Abstract
Sepsis is commonly caused by community-acquired pneumonia (CAP) and may develop into severe sepsis, characterized by multiple organ failure. The risk of severe sepsis among CAP patients and subsequent mortality increases sharply after the age of 65. The molecular mechanisms associated with this age-related risk are not fully understood. To better understand factors involved with increased incidence and mortality of severe sepsis in the elderly, we used a nested case-control study of patients enrolled in a multicenter observational cohort of 2,320 participants with CAP. We identified a total of 39 CAP patients 50-65 and 70-85 years old who did or did not develop severe sepsis. Plasma samples were obtained on presentation to the emergency department and prior to therapeutic interventions. A semi-quantitative plasma proteomics workflow was applied which incorporated tandem immunoaffinity depletion, iTRAQ labeling, strong cation exchange fractionation, and nanoflow-liquid chromatography coupled to high resolution mass spectrometry. In total, 772 proteins were identified, of which, 58 proteins exhibit statistically significant differences in expression levels amongst patients with severe sepsis as a function of age. Differentially-expressed proteins are involved in pathways such as acute phase response, coagulation signaling, atherosclerosis signaling, lipid metabolism, and production of nitric oxide and reactive oxygen species. This study provides insight into factors that may explain age-related differences in incidence of severe sepsis in the elderly.
Keywords: sepsis, severe sepsis, proteomics, CAP, plasma, aging, immunosenescence, pneumonia
Introduction
Sepsis is a systematic inflammatory state triggered by infection. The additional diagnosis of multiple organ failure and hypoperfusion (e.g., hypotension, decreased urine output) along with sepsis results in severe sepsis1. One of the leading causes of sepsis is community-acquired pneumonia (CAP)2-4. The presence and severity of organ failure is one of the most important determinants of mortality following sepsis5-10. Severe sepsis affects ∼750,000 persons annually in the United States6-11 and is one of the most common causes of death in intensive care units6-10.
Kellum and coworkers have shown that the incidence of severe sepsis and mortality rate increases sharply after the age of 6512, 13. For example, CAP patients ≥85 years old have a 2.8fold increased risk of severe sepsis and a 17-fold increased mortality rate compared to patients ≤50 years old2. The notable age-related differences in severe sepsis risk may be explained in part by immunosenescence underlying chronic disease. Immunosenescence is manifested in the elderly through decreased numbers of T cells, impaired B-cell function, increased apoptosis of neutrophils, and reduced bactericidal response of macrophages13-15. Most studies that examine age-related differences in immune response using animal models and humans have largely focused on either the adaptive immune response or select pathways (inflammatory, coagulation, and fibrinolysis markers) in the innate immune response14-20. A comprehensive assessment of differences in the immune response has not been conducted. Trials testing immunomodulating therapies for sepsis in broad populations have failed to consistently improve outcomes of sepsis patients21. An alternative approach is to personalize sepsis therapies based on host characteristics, such as age. To further the development of such a personalized approach, a better understanding of the differences in immune response due to age is necessary.
Herein, we conducted a nested case-control study using patients enrolled in an observational cohort of CAP. A semi-quantitative plasma proteomics workflow previously developed in our laboratory22, which included tandem immunoaffinity depletion, isobaric tags for relative and absolute quantitation (iTRAQ) labeling, strong cation exchange (SCX) fractionation, and nanoflow liquid chromatography (LC) coupled to high resolution mass spectrometry (MS), was applied to patient samples. We analyzed plasma proteins on presentation to the emergency department to compare the acute immune response. First, proteins were identified that differed between those who did or did not develop severe sepsis (within 90 days of hospitalization) among younger and older adults separately. We hypothesized that there may be differentially-expressed proteins that are unique to the younger or older adults which would help to explain the increased risk of severe sepsis in older adults. In addition to identifying these sets of proteins, differentially-expressed proteins that are common to the younger and older adult groups were also identified. Interestingly, the direction of fold-change observed for these common proteins varies depending on patient age and thus may explain higher risk of severe sepsis in older adults. These results and the implications of this study are presented herein.
Experimental procedures
Ethics statement
The Institutional Review Boards at the following hospitals approved the study: Pennsylvania: Allegheny General Hospital, Jefferson Hospital/SHHS, Mercy Hospital, St. Clair Memorial Hospital, St. Francis Medical Center, Sewickley Valley Hospital, University of Pittsburgh Medical Center (UPMC) Braddock, UPMC Horizon, UPMC Lee, UPMC McKeesport, UPMC Passavant, UPMC Presbyterian, UPMC Shadyside, UPMC Southside, UPMC St. Margaret, West Penn Hospital; Connecticut: Bridgeport Hospital, Hartford Hospital, Milford Hospital, New Britain General Hospital, Norwalk Hospital, Yale-New Haven Hospital; Tennessee: Methodist Health Care (single IRB approval for three Methodist University sites); Michigan: Henry Ford Health System, Detroit Receving/Sinai-Grace, Wayne State. Written, informed consent was obtained from all participants or by proxy.
Study design and patients
We conducted a nested case-control study using patients enrolled in the GenIMS study23. GenIMS is a prospective multicenter observational cohort of patients with CAP enrolled in emergency departments (EDs) of 28 academic and community hospitals in four US regions, including southwestern Pennsylvania, Connecticut, southern Michigan and western Tennessee. Details of this study including eligibility criteria have been described previously2, 23.
To compare differences in immune response across different age groups and between patients with and without severe sepsis, we identified a total of 39 patients from four groups. These included: 1) patients 50-65 years old who did not develop severe (hereafter referred to as young controls [YC]), 2) patients 70-85 years old who did not develop severe sepsis (hereafter referred to as old controls [OC]), 3) patients 50-65 years old who developed severe sepsis within 90 days after presentation to the ED (hereafter referred to as young severe sepsis [YS]), and 4) patients 70-85 years old who developed severe sepsis within 90 days after presentation to the ED (hereafter referred to as old severe sepsis [OS]). To ensure that differences in immune response are attributed to age and not to ethnic differences or underlying chronic diseases, only whites and matched patients according to chronic disease burden were included (Table S1). Approval for the participation of human patients was obtained by the Institutional Review Board of the University of Pittsburgh and other participating sites.
Plasma samples and tandem MARS depletion
Plasma samples were obtained on Day 1 when the CAP patients were admitted to the ED and prior to most interventions to ensure that differences in immune response are not affected by therapeutic strategies. Negative results were obtained in the blood culture test used to identify the pathogenic source of the samples obtained from CAP patients. The Hu 6 MARS column (Agilent; Santa Clara, CA) depletes serum albumin, IgG, α1-antitrypsin, IgA, transferrin, and haptoglobin proteins. An injection amount of 60 μL of crude plasma was applied to the MARS column and after the initial depletion flow-through fractions were concentrated with a 5K molecular weight cutoff concentrator (Agilent) at 4695 g at 4°C for 1.5 hours. Samples were then stored at -80 °C or re-injected onto the MARS column for tandem MARS depletion. The second flow-through fractions (hereafter referred to as TMD) were concentrated and protein concentrations were measured using the BCA protein assay.
Protein digestion
Protein was denatured with an extraction buffer (0.2 M Tris, 8 M urea, 10 mM CaCl2, pH 8.0), reduced with 1:40 molar excess of dithiothreitol (DTT) for 2 h at 37 °C, and then alkylated with 1:80 molar excess of iodoacetamide (IAM) for 2 h on ice. The alkylation reaction was quenched by adding 1:40 molar excess of Cysteine and the mixture was incubated at room temperature for 30 min. Tris buffer (0.2 M Tris, 10 mM CaCl2, pH 8.0) was added to dilute the urea concentration to 2 M. Each sample was incubated with bovine TPCK-heated trypsin at 1:50 substrate: enzyme ratio for 24 h at 37 °C.
iTRAQ labeling
Digested samples were desalted with an HLB cartridge (Waters; Milford, MA) and dried by centrifugal evaporation. Each sample was labeled with an iTRAQ reagent following the manufacturer's protocol (Applied Biosystems; Foster City, CA) with slight modifications. Briefly, each iTRAQ reagent was solubilized with 70 μL ethanol and transferred to peptide mixtures. After 1.5 h of incubation, the reaction was quenched with water. Labeled samples were mixed in 1:1:1:1 ratios for iTRAQ reagents that generate reporter ions at m/z 114:115:116:117, respectively.
