capacity (6–9). These data suggest that although TPN administration provides critical nutrition, it may also reduce the functional capability of the intestine, which may increase the risk of developing NEC during the initiation of enteral feeds. More important, piglet studies have also shown that administration of intravenous glucagon-like peptide-2 (GLP-2) prevented the mucosal atrophy and restored digestive function associated with TPN administration (6,10,11).
GLP-2 is a member of the glucagon superfamily and is produced by the enzymatic cleavage of proglucagon in entero-endocrine L cells and some neurons. The hormone has multiple actions in the intestine, including fortifying the intestinal barrier (12,13), reducing inflammation, and augmenting intestinal growth (14,15). There is a wealth of published literature on the intestinotrophic effects of GLP-2 (16). Given the beneficial effects of GLP-2, it has been studied in multiple GI diseases, including inflammatory bowel disease (IBD) and short bowel syndrome (SBS). In patients with IBD, circulating GLP-2 has been shown to be increased in patients with active disease (17), whereas animal models have found that either providing exogenous GLP-2 (18) or blocking degradation (19) reduces clinical and histological manifestations in experimental colitis. Vasoactive intestinal peptide (VIP), a secreted factor involved in intestinal motility, has been identified as the signal mediator for the anti-inflammatory actions of GLP-2 in rodent colitis models (20,21). GLP-2 has been most extensively tested in animal models and human patients with SBS in which it has shown promise to enhance intestinal adaptation and improve absorptive function (22–29). A GLP-2 analogue (teduglutide) is in clinical studies for the treatment of SBS, with early results finding teduglutide was well tolerated, reduced the amount of parenteral nutrition required in adults, and improved bowel morphology (25).
The ability of GLP-2 to suppress mucosal inflammation and increase intestinal growth makes it an attractive therapy for the prevention of NEC. Because preterm infants are provided with TPN to support growth, TPN also causes mucosal atrophy and may lead to an increased risk of developing NEC. Thus, we hypothesized that providing GLP-2 during TPN and before enteral feeds may be protective against NEC. The aim of this study was to test whether administration of GLP-2 in a preterm pig NEC-like model would lead to a reduction in both clinical and molecular NEC parameters through increased mucosal growth and reduction in inflammation. Our results show that although GLP-2 was able to delay the onset of NEC in our piglet model, it was unable to completely prevent the occurrence of NEC and had minimal effects on inflammation; however, GLP-2 administration did have protective effects on histological damage and increased morphometric intestinal endpoints.
METHODS
Study Protocol
All of the procedures were approved by the animal care and use committee of the Baylor College of Medicine. Pregnant Yorkshire pigs at 103 days’ gestation (term = 115 days) underwent cesarean section without induction of parturition, as previously described (30). Within 8 hours of birth, piglets had an umbilical artery catheter and orogastric feeding tube inserted. The piglets were housed individually in warmed cages and were immediately randomized to receive to either Control (CON, 0.45% saline, 0.1% human serum albumin) or GLP-2 (100 μg · kg−1 · day−1 human GLP-2 mixed in 0.45% saline, 0.1% human serum albumin) (California Peptide Inc, Napa, CA) (n = 12 per treatment group) via continuous infusion. For the first 2 days of life, all piglets received a continuous infusion of TPN at 5 mL · kg−1 · h−1. On day 3, TPN was discontinued and animals received enteral feeds via the orogastric catheter using Pepdite Junior formula (Nutricia, Gaithersburg, MD) every 3 hours at 15 to 25 mL/kg body weight. Animals were monitored continuously for signs of clinical NEC (lethargy, abdominal distention, bloody diarrhea, and vomiting) and were euthanized once signs were apparent. Forty-eight hours after the start of enteral feeds, all of the surviving animals were euthanized.
NEC Evaluation and Tissue Collection
Upon euthanasia, the GIT was then completely excised and graded using a previously described index (31) that gives a score to the stomach, small intestine, and colon based on macroscopic damage. Animals with a score >1 in any segment were considered positive for NEC. These individual segment scores were then totaled for the clinical score (maximum 24). All organs were quickly weighed; measurements were normalized to final body weight. Portions of proximal jejunum (PJ) and distal ileum (DI) were collected in formalin for histology, whereas adjacent regions in PJ and DI along with colon were flushed and snap frozen in liquid nitrogen. Arterial blood samples were collected, and plasma was isolated and snap frozen for further analysis.
