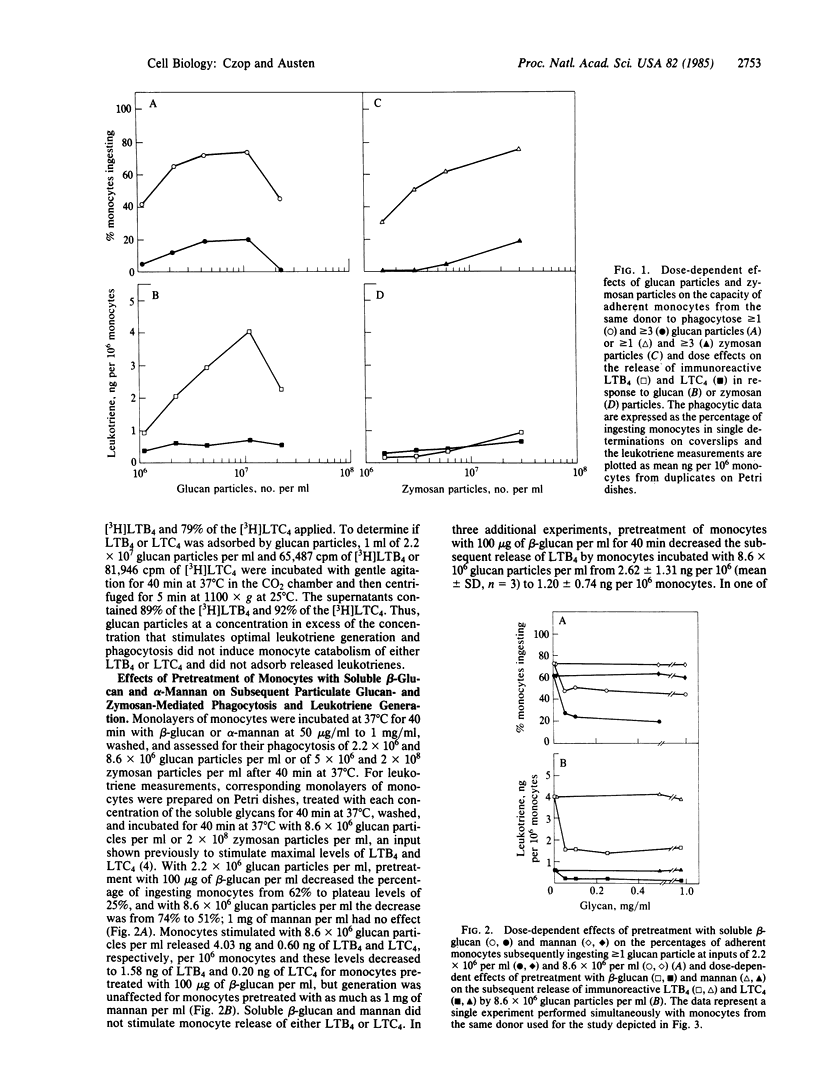
Abstract
Human monocytes possess a receptor for ingestion of particulate activators of the human alternative complement pathway that functions in the absence of plasma proteins and is distinct from the receptors for Fc-IgG and the major cleavage fragment of the third component of complement (C3b). Incubation of monolayers of monocytes with 1.1 X 10(6) to 2.2 X 10(7) glucan particles per ml initiated a phagocytic response comparable to that obtained with zymosan particles, of which beta-glucan is a constituent along with mannan. Maximal quantities of 4.93 +/- 3.43 ng of leukotriene B4 (LTB4) and 0.43 +/- 0.23 ng of leukotriene C4 (LTC4) (mean +/- SD, n = 3) were released by 10(6) monocytes stimulated with 1.1 X 10(7) glucan particles per ml. Preincubation of monocytes with 50 micrograms of soluble beta-glucan per ml reduced subsequent monocyte ingestion of 5 X 10(6) zymosan particles per ml and 2.2 X 10(6) glucan particles per ml by 52% and 55%, respectively, and diminished release of LTB4 by monocytes stimulated with 2 X 10(8) zymosan particles per ml and 8.6 X 10(6) glucan particles per ml by 73% and 61%, respectively. Preincubation with 1 mg of soluble mannan per ml had little effect on monocyte phagocytosis or LTB4 generation in response to either zymosan or glucan particles, and neither soluble beta-glucan nor mannan stimulated generation of LTB4 or LTC4. The effect of pretreatment of monocytes with soluble beta-glucan was time dependent, with the maximal effect being evident within 20 min of pretreatment, and was specific for zymosan or glucan particles in that the LTB4 and LTC4 release induced by 2.5 microM calcium ionophore A23187 was unaffected. That both phagocytosis and leukotriene generation are inhibited by soluble beta-glucan but not by mannan at a rate compatible with the phagocytic process of monocyte monolayers indicates ligand specificity for a beta-glucan receptor. As the beta-glucan receptor recognizes particulate activators of the alternative complement pathway, the nonimmune response to a single stimulus induces complement activation, phagocytosis, and leukotriene generation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. S., Farmer V. C., Jones D., Taylor I. F. The glucan components of the cell wall of baker's yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem J. 1969 Sep;114(3):557–567. doi: 10.1042/bj1140557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Augmentation of phagocytosis by a specific fibronectin fragment that links particulate activators to the fibronectin adherence receptor of human monocytes. J Immunol. 1982 Dec;129(6):2678–2681. [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Functional discrimination by human monocytes between their C3b receptors and their recognition units for particulate activators of the alternative complement pathway. J Immunol. 1980 Jul;125(1):124–128. [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Membrane sialic acid on target particles modulates their phagocytosis by a trypsin-sensitive mechanism on human monocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3831–3835. doi: 10.1073/pnas.75.8.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Opsonin-independent phagocytosis of activators of the alternative complement pathway by human monocytes. J Immunol. 1978 Apr;120(4):1132–1138. [PubMed] [Google Scholar]