SCX fractionation
SCX fractionation was carried out on a PolySulfoethyl A 100 mm × 2.1 mm, 5 μm, 200 Å column (The Nest Group, Inc.; Southborough, MA) with buffers as follows: mobile phase A was 5 mM monopotassium phosphate (25% v/v acetonitrile, pH 3.0), and mobile phase B was 5 mM phosphate, 350 mM potassium chloride, (25% v/v acetonitrile, pH 3.0). Dried iTRAQ labeled samples were resuspended in 300 μL of mobile phase A and injected onto the SCX column. The gradient for SCX was: 0-3 min, 0% B; 3-45 min, 0-75% B; 45-50 min, 75-100% B; 50-55 min, 100% mobile phase B; 55-56 min, 100-0% B; 56-106 min, 0% B. Thirteen SCX fractions were collected and each fraction was desalted with an HLB cartridge (Waters).
LC-MS/MS analysis
Online desalting and reversed phase chromatography was performed with a Nano2D-LC system equipped with an autosampler (Eksigent; Dublin, CA). Mobile phase A and B for these analyses were 3% (v/v) acetonitrile with 0.1% formic acid and 100% (v/v) acetonitrile with 0.1% formic acid, respectively. SCX fractions (5 μL)were loaded onto a trapping column (100 μm i.d. × 2 cm), which was packed in-house with C18 200 Å 3μm stationary phase material (Michrom Bioresource Inc.; Auburn, CA) at 3 μL.min−1 in 3% mobile phase B for 3 min. After desalting, the sample was loaded onto an analytical column (75 μm i.d. × 13.2 cm), which was packed in-house with C18 100 Å 3μm stationary phase material (Michrom Bioresource Inc.). The gradient was as follows: 0-5 min, 10% mobile phase B; 5-75 min, 10-30% B; 75-95 min, 30-60% B; 95-100 min, 60-90% B; 100-105 min, 90-10% B; 110-120 min, 10% B. The LC eluent was analyzed with positive ion nanoflow electrospray using a LTQ-Orbitrap Velos mass spectrometer (Thermo-Fisher Scientific, Waltham, MA). Data-dependent acquisition parameters were as follows: the MS survey scan in the Orbitrap was 60,000 resolution over 300-1800 m/z; the top six most intense peaks in the MS survey scan were isolated and fragmented with CID and HCD; CID was performed in the ion trap with normalized collision energy 35%; HCD was recorded in the Orbitrap with normalized collision energy 45% and 7,500 resolution; dynamic exclusion was enabled and a repeat count of 2 for a duration of 60 seconds was allowed and selected ions were placed on an exclusion list for 61seconds. Each SCX fraction was subject to triplicate LC-MS/MS analysis.
Data analysis
RAW files were analyzed with Proteome Discoverer 1.2 software (Thermo). Both CID and HCD spectra were used to obtain sequence information against the Uniprot human database (04/25/2010, 20295 sequences). Sequest search parameters were as follows: two maximum trypsin miscleavages; precursor mass tolerance 10 ppm; fragment mass tolerance 0.8 Da; static modifications were iTRAQ-4plex/+144.102 Da (N-terminus, Lys), and carbamidomethyl modification/+57.021 Da (Cys); dynamic modification of iTRAQ-4plex/+144.102 Da (Tyr). Decoy database searching was employed to generate medium (p<0.05) and high (p<0.01) confidence peptide lists. All the peptides with medium and high confidence were used to identify and quantify proteins. The reporter ions (i.e., m/z 114-117) were identified with the following parameters: centroid with smallest delta mass, 20 ppm for reporter ion mass tolerance. The isotope correction was employed according to the manufacturer's protocol (AB Sciex). Additionally, protein ratios in each experiment were normalized based on the protein median ratio option in the software (i.e., individual protein value is normalized against the median ratio value obtained across all proteins identified in a given experiment). Only proteins with at least two spectral counts in a technical replicate were considered for further analysis.
Statistics
Coefficient of variation (CV) values were calculated for reporter ion ratios (e.g., 115/114, 116/114, and 117/114) of proteins quantified in at least six iTRAQ experiments. The mean CV value across the iTRAQ experiments was calculated and used as the total biological variation, Sb, which was 0.60 in this study. The technical variation, St, was calculated for proteins quantified in at least two LC-MS/MS analyses within an individual iTRAQ experiment, which was 0.11 in this study. The relation between the fold change (F), random variation (S), biological variation (Sb), and technical variation St is expressed by the formula24
| (1) |
| (2) |
The quantities Z and T depend on the power of the test and the significance level, respectively. When the power of the test and significance level are set as 0.8 and 0.05, respectively, the value of (Z+T)2 will be ∼ 1024. n and m (i.e., m is set as 3 in these studies) are the number of biological and technical replicates, respectively. Such that formula (1) approximates to24
| (3) |
Solve formulas (2) and (3),
| (4) |
From this power analysis, fold-change cutoff was calculated based on the number of biological replicates (i.e., n) in which the proteins were quantified. Stringent filter criteria were applied to generate a list of statistically significant differentially-expressed proteins as follows: 1) proteins identified and quantified in at least six biological replicates, 2) CV values ≤ 0.60, and 3) fold-change cutoff dependent upon n as such a) ≥ 1.27 or ≤ 0.79 (n=10), b) ≥ 1.28 or ≤ 0.78 (n=9), c) ≥ 1.30 or ≤ 0.77 (n=8), d) ≥ 1.32 or ≤ 0.76 (n=9), e) ≥ 1.35 or ≤ 0.74 (n=6).
Western blotting analyses
The changes in the expression of C-reactive protein (CRP), apolipoprotein CIII (ApoCIII), and fibrinogen alpha chain (FAC) were subject to Western blotting analysis. Twenty μg of TMD proteins was denatured in an appropriate sample buffer and electrophoretically separated on a Criterion precast gel (Biorad Laboratories; Hercules, CA) at 140 V. Proteins from the gel were transferred onto a nitrocellulose membrane paper using a Fast-Transfer Blot System (Biorad). Blots were washed three times in Wash blot. BSA blocking solution (3%) was added to the membrane and incubated on a rocker for 2 h. A 1:5000 dilution of mouse monoclonal anti-CRP primary antibody (Sigma Aldrich; St. Louis, MO), 1:5000 dilution of rabbit polyclonal anti-Apo CIII primary antibody (Abcam; Cambridge, MA), or 1:2500 dilution of rabbit monoclonal anti-FAC primary antibody (Abcam) was added and incubated at 4 °C overnight. The blot was rinsed and incubated with a 1:7500 dilution of anti-mouse or anti-rabbit IgG alkaline phosphatase secondary antibody (Sigma Aldrich) for 1 h on a rocker. The blot was rinsed and colorometrically developed using 0.51mM 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (BCIP) and 0.24 mM nitrotetrazolium blue (NBT). The dried blot was scanned using a Canon scanner, saved as a .TIFF file, and densitometry analyses carried out with Scion Image Software. Within each experiment, the intensity for the sample from each group was normalized to the total blot intensity and used to generate mean and standard deviation values.
Ingenuity pathway analysis
Differentially-expressed proteins were analyzed using Ingenuity Pathway Analysis (IPA, www.ingenuity.com) to generate a list of pathways that are statistically relevant (p< 0.05).
Results
Data characterization and statistical analysis
An iTRAQ-based semi-quantitative proteomics workflow22 was employed to identify differentially-expressed proteins in 50-65 and 70-85 year old CAP patients who developed severe sepsis compared to those patients that did not (Table S1). The workflow employs tandem MARS depletion on a MARS Hu-6 column to effectively remove high abundance proteins and has been demonstrated to allow deeper probing into the plasma proteome22. Patient samples from the four groups (i.e., YC, YS, OC and OS) were randomly assigned to iTRAQ reagents in a blind fashion across ten SCX-LC-MS/MS experiments (Table 1).