Histological Sections
Formalin-fixed sections of PJ and DI were paraffin embedded, cut into 4-μm-thick sections and stained for Ki-67 as described previously (32). Positively staining nuclei were counted and normalized to total number of nuclei in 20 fields per animal. Additional paraffin-embedded sections (4 μm) were stained with hematoxylin and eosin (H&E). H&E sections were quantified for morphometric measures (crypt depth, villous height, villous area, and muscle thickness) using Scion Image software. H&E sections were also scored using a histological index developed for rat models of NEC (33,34). This index gives sections of scores of 0 to 4 based on the extent of tissue damage, necrosis, villus blunting, and pneumatosis. Positive histological NEC incidence was calculated as any segment with a score >1.
GLP-2 and VIP Assays
Plasma GLP-2 concentration was measured using a human GLP-2 antibody that recognizes both full-length human and porcine GLP-2 peptide as previously described (35). Plasma VIP concentrations were measured by radioimmunoassay as described previously after extraction of samples with 70% ethanol (36).
Myeloperoxidase Activity Assay and SAA Measurement
Myeloperoxidase (MPO) activity was measured using the method in Suzuki et al (37) with modifications. Briefly, full-thickness samples were homogenized in phosphate-buffered solution and centrifuged at 20,000g. The pellet fraction was then subject to an additional homogenization and centrifugation at 20,000g in a buffer containing hexadecyltrimethylammonium bromide (Sigma-Aldrich, St Louis, MO) to disrupt cell membranes (38). Supernatants were then assayed using a 96-well microplate reader (Molecular Devices, Sunnyvale, CA) for the colorimetric activity of tetramethylbenzidine (Sigma-Aldrich). Activity was calculated based on the standard curve of human macrophage–derived MPO (Sigma-Aldrich) standards at activities of 5 to 100 mU/mL. SAA was measured using a commercially available multispecies enzyme-linked immunosorbent assay kit (Tridelta Development Ltd, Maynooth, Ireland) according to the manufacturer’s instructions.
Statistical Analysis
All data are expressed as mean ± standard error of the mean. All of the comparisons were performed using a Student t test, with the exception of the incidence measures, which were calculated using the Fisher exact test. All of the analyses were completed using GraphPad Prism version 5 software (La Jolla, CA). Statistical significance was defined as P <0.05.
RESULTS
GLP-2 Delays but Does Not Prevent Mortality
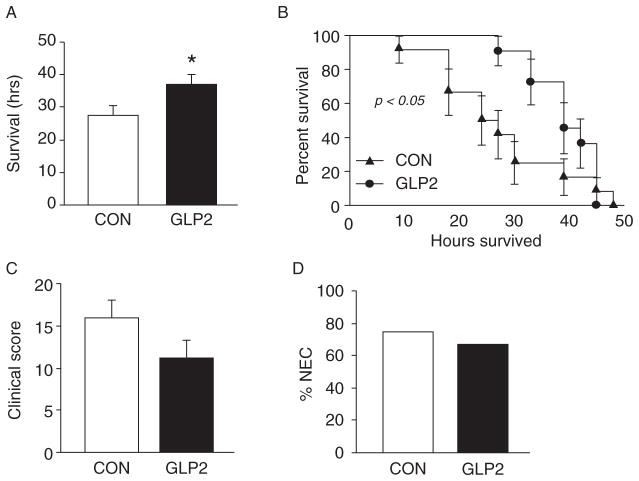
We began to see manifestations of clinical NEC approximately 10 hours after the first enteral feed in the CON group. GLP-2-infused piglets did not begin to develop NEC until approximately 25 hours postenteral feed induction. Figure 1A shows the mean survival time for both CON and GLP-2. The GLP-2 piglets had a significantly longer survival time compared with CON; however, overall survival was not different between the groups (Fig. 1B). Although GLP-2 piglets tended to have a lower clinical NEC score, this difference was not significant compared with CON (P = 0.13, Fig. 1C). CON piglets had a clinical NEC incidence of 75% (Fig. 2B), whereas GLP-2 was not significantly different at 67%.
FIGURE 1.
Glucagon-like peptide-2 (GLP-2) delays the onset but does not prevent necrotizing enterocolitis (NEC). Piglets delivered 12 days preterm were randomized to control (CON) or GLP-2 treatment and given TPN for 2 days. On day 3, TPN was withdrawn and enteral feeds were initiated. Animals were then monitored for clinical signs of NEC. A, GLP-2–infused animals had a significantly longer average survival time after the initiation of enteral feeds compared with CON. B, Kaplan-Meier analysis of both groups showed that although GLP-2 delayed the onset of NEC, mortality was not different between the groups. C, Clinical score, consisting of the presence or absence of abdominal distention, diarrhea, and vomiting, as well as the macroscopic appearance of damage in all segments of the gut. The GLP-2–infused animals tended to have a lower clinical score compared with control piglets. D, The overall incidence of NEC was not different between CON and GLP-2.
