Abstract
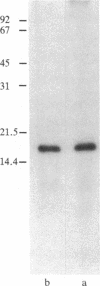
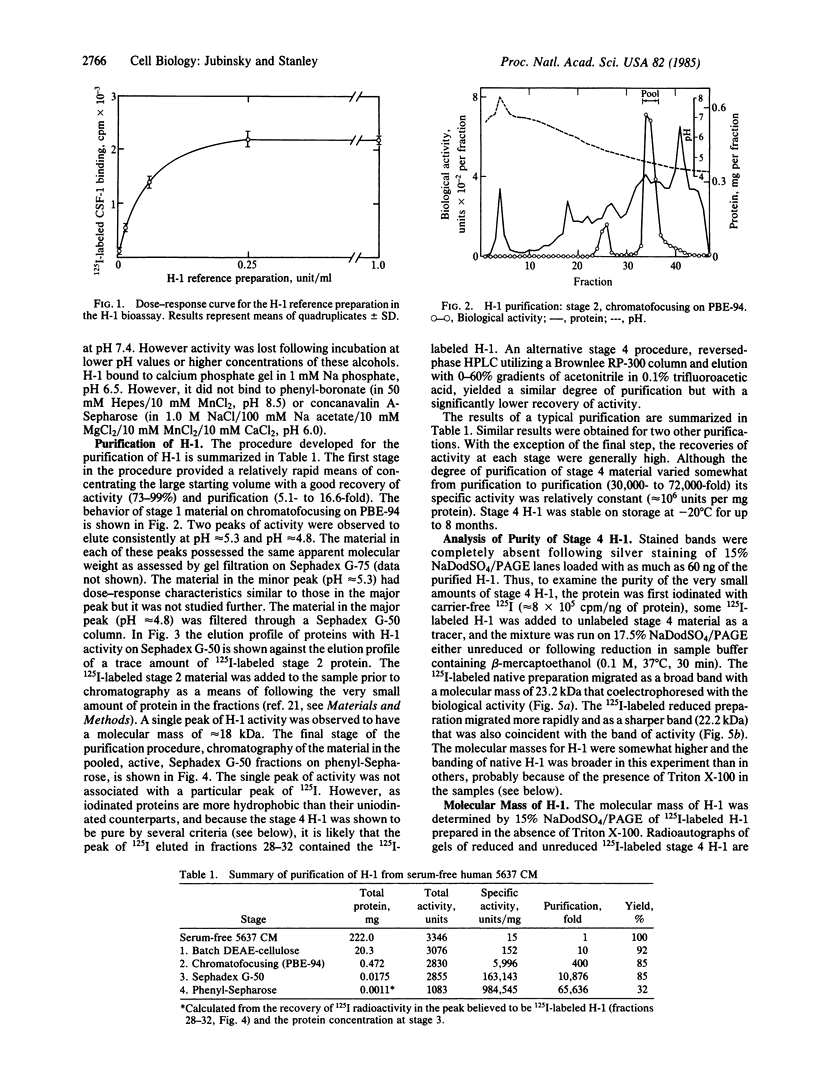
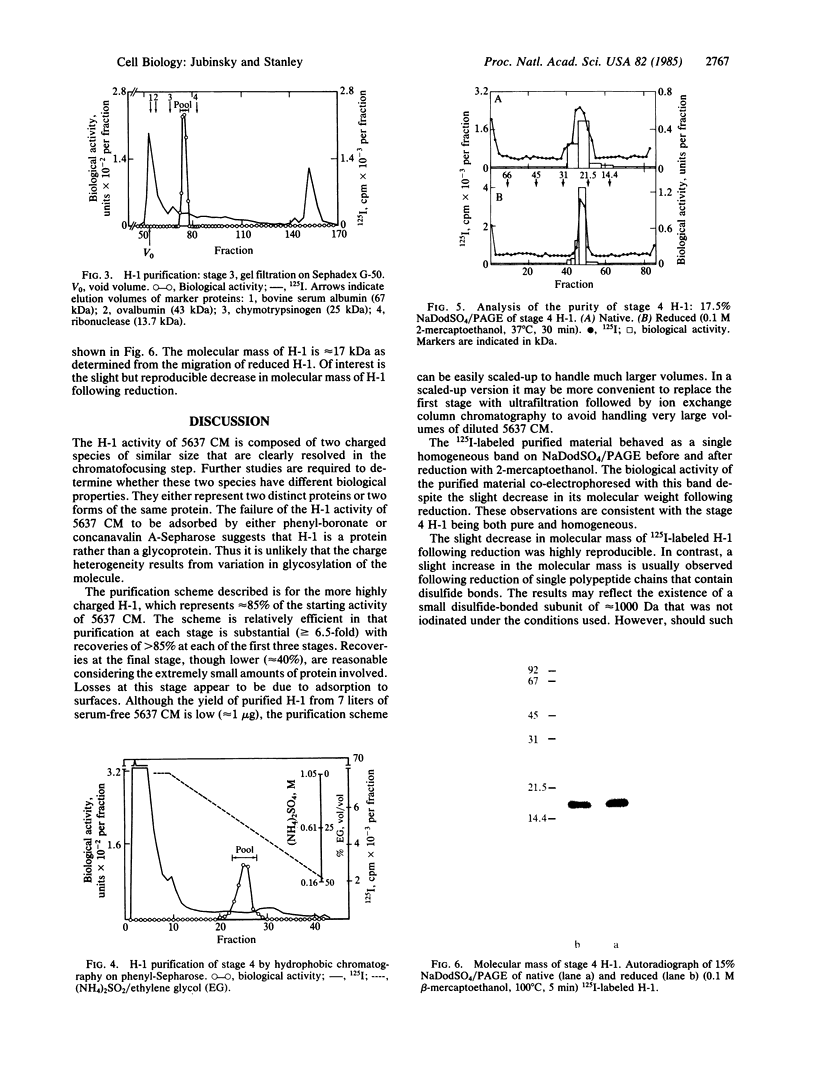
Hemopoietin 1 (H-1) and the mononuclear phagocyte specific growth factor CSF-1 act synergistically on developmentally early bone marrow cells to generate primitive CSF-1 receptor-bearing cells. The H-1 activity of the serum-free medium conditioned by the human urinary bladder carcinoma cell line 5637 was shown to result from the sum of the activities of two charged species (pI approximately equal to 4.8, approximately equal to 85%; pI approximately equal to 5.3, approximately equal to 15%) of similar size. No qualitative difference in the biological activity of these two species was detected. A four-step procedure, involving batch DEAE-cellulose chromatography, chromatofocusing, gel filtration, and hydrophobic chromatography has been developed for the major (pI approximately equal to 4.8) species. H-1 was purified approximately 65,000-fold and recovered as 32% of the total activity of the starting material. The lowest concentration yielding maximal biological activity was approximately equal to 0.25 ng/ml. The 125I-labeled purified H-1, in either native or reduced form, behaved as a homogeneous single band that coelectrophoresed with the biological activity of purified H-1 on sodium dodecyl sulfate gel electrophoresis (NaDodSO4/PAGE). The molecular mass of the purified reduced H-1, determined by NaDodSO4/PAGE was approximately equal to 17 kDa. Recent studies indicate that the purified H-1 is a multilineage hemopoietic growth factor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelmez S. H., Sacca R., Stanley E. R. Lineage specific receptors used to identify a growth factor for developmentally early hemopoietic cells: assay of hemopoietin-2. J Cell Physiol. 1985 Mar;122(3):362–369. doi: 10.1002/jcp.1041220305. [DOI] [PubMed] [Google Scholar]