Table 1. Experimental design and iTRAQ quantitation channel assignment.
| Experiment | Reporter ions | |||
|---|---|---|---|---|
|
| ||||
| 114 | 115 | 116 | 117 | |
| 1 | YSa | YCb | OSc | OCd |
| 2 | YS | YC | OS | OC |
| 3 | YS | YC | OS | OC |
| 4 | YS | YC | OS | OC |
| 5 | YC | OS | OC | YS |
| 6 | YC | OS | OC | YS |
| 7 | YC | OS | OC | YS |
| 8 | OS | OC | YS | YC |
| 9 | OS | OC | YS | YC |
| 10 | OS | OC | YS | YC |
Patients 50-65 years old who developed severe sepsis.
Patients 50-65 years old who did not develop severe sepsis.
Patients 70-85 years old who developed severe sepsis.
Patients 70-85 years old who did not develop severe sepsis.
Figure 1a shows an example of LC chromatograms for 13 SCX fractions of pooled iTRAQ 4-plex samples. The triplicate LC-MS/MS runs for each fraction are reproducible. Figures 1b-g show example spectra for peaks isolated and fragmented. Doubly-charged peptides at m/z 748.9046 (Figure 1b) and 742.9010 (Figure 1c) in SCX fractions seven and six were eluted from the column at tr57.20 min and 37.26 min, respectively. The CID MS/MS spectra (Figures 1d and e) display a consecutive series of b- and y-fragment ions used to assign the peptides as [YYTYLIMNK+2H]2+ of protein Complement C3 and [GWVTDGFSSLK+2H]2+of protein ApoCIII, respectively. A mass increase of 145 Da was observed for b1 and y1ions for each of the two peptides, indicating iTRAQ labeling at the N-terminus and the presence of lysine at the C-terminus. HCD MS/MS spectra (Figures 1f and g) also contain a series of b- and y- ions used to confirm the sequence of peptides. The lower m/z region of the HCD MS/MS spectra (inserts in Figure 1f and g) shows the intensity of the reporter ions which is used to quantify the peptides. As shown in Figures 1f and g, the ratios across the four groups are 1.0:0.9:1.0:1.0 and 1.0:1.9:2.3:1.4 for reporter ions 114:115:116:117 from Complement C3 and ApoCIII, respectively.
Figure 1.
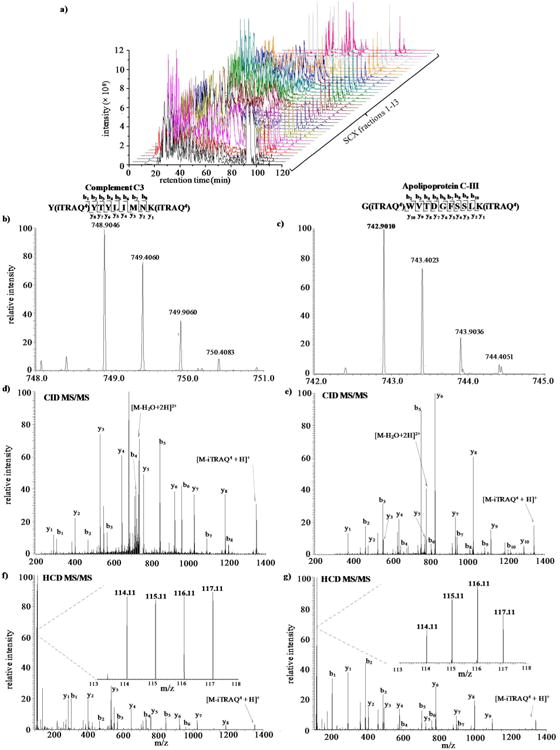
a) LC chromatograms for individual SCX fractions analyzed in triplicate. Example mass spectra of peptides eluted from b) SCX fraction 7 tr = 57.20 min with m/z 748.8046 and c) SCX fraction 6 tr= 37.26 min with m/z 742.9010. CID MS/MS spectra are shown in d) and e), respectively. HCD MS/MS are shown in f) and g). The inserts are zoom-ins of the f) and g) low m/z region.
Figure 2 shows a bar graph of the number of proteins and spectral counts (SCs) identified in each of ten biological replicate experiments. Also, the cumulative number of proteins identified in all of the ten experiments is also shown in Figure 2. An average of 283±14 proteins and 59645±4129 spectral counts were identified and similar results were obtained across individual experiments. The total number of proteins identified increases with each pooled sample experiment such that a total of 772 unique proteins were identified from all plasma samples. With more stringent criteria for protein filtering (i.e., at least two unique peptides), a total of 509 proteins were identified. A list of all identified proteins and peptides is provided in Supplementary Tables S2 and S3, respectively. Based on SCs, the three most abundant proteins are Complement C3 (49,749 SCs), α-2-macroglobulin (47,273 SCs) and ApoAI (28,261 SCs).
Figure 2.
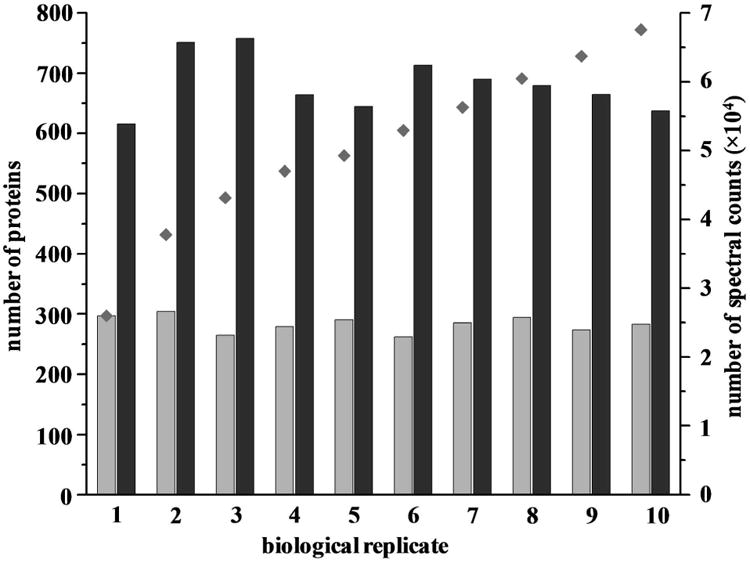
The number of proteins (
 ) and spectral counts (
) and spectral counts (
 ) identified in each experiments. The cumulative number of proteins identified with each subsequent experiment (
) identified in each experiments. The cumulative number of proteins identified with each subsequent experiment (
 ) is also shown.
) is also shown.
Western analysis verification
Western blotting analysis was employed to generate a secondary measurement for several differentially expressed proteins. Proteins involved in the acute phase response (i.e., CRP), coagulation pathway (i.e., FAC) pathway and lipid metabolism (i.e., ApoCIII) were selected. Figures 3a-c display Western blot data for CRP, FAC, ApoCIII, respectively. The histogram plots represent the normalized intensities corresponding to the density of the band spots for each group. The relative abundances (YS:YC:OS:OC) obtained from the Western blotting analysis of CRP, FAC and ApoCIII were 1.0:0.4:0.4:1.8, 1:0.3:0.1:0.5, and 1.0:1.9:2.5:1.2, respectively. These observed Western data were similar to the measured iTRAQ results for these proteins (Table 2). However, suppression of iTRAQ protein ratios in MS/MS experiments is a noted limitation of this quantitative approach25. Proteomics workflows to handle suppression issues at the MS3 level have been recently reported26-30.
Figure 3.
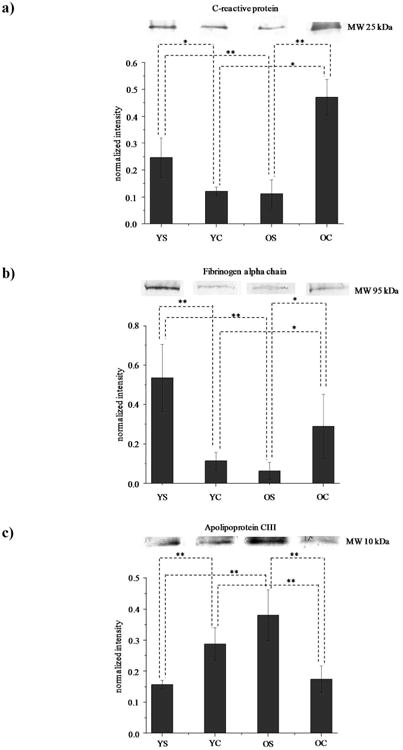
Western blotting images for a) C-reactive protein, b) fibrinogen alpha chain, and c) Apo CIII. The histogram under the images displays the normalized intensity ± standard deviation (n=6) of the proteins across each group. The intensity for each individual band is normalized to the total intensity of the blot.