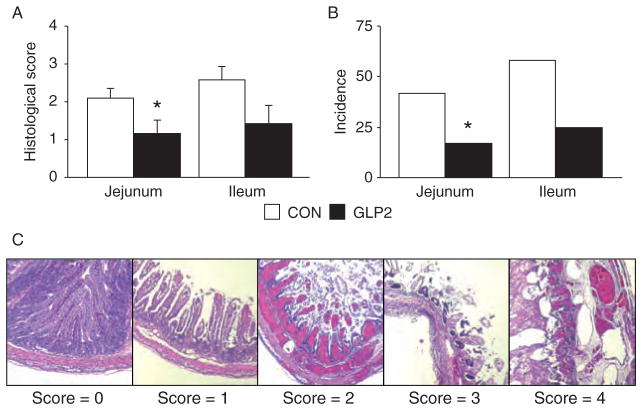
FIGURE 2.
Glucagon-like peptide-2 (GLP-2) reduced histological damage in proximal jejunum (PJ) and distal ileum (DI). Piglets delivered 12 days preterm were randomized to control or GLP-2 treatment and given total parenteral nutrition (TPN) for 2 days. On day 3, TPN was withdrawn and enteral feeds were initiated for 96 hours. Upon euthanasia, intestine was collected for histology. A, Histological necrotizing enterocolitis score was reduced in the jejunum and ileum of GLP-2 piglets compared with control. B, The incidence of histological necrotizing enterocolitis was reduced in both proximal jejunum and distal ileum segments in GLP-2–infused piglets. C, Representative images of the histological grading index.
Histological Damage Is Reduced in GLP-2 Piglets
In applying a rat NEC histological index, we found a significant reduction in histological damage in the PJ of GLP-2 piglets compared with CON (Fig. 2A). Scores in the DI tended to be lower in GLP-2 than CON but were not significant. When incidence was calculated, GLP-2 piglets had a significantly lower histological NEC incidence compared with CON in the PJ, with DI tending to be lower (Fig. 2B). Figure 3C shows representative sections for the rat NEC scoring system in the pig. We found excellent correlation (R2 = 0.62, P <0.0001) between the independent histological and clinical indices used to determine NEC. Using Ki-67 as a marker of proliferating cells, we found no significant difference in proliferation in either the PJ (Fig. 3) or DI (data not shown).
FIGURE 3.
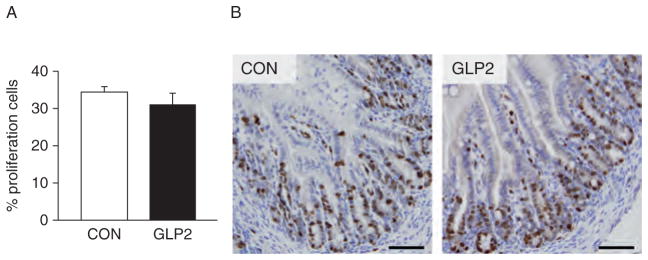
Crypt cell proliferation was not affected by glucagon-like peptide-2 (GLP-2) infusion. Piglets delivered 12 days preterm were randomized to control (CON) or GLP-2 treatment and given total parenteral nutrition (TPN) for 2 days. On day 3, TPN was withdrawn and enteral feeds were initiated for 96 hours. Upon euthanasia, intestine was collected and stained for Ki-67. No differences were seen between CON and GLP-2–infused piglets. Scale bar = 250 μm/L.
Serum Amyloid A Is Increased in GLP-2 Piglets
In order to assess the inflammatory response in our NEC model, we measured tissue MPO and plasma SAA. SAA concentrations in plasma were significantly increased in GLP-2 piglets compared with CON (Fig. 4A). MPO was measured in the PJ, DI, and colon. Although there was a trend for MPO to be lower in the GLP-2 piglets in the PJ and DI, this difference was not significant and there was no difference in colonic MPO (Fig. 4B).
FIGURE 4.
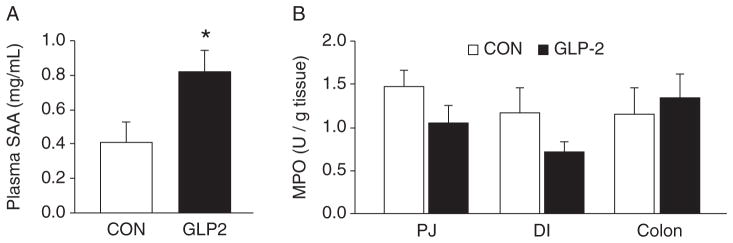
Serum amyloid A (SAA) was increased in glucagon-like peptide-2 (GLP-2) piglets. Piglets delivered 12 days preterm were randomized to control (CON) or GLP-2 treatment and given total parenteral nutrition (TPN) for 2 days. On day 3, TPN was withdrawn and enteral feeds were initiated for 96 hours. Upon euthanasia, arterial plasma and intestine were collected for analysis. A, SAA was significantly increased in GLP-2 piglets compared with control, whereas (B) tissue myeloperoxidase (MPO) was low and not different between the groups in any intestinal segment measured. DI = distal ileum; PJ = proximal jejunum.