- Czop J. K., Kadish J. L., Austen K. F. Purification and characterization of a protein with fibronectin determinants and phagocytosis-enhancing activity. J Immunol. 1982 Jul;129(1):163–167. [PubMed] [Google Scholar]
- Dahlén S. E., Björk J., Hedqvist P., Arfors K. E., Hammarström S., Lindgren J. A., Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. The human PMN leukocyte chemotactic activity of complex hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Oct;125(4):1789–1791. [PubMed] [Google Scholar]
- Hamuro J., Hadding U., Bitter-Suermann D. Solid phase activation of alternative pathway of complement by beta-1,3-glucans and its possible role for tumour regressing activity. Immunology. 1978 Apr;34(4):695–705. [PMC free article] [PubMed] [Google Scholar]
- Johnson E., Eskeland T., Bertheussen K. Phagocytosis by human monocytes of particles activating the alternative pathway of complement. Scand J Immunol. 1984 Jan;19(1):31–39. doi: 10.1111/j.1365-3083.1984.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Levine L., Morgan R. A., Lewis R. A., Austen K. F., Clark D. A., Marfat A., Corey E. J. Radioimmunoassay of the leukotrienes of slow reacting substance of anaphylaxis. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7692–7696. doi: 10.1073/pnas.78.12.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Mencia-Huerta J. M., Soberman R. J., Hoover D., Marfat A., Corey E. J., Austen K. F. Radioimmunoassay for leukotriene B4. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7904–7908. doi: 10.1073/pnas.79.24.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., ROSS O. A., TODD E. W., WARDLAW A. C. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Merler E. Increased prostaglandin production by human monocytes after membrane receptor activation. J Immunol. 1979 Jul;123(1):115–120. [PubMed] [Google Scholar]
- Rabinovitch M., De Stefano M. J. Particle recognition by cultivated macrophages. J Immunol. 1973 Mar;110(3):695–701. [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Hamill A. L., Cohn Z. A. Dynamics of leukotriene C production by macrophages. J Exp Med. 1980 Nov 1;152(5):1236–1247. doi: 10.1084/jem.152.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Chen L. C. A single column packing for the gas-liquid chromatographic separation of various biologically important compounds. Prep Biochem. 1976;6(1):27–56. doi: 10.1080/00327487608061598. [DOI] [PubMed] [Google Scholar]
- Seijelid R., Bögwald J., Lundwall A. Glycan stimulation of macrophages in vitro. Exp Cell Res. 1981 Jan;131(1):121–129. doi: 10.1016/0014-4827(81)90413-4. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Campbell E. J., Senior R. M., Stahl P. D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982 Dec;32(6):423–431. [PubMed] [Google Scholar]
- Smith M. J., Ford-Hutchinson A. W., Bray M. A. Leukotriene B: a potential mediator of inflammation. J Pharm Pharmacol. 1980 Jul;32(7):517–518. doi: 10.1111/j.2042-7158.1980.tb12985.x. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Czop J. K., Austen K. F. Release of leukotrienes by human monocytes on stimulation of their phagocytic receptor for particulate activators. J Immunol. 1984 Jun;132(6):3034–3040. [PubMed] [Google Scholar]