Table 2.
The list of proteins which are differently expressed between groups.
| Acc. No.a | Protein Name | YS/YC (mean ± SDb) | OS/OC (mean ± SD) | nc |
|---|---|---|---|---|
| O14791 | Apolipoprotein L1 | / | 1.39 ± 0.70 | 10 |
| O95445 | Apolipoprotein M | / | 1.44 ± 0.48 | 8 |
| P01008 | Antithrombin-III | 0.60 ± 0.06 | / | 10 |
| P01011 | Alpha-1-antichymotrypsin | 1.58 ± 0.87 | 0.68 ± 0.18 | 10 |
| P02649 | Apolipoprotein E | 2.11 ± 0.30 | 0.65 ± 0.17 | 10 |
| P02652 | Apolipoprotein A-II | 0.50 ± 0.08 | 1.45 ± 0.46 | 10 |
| P02654 | Apolipoprotein C-I | / | 2.17 ± 1.02 | 10 |
| P02656 | Apolipoprotein C-III | 0.69 ± 0.29 | 1.43 ± 0.77 | 10 |
| P02671 | Fibrinogen alpha chain | 2.44 ± 0.72 | 0.60 ± 0.13 | 10 |
| P02675 | Fibrinogen beta chain | 2.10 ± 0.58 | / | 10 |
| P02679 | Fibrinogen gamma chain | 2.08 ± 0.72 | / | 10 |
| P02735 | Serum amyloid A protein | 3.06 ± 1.09 | / | 10 |
| P02741 | C-reactive protein | 3.27 ± 1.07 | 0.52 ± 0.22 | 10 |
| P02749 | Beta-2-glycoprotein 1 | / | 1.58 ± 0.61 | 10 |
| P02750 | Leucine-rich alpha-2-glycoprotein | 2.14 ± 0.30 | / | 10 |
| P02751 | Fibronectin | / | 0.62 ± 0.31 | 10 |
| P02753 | Retinol-binding protein 4 | 0.53 ± 0.09 | / | 10 |
| P02763 | Alpha-1-acid glycoprotein 1 | 2.03 ± 0.43 | / | 10 |
| P02766 | Transthyretin | 0.44 ± 0.09 | / | 10 |
| P02774 | Vitamin D-binding protein | 0.57 ± 0.08 | / | 10 |
| P02775 | Platelet basic protein | / | 0.76 ± 0.21 | 9 |
| P04114 | Apolipoprotein B-100 | 1.52 ± 0.25 | / | 7 |
| P04259 | Keratin, type II cytoskeletal 6B | 0.52 ± 0.06 | / | 7 |
| P04275 | von Willebrand factor | 2.05 ± 0.34 | / | 10 |
| P04278 | Sex hormone-binding globulin | 0.70 ± 0.26 | / | 7 |
| P05090 | Apolipoprotein D | / | 1.47 ± 0.32 | 10 |
| P05154 | Plasma serine protease inhibitor | 0.73 ± 0.46 | / | 6 |
| P05452 | Tetranectin | 0.64 ± 0.10 | / | 10 |
| P05546 | Heparin cofactor 2 | 0.71 ± 0.26 | / | 10 |
| P06276 | Cholinesterase | / | 1.45 ± 0.47 | 9 |
| P06396 | Gelsolin | 0.55 ± 0.10 | / | 10 |
| P07996 | Thrombospondin-1 | / | 0.65 ± 0.34 | 6 |
| P10909 | Clusterin | 0.73 ± 0.30 | / | 10 |
| P13645 | Keratin, type I cytoskeletal 10 | 0.58 ± 0.06 | / | 10 |
| P15169 | Carboxypeptidase N catalytic chain | 1.31 ± 0.36 | / | 10 |
| P16070 | CD44 antigen | 1.62 ± 0.55 | / | 7 |
| P18428 | Lipopolysaccharide-binding protein | 2.20 ± 0.50 | 0.76 ± 0.32 | 10 |
| P19652 | Alpha-1-acid glycoprotein 2 | 2.17 ± 0.60 | / | 10 |
| P19823 | Inter-alpha-trypsin inhibitor heavy chain H2 | / | 1.40 ± 0.53 | 10 |
| P19827 | Inter-alpha-trypsin inhibitor heavy chain H1 | / | 1.58 ± 0.58 | 10 |
| P20851 | C4b-binding protein beta chain | 1.39 ± 0.46 | / | 6 |
| P29622 | Kallistatin | / | 1.69 ± 0.57 | 10 |
| P35542 | Serum amyloid A-4 protein | 0.48 ± 0.04 | / | 10 |
| P35858 | Insulin-like growth factor-binding protein complex acid labile subunit | / | 1.39 ± 0.46 | 9 |
| P61626 | Lysozyme C | 1.91 ± 0.96 | / | 8 |
| P61769 | Beta-2-microglobulin | 1.89 ± 1.02 | / | 6 |
| P80108 | Phosphatidylinositol-glycan-specific phospholipase D | 0.39 ± 0.06 | / | 9 |
| Q03591 | Complement factor H-related protein 1 | 1.31 ± 0.60 | / | 10 |
| Q04756 | Hepatocyte growth factor activator | / | 1.81 ± 0.68 | 10 |
| Q15431 | Synaptonemal complex protein 1 | / | 1.37 ± 0.62 | 6 |
| Q6UXB8 | Peptidase inhibitor 16 | / | 2.03 ± 0.78 | 6 |
| Q86UD1 | Out at first protein homolog | / | 0.74 ± 0.19 | 6 |
| Q86UV6 | Tripartite motif-containing protein 74 | / | 1.85 ± 0.79 | 6 |
| Q96KN2 | Beta-Ala-His dipeptidase | / | 2.06 ± 0.93 | 6 |
| Q96PD5 | N-acetylmuramoyl-L-alanine amidase | 0.58 ± 0.06 | 1.35 ± 0.39 | 10 |
| Q9BXR6 | Complement factor H-related protein 5 | / | 0.72 ± 0.18 | 10 |
| Q9NXD2 | Myotubularin-related protein 10 | / | 1.78 ± 0.69 | 7 |
| Q9Y6R7 | IgGFc-binding protein | / | 0.70 ± 0.26 | 9 |
accession number provided from the uniprot human database (04/25/2010, 20295 sequences).
mean and SD values are calculated based on the reporter ion ratios for proteins quantified in at least 6 experiments.
the number of experiments in which the corresponding proteins are quantified.
Differentially-expressed proteins
Proteins ratios were obtained from the two age groups: YS/YC in 50-65 years old group and OS/OC in 70-85 years old group using YC and OC as reference channels, respectively. Fifty-eight differentially-expressed proteins (Table 2) were identified in comparisons from both age groups (i.e., YS/YC and OS/OC). Proteins differentially expressed between younger and older adults within each disease group (i.e., OC/YC and OS/YS) are provided in Supplemental Table S5 for interested readers. The measured fold-change values reported in the table include the mean and SD for each protein based on the ratios averaged across all biological replicates. As shown in the Venn diagram (Figure 4), 28 proteins are differentially-expressed in the population of 50-65 year olds (i.e., YS/YC) in which 14 have higher levels and 14 have lower levels in patients with severe sepsis compared to those with CAP. In the population of 70-85 year olds (i.e., OS/OC), 23 proteins are differentially-expressed, in which 16 and seven have higher and lower levels in patients with severe sepsis, respectively. Of the 58 total differentially-expressed proteins, eight are in common amongst both age groups. Interestingly, however the direction of the fold change differs in younger and older adults. Specifically, α-1-antichymotrypsin (A1ACT), ApoE, fibrinogen α chain, C-reactive protein (CRP), and LPS binding protein (LBP) levels were higher in younger adults with severe sepsis but lower in older adults with severe sepsis relative to age-matched controls, suggesting that lower levels of these proteins is associated with increased risk and incidence of severe sepsis in older adults. On the other hand, Apo AII, Apo CIII, and N-acetylmuranoyl-L-alanineamidase have lower and higher levels in younger and older with severe sepsis, respectively, suggesting that higher levels of these proteins may be factors for increased severe sepsis risk in older adults.