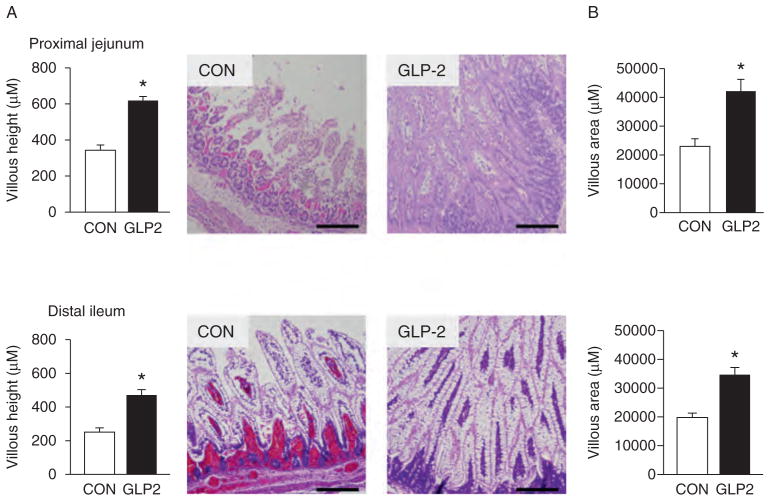
GLP-2 Significantly Increases Villous Height and Area
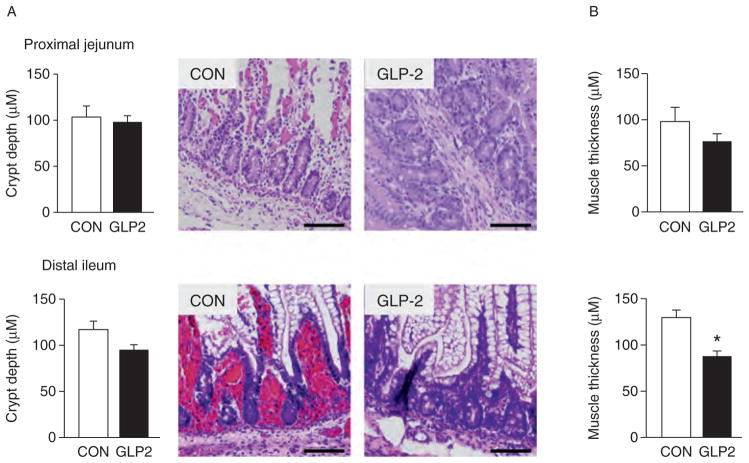
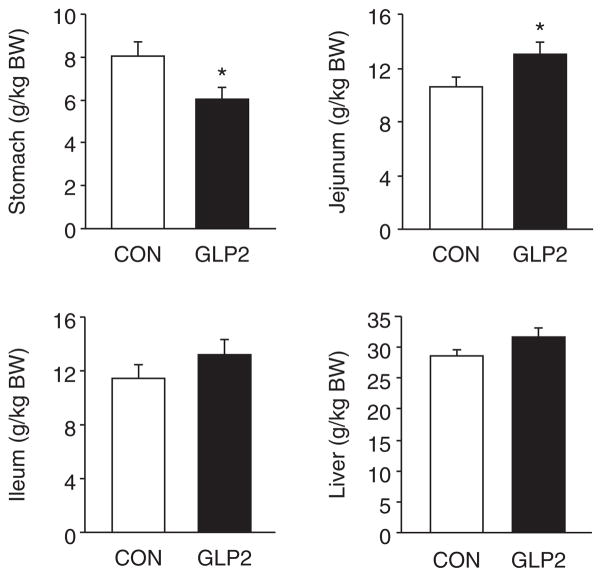
As expected, the GLP-2-infused piglets experienced a significant increase in circulating GLP-2 compared with CON (Fig. 5A). For VIP (Fig. 5B), there was no difference between CON and GLP-2. Using H&E-stained sections of both PJ and DI, we measured morphological changes in villous height, villous area, crypt depth, and muscle thickness. Figure 6 shows the changes in villous height and villous area for PJ and DI and representative images for each small intestine segment. Both villous height and villous area are significantly increased in GLP-2 piglet compared with CON. In Figure 7, there is no change in crypt depth or muscle thickness in the PJ, whereas GLP-2 piglets had a significantly reduced muscle thickness in the DI. We also found significant decrease in stomach weight in GLP-2 piglets compared with CON, whereas the PJ was significantly larger in the GLP-2 (Fig. 8). There was no difference in DI or liver between the groups.