- Bartelmez S. H., Stanley E. R. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: assay of hemopoietin-1. J Cell Physiol. 1985 Mar;122(3):370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979 Dec;54(6):1446–1450. [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Fogh J. Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors. Natl Cancer Inst Monogr. 1978 Dec;(49):5–9. [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. Modulation of receptors for the colony-stimulating factor, CSF-1, by bacterial lipopolysaccharide and CSF-1. J Immunol Methods. 1984 Oct 12;73(1):17–28. doi: 10.1016/0022-1759(84)90027-9. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Iscove N. N., Roitsch C. A., Williams N., Guilbert L. J. Molecules stimulating early red cell, granulocyte, macrophage, and megakaryocyte precursors in culture: similarity in size, hydrophobicity, and charge. J Cell Physiol Suppl. 1982;1:65–78. doi: 10.1002/jcp.1041130412. [DOI] [PubMed] [Google Scholar]
- Kriegler A. B., Bradley T. R., Januszewicz E., Hodgson G. S., Elms E. F. Partial purification and characterization of a growth factor for macrophage progenitor cells with high proliferative potential in mouse bone marrow. Blood. 1982 Aug;60(2):503–508. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNiece I. K., Bradley T. R., Kriegler A. B., Hodgson G. S. A growth factor produced by WEHI-3 cells for murine high proliferative potential GM-progenitor colony forming cells. Cell Biol Int Rep. 1982 Mar;6(3):243–251. doi: 10.1016/0309-1651(82)90076-5. [DOI] [PubMed] [Google Scholar]
- Myers C. D., Katz F. E., Joshi G., Millar J. L. A cell line secreting stimulating factors for CFU-GEMM culture. Blood. 1984 Jul;64(1):152–155. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J. Methods for the purification, assay, characterization and target cell binding of a colony stimulating factor (CSF-1). J Immunol Methods. 1981;42(3):253–284. doi: 10.1016/0022-1759(81)90156-3. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Hansen G., Woodcock J., Metcalf D. Colony stimulating factor and the regulation of granulopoiesis and macrophage production. Fed Proc. 1975 Dec;34(13):2272–2278. [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Stanley E. R., Jubinsky P. T. Factors affecting the growth and differentiation of haemopoietic cells in culture. Clin Haematol. 1984 Jun;13(2):329–348. [PubMed] [Google Scholar]