Figure 4.
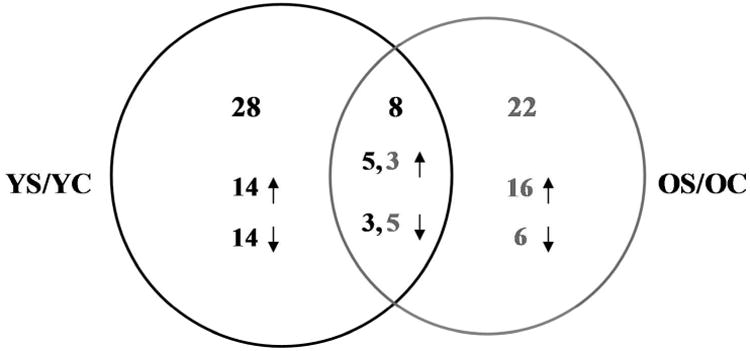
Venn diagram of differentially-expressed proteins for each age group (i.e., YS/YC 50-65 years old and OS/OC 70-85 years old). The number of proteins that have higher (↑) or lower (↓) fold-change values in each comparison are also shown.
Pathway analysis
Using IPA analysis, 19 biological pathways (Figure 5) are significantly over-represented (p<0.05) for the 58 differentially-expressed proteins identified in these studies. The most represented pathways include LXR/RXR activation and acute phase response signaling where by 18 proteins are associated with each of these pathways. Next many differentially-expressed proteins are involved in atherosclerosis signaling, interleukin (IL)-12 signaling and production of NO and reactive oxygen species in macrophages, and endocytosis signaling. Fewer proteins are involved in the remaining biological pathways shown in Figure 5 such as actin cytoskeleton and IL-6 signaling. A list of the proteins associated with specific pathways is provided in Supplemental Table S4.
Figure 5.
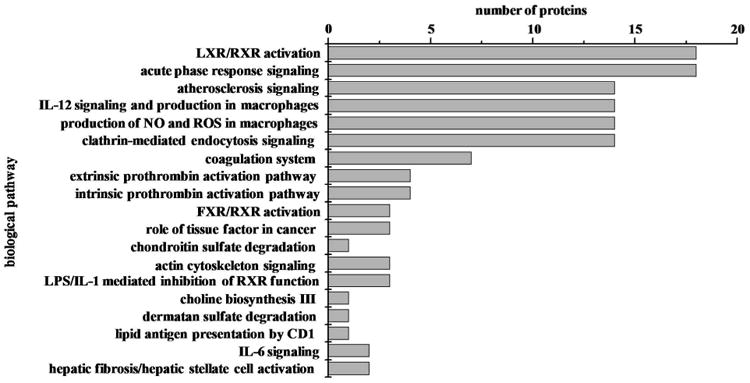
Histogram plot of biological pathways associated with differentially expressed proteins as a function of severity of sepsis (N=58). The p value cutoff for the IPA pathways is p<0.05.
Discussion
This work investigates the effects of aging on the risk of severe sepsis in the acute plasma proteome of elderly CAP patients. While sepsis can occur as early as the neonatal stage31, 32, incidence and morbidity increases with age and rises sharply after 65 years13, 33, 34 presumably due to immunosenescence14, 34, 35 and high levels of inflammatory proteins17-20, 36. However, no significant differences with age have been reported for inflammatory and coagulation proteins and cell surface markers in patients with severe sepsis2.
Our plasma proteomics study identified 58 differentially-expressed proteins in CAP patients that subsequently developed severe sepsis relative to those who did not in groups of elderly adults (Table 2). We note that some of the differences observed may be due to both a mixture of pre-existing conditions and differences in the initial acute phase response, as our study groups did not include a nonseptic subset. Below is a discussion of the proteins involved the biological pathways such as acute phase signaling, coagulation pathway, and lipid metabolism. Other pathways are briefly discussed and the implications of these changes for understanding aging and severe sepsis in the elderly are presented.
Acute phase response
Sepsis is a pro-inflammatory state which is characterized by elevated levels of pro-inflammatory cytokines, such as IL-6, IL-10 and tumor necrosis factor (TNF)-α11. These pro-inflammatory cytokines regulate acute phase responses and the expression of acute phase proteins (APPs)37. Altered expression of APPs has been demonstrated in sepsis16, 38-42. For example, increased levels of α-1-acid glycoprotein (A1AG), A1ACT, and LBP and decreased levels of transthyretin (TTR) are observed in 65 year old sepsis patients39, 40 and cecal ligand and puncture (CLP) mouse41 and pig models42. It also appears that acute phase response increases with the disease severity. For example, sepsis patients have higher levels of CRP and lower levels of serum amyloid A4 relative to patients with systemic inflammatory response syndrome (SIRS), another precursor to sepsis16, 38.
In our studies, 18 differentially-expressed proteins including CRP, LBP, A1ACT, and TTR are involved in acute phase response (Figure 5 and Table S4) and the levels in younger adults are consistent with other studies16, 38-42. For example, CRP (fold-change YS/YC 3.268±1.070), LBP (2.201±0.502), A1ACT (1.577±0.867), and A1AG (2.033±0.426) have higher concentrations while TTR (0.440±0.094) has lower concentrations in younger adults who developed severe sepsis compared to those did not. Also evidenced in our results is that acute phase response also varies depending on patient age. The proteins CRP (0.521±0.221), LBP (0.762±0.321), and A1ACT (0.683±0.183) have lower concentrations in older adults who later developed severe sepsis suggesting that lower expression of these proteins at older age leads to severe sepsis. CRP and LBP have protective effects by neutralizing the toxicity of pathogens39, 43-46, such that decreased expression of these proteins in older patients may help explain higher risk and mortality of severe sepsis. Both aging and severe sepsis alter the immune system and response to infection47. Taken together, these results of acute phase response imply that a hypoinflammatory response in older adults may increase risk of severe sepsis, consistent with other literature reports11, 48, 49.
Coagulation pathway
Pro-inflammatory cytokines can also activate the coagulation pathway. Briefly, the activation of a series of coagulation factors cleave prothrombin to thrombin, which in turn converts soluble fibrinogen into fibrin, upon which cross-linked fibrin forms blood clots11, 50.In sepsis patients, pro-coagulation overwhelms fibrinolysis (or anti-coagulation) leading to the presence of more blood clots11, 50.This has been demonstrated by decreased levels of anti- coagulant proteins [e.g., antithrombin III (ATIII), and heparin cofactor II] and increased levels of pro-coagulant proteins [e.g., fibrinogen, Von Willebrand factor (VWF)] in sepsis patients41, 51-53.
In these studies, elevated levels of fibrinogen alpha chain (2.441±0.579), fibrinogen beta chain (2.102±0.579), fibrinogen gamma chain (2.077±0.720) and VWF (2.047±0.340) were found in younger adults who developed severe sepsis. Fibrinogen has higher concentrations and ATIII has lower concentrations in sepsis41, 51. Higher levels of VWF are associated with mortality in sepsis52. Higher levels of fibrinogen and VWF may indicate the presence of more blood clots in younger patients, consistent with literature reports53-55, whom developed severe sepsis. This notion is further supported by decreased concentrations of ATIII (0.596±0.064) and heparin cofactor II (0.707±0.255) in these studies. Interestingly, fibrinogen (0.597±0.129) had lower levels in older adults who developed severe sepsis. Our proteomics results support the notion that an acute response of reduced coagulation activity may be a contributing factor to higher incidence and mortality of severe sepsis found in older adults.