FIGURE 5.
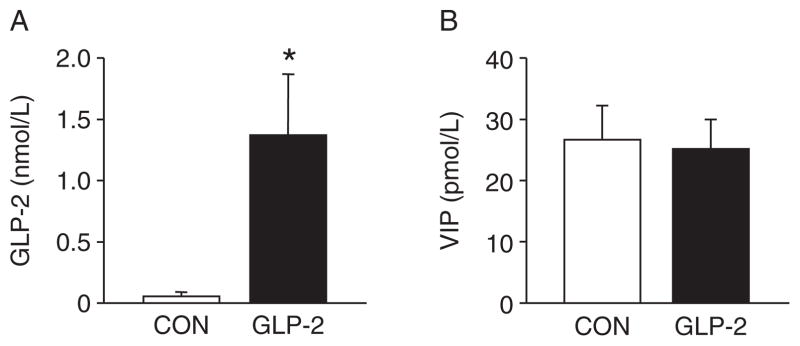
Glucagon-like peptide-2 (GLP-2) infusion significantly increased circulating GLP-2 concentrations. Piglets delivered 12 days preterm were randomized to control (CON) or GLP-2 treatment and given total parenteral nutrition (TPN) for 2 days. On day 3, TPN was withdrawn and enteral feeds were initiated for 96 hours. Upon euthanasia, arterial plasma was collected for analysis. A, As expected, continuous infusion of GLP-2 increased the circulating GLP-2 concentration compared with controls. B, Vasoactive intestinal peptide (VIP) was not different between the groups.
FIGURE 6.
Glucagon-like peptide-2 (GLP-2) increased villous height (A) and area (B). Piglets delivered 12 days preterm were randomized to control or GLP-2 treatment and given total parenteral nutrition (TPN) for 2 days. On day 3, total parenteral nutrition (TPN) was withdrawn and enteral feeds were initiated for 96 hours. Upon euthanasia, intestine was collected for histology. GLP-2 stimulated increased villous height and area in both the proximal jejunum and distal ileum. Scale bar = 200 μm/L.
FIGURE 7.
Crypt depth was not different between control (CON) and glucagon-like peptide-2 (GLP-2). Piglets delivered 12 days preterm were randomized to CON or GLP-2 treatment and given total parenteral nutrition (TPN) for 2 days. On day 3, TPN was withdrawn and enteral feeds were initiated for 96 hours. Upon euthanasia, intestine was collected for histology. Crypt depth (A) was not different in either proximal jejunum or distal ileum, whereas distal ileum muscle thickness (B) was significantly reduced in GLP-2 piglets compared with CON. Scale bar = 200 μm/L.
FIGURE 8.
Glucagon-like peptide-2 (GLP-2) increased proximal jejunum total weight. Piglets delivered 12 days preterm were randomized to control (CON) or GLP-2 treatment and given TPN for 2 days. On day 3, total parenteral nutrition was withdrawn and enteral feeds were initiated for 96 hours. GLP-2 significantly increased proximal jejunum weight, whereas stomach weight was significantly reduced compared with CON.
DISCUSSION
The aim of this study was to determine whether GLP-2 administration to preterm piglets would prevent the onset or severity of NEC. Given the substantial evidence showing that GLP-2 induces anti-inflammatory and intestinotrophic effects, we expected the GLP-2–treated piglets to have fewer clinical and molecular manifestations of an NEC-like disease. Although the GLP-2–treated piglets had a significantly longer mean survival time and tended to have a lower clinical score, neither the overall survival nor the incidence of NEC was different between the groups.