Lipid metabolism
Apolipoproteins play important roles in liver X receptor/retinoid X receptor (LXR/RXR) activation and atherosclerosis signaling. For example, Apo B-100 can suppress the activation of LXR/RXR56. The activated LXR/RXR complex enhances the process of reverse cholesterol transport and cholesterol efflux, which transfers accumulated cholesterol from the blood vessel walls to the liver for excretion57, 58. Additionally, activated LXR/RXR can reduce the expression of pro-inflammatory cytokines (e.g., IL-6, IL-1β) by inhibition of the activity of transcription factor NF-κB59. The acute phase response which is activated during aging and severe sepsis can inhibit LXR/RXR activation60. Failed activation of the LXR/RXR complex may lead to atherosclerosis61, 62, which is associated with severe sepsis63, 64. Apolipoproteins may have various biological functions by forming different lipoproteins [e.g., low density lipoproteins (LDL) and high density lipoproteins (HDL)]. For example, Apo B-100 is the protein component for LDL which is a cholesterol transporter65. Whereas HDL formed by Apo A and Apo CIII can inhibit oxidation and inflammation66.
Altered levels of apoliproteins have been reported in sepsis. For example, elevated levels of ApoB-10067 and lower concentrations of Apo CIII in response to inflammation68 have been reported. Consistent with this, we observed increased expression of ApoB-100 (1.523±0.253) and decreased expression of Apo CIII (0.690±0.294) in younger adults who developed severe sepsis. These changes may suggest suppressed activation of the LXR/RXR pathway and subsequent higher risk of atherosclerosis in younger adults (although we do not have data to support this notion). Additionally, Apo CIII has different isoforms based on the number (i.e., 0, 1, 2, and 3) of sialic acids per protein: Apo CIII-0, Apo CIII-1, Apo CIII-2, and Apo CIII-369. The Apo CIII isoform ratios have been demonstrated to be correlated with mortality in younger severe sepsis patients (∼ 49 years old)69. For example, the ratio of Apo CIII-2/Apo CIII-1 higher than 1.25 may indicate increased risk of death in the younger severe sepsis patients69. ApoE also has different isoforms: Apo E2, Apo E3, Apo E4. It has been shown that the levels of Apo E2 correlate positively with that of factor VIII, which is necessary for blood clotting70. Also, higher levels of Apo E3 may reduce the risk of severe sepsis70. In this study, higher levels of Apo E (2.105±0.301) were found in younger adults consistent with a rat model71, however lower levels (0.646±0.170) were detected in older adults in our study. Also, higher levels of Apo CI (2.165±1.022) and Apo M (1.438±0.483) were detected in older adults who developed severe sepsis. Although Apo CI levels correlate with the survivorship in ∼25 year old severe sepsis patients72, higher concentration of Apo CI has been reported to promote atherosclerosis73, 74. Apo M has also been reported to have lower concentrations in young adults severe sepsis patients 75, 76 and contributes to the anti-inflammatory response in sepsis76. Higher levels of Apo M in older adults who developed severe sepsis in our studies suggest that these patients have a reduced inflammatory state during acute response. That lipid metabolism is altered in these studies points to atherosclerosis as a contributing factor of severe sepsis incidence; however further analyses are necessary to support this hypothesis.
Other pathways
Other key pathways identified in these studies such as IL-12 and IL-6 signaling support a hypoinflammatory response in older adults (Table S4, Table 2)77.Enhanced production of nitric oxide (NO) and reactive oxygen species (ROS) in macrophages has been previously reported in aging and sepsis78, 79. We detect differentially-expressed proteins involved in the production of NO and ROS (i.e., retinol binding protein 1, lysozyme C and clusterin) which support elevated oxidative stress in the elderly79, 80 and severe sepsis patients78, 81.
The nested-case control study design employed herein has identified many potential factors that may contribute to higher incidence of severe sepsis in adults older than 65 years old. There is a substantial role of inflammation and coagulation processes in acute host response and our proteomics results suggest that older individuals have hypoinflammatory and reduced coagulation responses. While other factors such as lipid metabolism and production of ROS are implicated, additional experiments are necessary in order to determine better the effects of age on these processes. For example, it would be worthwhile in future studies to measure oxidative stress markers in this CAP cohort and to examine the correlation between incidence of severe sepsis and age with atherosclerosis. A limitation to these studies is the small sample populations (N=10) used to measure a wide range of proteins that are known to vary substantially in plasma tissue82. Future studies include selecting key proteins to follow their expression levels in a larger cohort using an independent method, such as ELISA.
Conclusions
The incidence and mortality of severe sepsis increases with aging, especially after 65 years. This study investigated the effects of aging on initial host response to sepsis in CAP patients who eventually develop severe sepsis after hospitalization. Using a proteomics approach, several altered biological pathways were identified. Acute phase response and coagulation were highly represented in these studies, and novel pathways such as lipid metabolism, atherosclerosis, and production of NO and ROS were observed. Interestingly, differentially-expressed proteins involved in these pathways show opposite expression levels of change dependent on patient age (i.e., 50-65 and 70-85 years old). These findings provide more insight to factors that may explain higher risk, increased incidence, and mortality in older adults with severe sepsis. Future studies on a larger cohort of patients (e.g., N>50) will be performed to further understand our results and to assess proteins with low concentrations and important biological roles (e.g., acute phase response, coagulation, and lipid metabolism). Such insight will be helpful for the development of age-specific and personalized severe sepsis treatments.
Supplementary Material
Acknowledgments
The authors acknowledge the University of Pittsburgh Start-up and Central Medical Research Funds for support of this work and Lisa Weissfield for assistance with patient sampling. GenIMS was funded by National Institute of General Medical Science (NIGMS) R01 GM61992 and with additional support from GlaxoSmithKline for enrollment and clinical data collection, Diagnostic Products Corporation for the cytokine assays.
Footnotes
Supporting Information Available: This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Kale S, Yende S, Kong L, Perkins A, Kellum JA, Newman AB, Vallejo AN, Angus DC for the Gen, I. M. S. I. The effects of age on inflammatory and coagulation-fibrinolysis response in patients hospitalized for pneumonia. PLoS ONE. 2010;5(11):e13852. doi: 10.1371/journal.pone.0013852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dremsizov T, Clermont G, Kellum JA, Kalassian KG, Fine MJ, Angus DC. Severe sepsis in community-acquired pneumonia. Chest. 2006;129(4):968–978. doi: 10.1378/chest.129.4.968. [DOI] [PubMed] [Google Scholar]
- 4.Beutz MA, Abraham E. Community-acquired pneumonia and sepsis. Clinics in chest medicine. 2005;26(1):19–28. doi: 10.1016/j.ccm.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Sakr Y. SOFA so good for predicting long-term outcomes. Resuscitation. 2012;83(5):537–538. doi: 10.1016/j.resuscitation.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Carrigan SD, Scott G, Tabrizian M. Toward resolving the challenges of sepsis diagnosis. Clinical Chemistry. 2004;50(8):1301–1314. doi: 10.1373/clinchem.2004.032144. [DOI] [PubMed] [Google Scholar]
- 7.Davis BH. Improved diagnostic approaches to infection/sepsis detection. Expert Review of Molecular Diagnostics. 2005;5(2):193–207. doi: 10.1586/14737159.5.2.193. [DOI] [PubMed] [Google Scholar]
- 8.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 9.Balk RA. Severe sepsis and septic shock: definitions, epidemiology, and clinical manifestations. Critical care clinics. 2000;16(2):179–192. doi: 10.1016/s0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- 10.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 12.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Critical Care Medicine. 2006;34(1):15–21. doi: 10.1097/01.CCM.0000194535.82812.BA. [DOI] [PubMed] [Google Scholar]
- 14.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clinical Infectious Diseases. 2005;41(Supplement 7):S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 15.Wick G, Grubeck-Loebenstein B. The aging immune system: primary and secondary alterations of immune reactivity in the elderly. Experimental Gerontology. 1997;32(4–5):401–413. doi: 10.1016/s0531-5565(96)00152-0. [DOI] [PubMed] [Google Scholar]
- 16.Póvoa P. C-reactive protein: a valuable marker of sepsis. Intensive Care Medicine. 2002;28(3):235–243. doi: 10.1007/s00134-002-1209-6. [DOI] [PubMed] [Google Scholar]
- 17.McDonald AP, Meier TR, Hawley AE, Thibert JN, Farris DM, Wrobleski SK, Henke PK, Wakefield TW, Myers DD., Jr Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thrombosis Research. 2010;125(1):72–78. doi: 10.1016/j.thromres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Critical Care Medicine. 2009;37(3):1018–1023. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto K, Shimokawa T, Yi H, Isobe Ki, Kojima T, Loskutoff DJ, Saito H. Aging accelerates endotoxin-induced thrombosis: increased responses of plasminogen activator inhibitor-1 and lipopolysaccharide signaling with aging. The American Journal of Pathology. 2002;161(5):1805–1814. doi: 10.1016/s0002-9440(10)64457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. The American Journal of Medicine. 2003;114(3):180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. New England Journal of Medicine. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 22.Cao Z, Yende S, Kellum JA, Robinson RAS. Additions to the human plasma proteome via a tandem MARS depletion iTRAQ-based workflow. International Journal of Proteomics. 2013;2013:8. doi: 10.1155/2013/654356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC for the GenIMS Investigators. Understanding the Inflammatory Cytokine Response in Pneumonia and Sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horgan GW. Sample size and replication in 2D gel electrophoresis studies. Journal of Proteome Research. 2007;6(7):2884–2887. doi: 10.1021/pr070114a. [DOI] [PubMed] [Google Scholar]
- 25.Karp NA, Huber W, Sadowski PG, Charles PD, Hester SV, Lilley KS. Addressing Accuracy and Precision Issues in iTRAQ Quantitation. Molecular & Cellular Proteomics. 2010;9(9):1885–1897. doi: 10.1074/mcp.M900628-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wühr M, Haas W, McAlister GC, Peshkin L, Rad R, Kirschner MW, Gygi SP. Accurate multiplexed proteomics at the MS2 level using the complement reporter ion cluster. Analytical Chemistry. 2012;84(21):9214–9221. doi: 10.1021/ac301962s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenger CD, Lee MV, Hebert AS, McAlister GC, Phanstiel DH, Westphall MS, Coon JJ. Gas-phase purification enables accurate, multiplexed proteome quantification with isobaric tagging. Nat Meth. 2011;8(11):933–935. doi: 10.1038/nmeth.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting L, Rad R, Gygi SP, Haas W. MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Meth. 2011;8(11):937–940. doi: 10.1038/nmeth.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans AR, Robinson RAS. Global combined precursor isotopic labeling and isobaric tagging (cPILOT) approach with selective MS3 acquisition. Proteomics. 2013 doi: 10.1002/pmic.201300198. Accepted. [DOI] [PubMed] [Google Scholar]
- 30.Cao Z, Evans AR, Robinson RAS. MS3 based quantitative proteomics using pulsed-Q dissociation (PQD) Manuscript in preparation. 2013 doi: 10.1002/rcm.7192. [DOI] [PubMed] [Google Scholar]
- 31.Downie L, Armiento R, Subhi R, Kelly J, Clifford V, Duke T. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics—systematic review and meta-analysis. Archives of Disease in Childhood. 2013;98(2):146–154. doi: 10.1136/archdischild-2012-302033. [DOI] [PubMed] [Google Scholar]
- 32.Karumbi J, Mulaku M, Aluvaala J, English M, Opiyo N. Topical umbilical cord care for prevention of infection and neonatal mortality. Pediatric Infectious Disease Journal. 2013;32(1):78–83. doi: 10.1097/INF.0b013e3182783dc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott WR, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly. American Journal of Respiratory and Critical Care Medicine. 2002;165(6):766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 34.Girard TD, Ely EW. Bacteremia and sepsis in older adults. Clinics in geriatric medicine. 2007;23(3):633–647. doi: 10.1016/j.cger.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Fulop T, Castle S, Larbi A, Fortin C, Lesur O, Pawelec G. Role of immunosenescence in infections and sepsis in the elderly. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, editors. Handbook on Immunosenescence. Springer Netherlands; 2009. pp. 965–977. [Google Scholar]
- 36.Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clinical and Experimental Immunology. 1999;118(2):235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Critical Care. 2010;14(1):R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Z, Want EJ, Chen W, Keating W, Nussbaumer W, Moore R, Gentle TM, Siuzdak G. Sepsis Plasma Protein Profiling with Immunodepletion, Three-Dimensional Liquid Chromatography Tandem Mass Spectrometry, and Spectrum Counting. Journal of Proteome Research. 2006;5(11):3154–3160. doi: 10.1021/pr060327k. [DOI] [PubMed] [Google Scholar]
- 39.Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001;98(13):3800–3808. doi: 10.1182/blood.v98.13.3800. [DOI] [PubMed] [Google Scholar]
- 40.Kalenka A, Feldmann RE, Otero K, Maurer MH, Waschke KF, Fiedler F. Changes in the serum proteome of patients with sepsis and septic shock. Anesthesia & Analgesia. 2006;103(6):1522–1526. doi: 10.1213/01.ane.0000242533.59457.70. [DOI] [PubMed] [Google Scholar]
- 41.Ren Y, Wang J, Xia J, Jiang C, Zhao K, Li R, Xu N, Xu Y, Liu S. The alterations of mouse plasma proteins during septic development. Journal of Proteome Research. 2007;6(7):2812–2821. doi: 10.1021/pr070047k. [DOI] [PubMed] [Google Scholar]
- 42.Thongboonkerd V, Chiangjong W, Mares J, Moravec J, Tuma Z, Karvunidis T, Sinchaikul S, Chen ST, Opatrný K, Matejovic M. Altered plasma proteome during an early phase of peritonitis-induced sepsis. Clinical Science. 2009;116(9):721–730. doi: 10.1042/CS20080478. [DOI] [PubMed] [Google Scholar]
- 43.Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, Sabino H. C-reactive protein as a marker of infection in critically ill patients. Clinical Microbiology and Infection. 2005;11(2):101–108. doi: 10.1111/j.1469-0691.2004.01044.x. [DOI] [PubMed] [Google Scholar]
- 44.Masiá M, Gutiérrez F, Llorca B, Navarro JC, Mirete C, Padilla S, Hernández I, Flores E. Serum concentrations of lipopolysaccharide-binding protein as a biochemical marker to differentiate microbial etiology in patients with community-acquired pneumonia. Clinical Chemistry. 2004;50(9):1661–1664. doi: 10.1373/clinchem.2004.031294. [DOI] [PubMed] [Google Scholar]
- 45.Tschaikowsky K, Hedwig-Geissing M, Schmidt J, Braun GG. Lipopolysaccharide-binding protein for monitoring of postoperative sepsis: complemental to C-reactive protein or redundant? PLoS ONE. 2011;6(8):e23615. doi: 10.1371/journal.pone.0023615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villar J, Pérez-Méndez L, Espinosa E, Flores C, Blanco J, Muriel A, Basaldúa S, Muros M, Blanch L, Artigas A, Kacmarek RM for the, G.; groups, G.-S. Serum lipopolysaccharide binding protein levels predict severity of lung injury and mortality in patients with severe sepsis. PLoS ONE. 2009;4(8):e6818. doi: 10.1371/journal.pone.0006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadeghi HM, Schnelle JF, Thomas JK, Nishanian P, Fahey JL. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Experimental Gerontology. 1999;34(8):959–970. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 48.Welty-Wolf KE, Carraway MS, Ghio A, Kantrow SP, Huang YCT, Piantadosi CA. Proinflammatory cytokines increase in sepsis after anti-adhesion molecule therapy. Shock. 2000;13(5):404–409. doi: 10.1097/00024382-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Gustot T. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Current Opinion in Critical Care. 2011;17(2):153–159. doi: 10.1097/MCC.0b013e328344b446. [DOI] [PubMed] [Google Scholar]