In addition to the clinical assessment of NEC, we also examined the extent of histological evidence of mucosal tissue injury using an index described for a rat model of NEC (33,34); we found good correlation between the histological index and the clinical score. In contrast to the clinical score, however, we observed a significant reduction in histological damage in the PJ and DI of GLP-2–treated compared with CON pigs. This reduction in score translated to a reduction in histological NEC incidence in the PJ. Thus, we further examined whether the histological evidence of injury was associated with tissue inflammation, based on measures of MPO and SAA. MPO is an enzyme found predominately in the granules of neutrophils and is a marker of neutrophil infiltration (37). MPO is routinely used in other GI injury/illness models as an inflammatory marker. We found that MPO activity tended to be lower in GLP-2 versus CON in the PJ and DI but not colon, yet these were not statistically significant. Interestingly, the amount of MPO found in either treatment group was extremely low (>2 U/g tissue). Other groups have examined MPO in rat models of NEC and have reported a wide range of MPO activity (3–168 U/g tissue) (39,40). Visual examination of histological sections for the presence of neutrophils suggests that extremely few are present in the remnant piglet intestine after the onset of NEC. Given this apparent lack of neutrophils and the wide variation of reported MPO values, the use of MPO as a marker of inflammation in NEC may not be appropriate. Thus, serum amyloid A (SAA) has recently been shown as a biomarker in human NEC (41). SAA is an acute phase protein whose production is stimulated by proinflammatory cytokines (42). SAA was significantly increased in GLP-2 piglets compared with CON, indicating that GLP-2 was unable to dampen the inflammatory response in these animals. Of note, the level SAA was elevated in both groups compared with a baseline sample group (non-NEC, 107.01 ± 66.22). These data suggest that SAA is an appropriate biomarker in our model. We further examined the circulating concentration of VIP because our previous studies showed the GLP-2 receptor to be colocalized in VIP-positive enteric neurons. Moreover, studies have linked VIP as a potential signal that mediates the anti-inflammatory effects of GLP-2 in rodent models of colitis (20,21); however, we found no differences in the circulating VIP levels between the groups.
Given the limited preventive effect of GLP-2 on NEC and tissue inflammation, we sought to confirm the intestinotrophic effects that we had reported in several previous studies (6,10,11,43). The PJ wet weight normalized to body weight was significantly greater in the GLP-2–treated compared with CON piglets, similar to our previous reports (10). Likewise, morphometric measures of H&E-stained sections indicated an increase in villous height and total villous area in both PJ and DI of GLP-2 piglets, whereas crypt depth was not different. Previously published work shows that a similar dose of GLP-2 given to TPN-fed piglets increases proliferation compared with TPN alone (10). Additionally, studies of enterally fed piglets compared with TPN or TPN+GLP-2 show increases in proliferation in the enterally fed group, with no difference between TPN or TPN+GLP-2 (6). We expected that enteral feeding would stimulate proliferation in both groups with a further increase in proliferation in the GLP-2–infused animals. Staining with Ki-67 showed no differences between CON and GLP-2 in either the PJ or DI. One possible explanation is that the rate of proliferation was maximally stimulated by enteral feeding and limited any effect of GLP-2. Taken together, the results suggest that the preterm pig was responsive to the intestinotrophic effects GLP-2 consistent with previous evidence of GLP-2 receptor expression (44).
One caveat to the present study was the relatively high NEC incidence (~70%) compared with those in previous reports (~50%–60%), which may be caused by the slightly younger gestational age of piglet used (12 vs 10 days preterm). We postulate that this high NEC incidence reflected a rather severe condition of inflammation and tissue injury in both groups and thus may have limited any protective effect of GLP-2 on NEC incidence. This coupled with the relatively short (2 days) GLP-2 treatment period before the onset of enteral formula feeding may have limited its effectiveness.
The choice of dose for GLP-2 was based on several previous studies. We previously administered a dose of 50 mg/kg via IP injection every 6 hours. We found no effect of GLP-2 on the incidence of NEC (31). Interestingly, we also saw no intestinotrophic effects of GLP-2 at this dose. We used a higher dose of GLP-2 that had been previously shown to increase cell survival and proliferation in TPN-fed piglets (10); however, in the context of an inflammatory response because of NEC, it is possible that the dose chosen was too high. Indeed, human studies have suggested that a higher dose of GLP-2 is not always the best. For example, a dose of 0.1 mg/kg of teduglutide given to patients who are dependent on parenteral nutrition was not different from placebo in the context of reducing parenteral nutrition volume; however, a dose of 0.05 mg/kg was effective at reducing dependence on parenteral nutrition (25). Given the differential response of the 2 doses of GLP-2 suggests that a series of studies examining different combinations of GLP-2 dose and perhaps length of administration will determine the optimal conditions for this study. Indeed, our providing GLP-2 to animals for a longer duration before the induction of NEC support this theory.
An additional confounding factor is the subjectivity with which the clinical scoring system was used and the time of euthanasia. Although the observers were not blinded to the identification of study animals, every effort was made to ensure that all of the animals were considered without bias toward their treatment group. The large difference in mean survival time suggests that animals were cared for in an unbiased way, because the time between presenting symptoms and euthanasia was usually no more than a few hours (Fig. 1). In an effort to reduce subjectivity in the clinical scoring system, we adhered to the grading assignments described in Sangild et al (31). These grading assignments offered concrete markers (hemorrhagic mucosa/transmural necrosis) that minimized bias toward either group. The histological NEC scoring was performed by a person blinded to the treatment groups, however.
In summary, continuously infused GLP-2 given to preterm-derived piglets before and after the initiation of enteral feeds was able to delay but not prevent the onset of clinical NEC. We also showed that GLP-2 treatment provided some limited protection based on histological evidence of tissue injury, but that this was not associated with significant changes in levels of proinflammatory markers. Additional studies are necessary to determine whether longer-term pretreatment with GLP-2 can prevent NEC in neonatal animal models.
Acknowledgments
This work was supported by federal funds from the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001, and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338).
The authors acknowledge Liwei Cui, Dr Amy Hair, Dr Milton Finegold, and the staff of the Texas Medical Center Digestive Disease Center Cellular and Molecular Morphology Core for technical assistance.
Footnotes
The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The authors report no conflicts of interest.
References
- 1.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 2.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Christensen RD, Henry E, Wiedmeier SE, et al. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol. 2007;27:284–90. doi: 10.1038/sj.jp.7211686. [DOI] [PubMed] [Google Scholar]
- 4.Christensen RD, Henry E, Kiehn TI, et al. Pattern of daily weights among low birth weight neonates in the neonatal intensive care unit: data from a multihospital health-care system. J Perinatol. 2006;26:37–43. doi: 10.1038/sj.jp.7211431. [DOI] [PubMed] [Google Scholar]
- 5.Thureen PJ, Hay WW., Jr Early aggressive nutrition in preterm infants. Semin Neonatol. 2001;6:403–15. doi: 10.1053/siny.2001.0061. [DOI] [PubMed] [Google Scholar]
- 6.Burrin DG, Stoll B, Jiang R, et al. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1249–56. doi: 10.1152/ajpgi.2000.279.6.G1249. [DOI] [PubMed] [Google Scholar]
- 7.Stoll B, Chang X, Fan MZ, et al. Enteral nutrient intake level determines intestinal protein synthesis and accretion rates in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2000;279:G288–94. doi: 10.1152/ajpgi.2000.279.2.G288. [DOI] [PubMed] [Google Scholar]
- 8.Shulman RJ. Effect of different total parenteral nutrition fuel mixes on small intestinal growth and differentiation in the infant miniature pig. Gastroenterology. 1988;95:85–92. doi: 10.1016/0016-5085(88)90294-6. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein RM, Hebiguchi T, Luk GD, et al. The effects of total parenteral nutrition on gastrointestinal growth and development. J Pediatr Surg. 1985;20:785–91. doi: 10.1016/s0022-3468(85)80044-0. [DOI] [PubMed] [Google Scholar]
- 10.Burrin DG, Stoll B, Guan X, et al. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology. 2005;146:22–32. doi: 10.1210/en.2004-1119. [DOI] [PubMed] [Google Scholar]
- 11.Cottrell JJ, Stoll B, Buddington RK, et al. Glucagon-like peptide-2 protects against TPN-induced intestinal hexose malabsorption in enterally refed piglets. Am J Physiol Gastrointest Liver Physiol. 2006;290:G293–300. doi: 10.1152/ajpgi.00275.2005. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin MA, McKay DM, Yang PC, et al. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–9. doi: 10.1136/gut.47.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrin DG, Petersen Y, Stoll B, et al. Glucagon-like peptide 2: a nutrient-responsive gut growth factor. J Nutr. 2001;131:709–12. doi: 10.1093/jn/131.3.709. [DOI] [PubMed] [Google Scholar]
- 15.Drucker DJ. Glucagon-like peptide 2. Trends Endocrinol Metab. 1999;10:153–6. doi: 10.1016/s1043-2760(98)00136-2. [DOI] [PubMed] [Google Scholar]
- 16.Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu Rev Nutr. 2006;26:391–411. doi: 10.1146/annurev.nutr.26.061505.111223. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Q, Boushey RP, Cino M, et al. Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1057–63. doi: 10.1152/ajpregu.2000.278.4.R1057. [DOI] [PubMed] [Google Scholar]
- 18.Alavi K, Schwartz MZ, Palazzo JP, et al. Treatment of inflammatory bowel disease in a rodent model with the intestinal growth factor glucagon-like peptide-2. J Pediatr Surg. 2000;35:847–51. doi: 10.1053/jpsu.2000.6861. [DOI] [PubMed] [Google Scholar]
- 19.Yazbeck R, Sulda ML, Howarth GS, et al. Dipeptidyl peptidase expression during experimental colitis in mice. Inflamm Bowel Dis. 2010;16:1340–51. doi: 10.1002/ibd.21241. [DOI] [PubMed] [Google Scholar]
- 20.Sigalet DL, Wallace L, De Heuval E, et al. The effects of glucagon-like peptide 2 on enteric neurons in intestinal inflammation. Neurogastroenterol Motil. 2010;22:1318–50. doi: 10.1111/j.1365-2982.2010.01585.x. [DOI] [PubMed] [Google Scholar]