- 50.Amaral A, Opal S, Vincent JL. Coagulation in sepsis. Intensive Care Medicine. 2004;30(6):1032–1040. doi: 10.1007/s00134-004-2291-8. [DOI] [PubMed] [Google Scholar]
- 51.Soares AJC, Santos MF, Trugilho MRO, Neves-Ferreira AGC, Perales J, Domont GB. Differential proteomics of the plasma of individuals with sepsis caused by Acinetobacter baumannii. Journal of Proteomics. 2009;73(2):267–278. doi: 10.1016/j.jprot.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA Network, T. A. R. D. S. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2004;170(7):766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 53.Kaspereit F, Doerr B, Dickneite G. The effect of fibrinogen concentrate administration on coagulation abnormalities in a rat sepsis model. Blood Coagulation & Fibrinolysis. 2004;15(1):39–43. doi: 10.1097/00001721-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 54.van't Veer C, van der Poll T. Keeping blood clots at bay in sepsis. Nat Med. 2008;14(6):606–608. doi: 10.1038/nm0608-606. [DOI] [PubMed] [Google Scholar]
- 55.Sunder-Plassmann G SW, Korninger C, Stain M, Bettelheim P, Pabinger-Fasching I, Lechner K. Disseminated intravascular coagulation and decrease in fibrinogen levels induced by vincristine/prednisolone therapy of lymphoid blast crisis of chronic myeloid leukemia. Ann Hematol. 1991;62(5):169–173. doi: 10.1007/BF01703143. [DOI] [PubMed] [Google Scholar]
- 56.Chan DC, Hoang A, Barrett PHR, Wong ATY, Nestel PJ, Sviridov D, Watts GF. Apolipoprotein B-100 and ApoA-II Kinetics as Determinants of Cellular Cholesterol Efflux. Journal of Clinical Endocrinology & Metabolism. 2012;97(9):E1658–E1666. doi: 10.1210/jc.2012-1522. [DOI] [PubMed] [Google Scholar]
- 57.Larrede S, Quinn CM, Jessup W, Frisdal E, Olivier M, Hsieh V, Kim MJ, Van Eck M, Couvert P, Carrie A, Giral P, Chapman MJ, Guerin M, Le Goff W. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(11):1930–1936. doi: 10.1161/ATVBAHA.109.194548. [DOI] [PubMed] [Google Scholar]
- 58.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. Journal of Endocrinology. 2010;204(3):233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 59.Myhre AE, Ågren J, Dahle MK, Tamburstuen MV, Lyngstadaas SP, Collins JL, Foster SJ, Thiemermann C, Aasen AO, Wang JE. Liver X receptor is a key regulator of cytokine release in human monocytes. Shock. 2008;29(4):468–474. doi: 10.1097/SHK.0b013e31815073cb. [DOI] [PubMed] [Google Scholar]
- 60.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Thematic review series: the pathogenesis of atherosclerosis. effects of infection and inflammation on lipid and lipoprotein metabolism mechanisms and consequences to the host. Journal of Lipid Research. 2004;45(7):1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Brazil M. Macrophage LXRs inhibit atherosclerosis. Nat Rev Drug Discov. 2002;1(11):840–840. [Google Scholar]
- 62.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proceedings of the National Academy of Sciences. 2002;99(11):7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hackam DG, Mamdani M, Li P, Redelmeier DA. Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet. 2006;367(9508):413–418. doi: 10.1016/S0140-6736(06)68041-0. [DOI] [PubMed] [Google Scholar]
- 64.Podnos YD, Jimenez JC, Wilson SE. Intra-abdominal Sepsis in Elderly Persons. Clinical Infectious Diseases. 2002;35(1):62–68. doi: 10.1086/340866. [DOI] [PubMed] [Google Scholar]
- 65.Segrest JP, Jones MK, De Loof H, Dashti N. Structure of apolipoprotein B-100 in low density lipoproteins. Journal of Lipid Research. 2001;42(9):1346–1367. [PubMed] [Google Scholar]
- 66.Wu A, Hinds CJ, Thiemermann C. High-density lipoproteins in sepsis and septic shock: metabolism, actions, and therapeutic applications. Shock. 2004;21(3):210–221. doi: 10.1097/01.shk.0000111661.09279.82. [DOI] [PubMed] [Google Scholar]
- 67.Phetteplace HW, S N, Hirano KI, Davidson NO, Lanza-Jacoby SP. Escherichia coil sepsis increases hepatic apolipoprotein B secrection by inhibiting degradation 2000. 2000;35(10):1079–1085. doi: 10.1007/s11745-000-0622-y. [DOI] [PubMed] [Google Scholar]
- 68.Lacorte JM, Beigneux A, Parant M, Chambaz J. Repression of apoC-III gene expression by TNFα involves C/EBPδ/NF-IL6β via an IL-1 independent pathway. FEBS Letters. 1997;415(2):217–220. doi: 10.1016/s0014-5793(97)01127-7. [DOI] [PubMed] [Google Scholar]
- 69.Harvey SB, Zhang Y, Wilson-Grady J, Monkkonen T, Nelsestuen GL, Kasthuri RS, Verneris MR, Lund TC, Ely EW, Bernard GR, Zeisler H, Homoncik M, Jilma B, Swan T, Kellogg TA. O-Glycoside biomarker of apolipoprotein C3: responsiveness to obesity, bariatric surgery, and therapy with metformin, to chronic or severe liver disease and to mortality in severe sepsis and graft vs host disease. Journal of Proteome Research. 2008;8(2):603–612. doi: 10.1021/pr800751x. [DOI] [PubMed] [Google Scholar]
- 70.Conlan MG, Folsom AR, Finch A, Davis CE, Sorlie P, Marcucci G, Wu KK. Associations of factor-VIII and von-Willebrand-factor with age, race, sex, and risk-factors for atherosclerosis-the atherosclerosis risk in communities (ARIC) study. Thrombosis and Haemostasis. 1993;70(3):380–385. [PubMed] [Google Scholar]
- 71.Kattan O, Kasravi F, Elford E, Schell M, Harris H. Apolipoprotein E-mediated immune regulation in sepsis. J Immunol. 2008;181:1399–1408. doi: 10.4049/jimmunol.181.2.1399. [DOI] [PubMed] [Google Scholar]
- 72.Berbée JP, Hoogt C, Haas CC, Kessel KM, Dallinga-Thie G, Romijn J, Havekes L, Leeuwen H, Rensen PN. Plasma apolipoprotein CI correlates with increased survival in patients with severe sepsis. Intensive Care Medicine. 2008;34(5):907–911. doi: 10.1007/s00134-008-1006-y. [DOI] [PubMed] [Google Scholar]
- 73.Westerterp M, Van Eck M, de Haan W, Offerman EH, Van Berkel TJC, Havekes LM, Rensen PCN. Apolipoprotein CI aggravates atherosclerosis development in ApoE-knockout mice despite mediating cholesterol efflux from macrophages. Atherosclerosis. 2007;195(1):e9–e16. doi: 10.1016/j.atherosclerosis.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 74.Westerterp M, Berbée JF, Pires NM, van Mierlo GJ, Kleemann R, Romijn JA, Havekes LM, Rensen PC. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116(19):2173–2181. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
- 75.Christoffersen C, Nielsen L. Apolipoprotein M - a new biomarker in sepsis. Critical Care. 2012;16(3):126. doi: 10.1186/cc11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumaraswamy S, Linder A, Akesson P, Dahlback B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflamatory response syndromes. Crit Care. 2012;16:R60. doi: 10.1186/cc11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diefenbach A, Schindler H, Röllinghoff M, Yokoyama WM, Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science. 1999;284(5416):951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 78.Kolls JK. Oxidative stress in sepsis: a redox redux. The Journal of Clinical Investigation. 2006;116(4):860–863. doi: 10.1172/JCI28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berr C. Cognitive impairment and oxidative stress in the elderly: Results of epidemiological studies. Biofactors. 2000;13(1-4):205–209. doi: 10.1002/biof.5520130132. [DOI] [PubMed] [Google Scholar]
- 80.Karolkiewicz J, Szczêsniak L, Deskur-Smielecka E, Nowak A, Stemplewski R, Szeklicki R. Oxidative stress and antioxidant defense system in healthy, elderly men: relationship to physical activity. The Aging Male. 2003;6(2):100–105. [PubMed] [Google Scholar]
- 81.Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. British Journal of Anaesthesia. 2003;90(2):221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 82.Anderson NL, Anderson NG. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Molecular & Cellular Proteomics. 2002;1(11):845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