- 21.Flock G, Holland D, Seino Y, et al. GPR119 regulates murine glucose homeostasis through incretin receptor-dependent and independent mechanisms. Endocrinology. 2011;152:374–83. doi: 10.1210/en.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–15. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 23.Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224–31. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeppesen PB, Lund P, Gottschalck IB, et al. Short bowel patients treated for two years with glucagon-like peptide 2: effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol Res Pract. 2009;2009:616054. doi: 10.1155/2009/616054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeppesen PB, Gilroy R, Pertkiewicz M, et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–14. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitchen PA, Goodlad RA, FitzGerald AJ, et al. Intestinal growth in parenterally-fed rats induced by the combined effects of glucagon-like peptide 2 and epidermal growth factor. JPEN J Parenter Enteral Nutr. 2005;29:248–54. doi: 10.1177/0148607105029004248. [DOI] [PubMed] [Google Scholar]
- 27.Mouksassi MS, Marier JF, Cyran J, et al. Clinical trial simulations in pediatric patients using realistic covariates: application to teduglutide, a glucagon-like peptide-2 analog in neonates and infants with short-bowel syndrome. Clin Pharmacol Ther. 2009;86:667–71. doi: 10.1038/clpt.2009.199. [DOI] [PubMed] [Google Scholar]
- 28.Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery. 2011;150:217–23. doi: 10.1016/j.surg.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigalet DL, Bawazir O, Martin GR, et al. Glucagon-like peptide-2 induces a specific pattern of adaptation in remnant jejunum. Dig Dis Sci. 2006;51:1557–66. doi: 10.1007/s10620-006-9077-5. [DOI] [PubMed] [Google Scholar]
- 30.Sangild PT, Petersen YM, Schmidt M, et al. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J Nutr. 2002;132:3786–94. doi: 10.1093/jn/132.9.2673. [DOI] [PubMed] [Google Scholar]
- 31.Sangild PT, Siggers RH, Schmidt M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. 2006;130:1776–92. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Bauchart-Thevret C, Stoll B, Chacko S, et al. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab. 2009;296:E1239–50. doi: 10.1152/ajpendo.91021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Radulescu A, Zorko N, et al. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221–30. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan MS, Hedlund E, Adler L, et al. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–28. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 35.Burrin DG, Stoll B, Jiang R, et al. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–10. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 36.Fahrenkrug J, Schaffalitzky de Muckadell OV. Radioimmunoassay of vasoactive intestinal polypeptide (VIP) in plasma. J Lab Clin Med. 1977;89:1379–88. [PubMed] [Google Scholar]
- 37.Suzuki K, Ota H, Sasagawa S, et al. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–52. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 38.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 39.Tayman C, Tonbul A, Kosus A, et al. Protective effects of caffeic acid phenethyl ester (CAPE) on intestinal damage in necrotizing enterocolitis. Pediatr Surg Int. 2011;27:1179–89. doi: 10.1007/s00383-011-2942-0. [DOI] [PubMed] [Google Scholar]
- 40.Musemeche CA, Baker JL, Feddersen RM. A model of intestinal ischemia in the neonatal rat utilizing superior mesenteric artery occlusion and intraluminal platelet-activating factor. J Surg Res. 1995;58:724–7. doi: 10.1006/jsre.1995.1114. [DOI] [PubMed] [Google Scholar]
- 41.Ng PC, Ang IL, Chiu RW, et al. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. J Clin Invest. 2010;120:2989–3000. doi: 10.1172/JCI40196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maury CP. Comparative study of serum amyloid A protein and C-reactive protein in disease. Clin Sci (Lond) 1985;68:233–8. doi: 10.1042/cs0680233. [DOI] [PubMed] [Google Scholar]
- 43.Taylor-Edwards CC, Burrin DG, Holst JJ, et al. Glucagon-like peptide-2 (GLP-2) increases small intestinal blood flow and mucosal growth in ruminating calves. J Dairy Sci. 2011;94:888–98. doi: 10.3168/jds.2010-3540. [DOI] [PubMed] [Google Scholar]
- 44.Petersen YM, Burrin DG, Sangild PT. GLP-2 has differential effects on small intestine growth and function in fetal and neonatal pigs. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1986–93. doi: 10.1152/ajpregu.2001.281.6.R1986. [DOI] [PubMed] [Google Scholar]