Abstract
The recent description of infertility in humans with loss-of-function mutations in genes for neurokinin B (NKB) or its receptor (NK3R) has focused attention on the importance of this tachykinin in the control of GnRH secretion. In a number of species, NKB neurons in the arcuate nucleus also produce two other neuropeptides implicated in the control of GnRH secretion: 1) kisspeptin, which is also essential for fertility in humans, and 2) dynorphin, an inhibitory endogenous opioid peptide. A number of characteristics of this neuronal population led to the hypothesis that they may be responsible for driving synchronous release of GnRH during episodic secretion of this hormone and there is now considerable evidence to support this hypothesis in sheep and goats. In this article, we briefly review the history of work on the NKB system in sheep and then review the anatomy of NKB signaling in the sheep. We next describe evidence from a number of species that led to development of a model for the role of these neurons in episodic GnRH secretion. Finally, we discuss recent experiments in sheep and goats that tested this hypothesis and led to a modified version of the model, and then broaden our focus to briefly consider the possible roles of NKB in other species and systems.
Keywords: NKB, GnRH pulse generator, kisspeptin, LH
Introduction
Studies in sheep on neurokinin B (NKB) and the control of GnRH secretion have a relatively brief history because workers using this animal model, like most other reproductive neuroendocrinologists, largely ignored the pioneering work of Naomi Rance and her colleagues correlating NKB expression with LH secretion in humans [1], primates [2], and rats [3]. Thus the first paper on the ovine NKB system did not appear until 2000 [4]. It described a population of NKB neurons in the arcuate nucleus (ARC), almost all (97%) of which contained estrogen receptor-α (ERα). A subset of this population was also sexually differentiated, with twice as many neurons in the caudal ARC of females than males and prenatally-androgenized females, and appeared to project to GnRH neurons in the preoptic area (POA) based on light microscopic analysis. We were struck by the close anatomical similarities of these NKB neurons to a group of ARC dynorphin neurons that we were testing for a role in progesterone negative feedback in the ewe. Specifically, they showed the same distribution within the ARC (concentrated in the more posterior regions) and over 90% of them contained progesterone receptors (PR) [5]. We could infer that these dynorphin neurons contained ERα because all ovine PR-positive neurons also express this estrogen receptor [6]. The high expression of steroid receptors in these NKB and dynorphin neurons contrasted with the relatively low percentage (15-20%) co-localization of ERα with other ARC neurons containing β-endorphin, neuropeptide Y, or dopamine [7, 8], and raised the possibility that NKB and dynorphin were contained in the same neurons. This co-localization was confirmed by a dual-label immunocytochemical (ICC) analysis, which also reported the frequent presence of close appositions containing both NKB and dynorphin onto NKB-dynorphin cells [9] suggesting that these cells form a reciprocally-interconnected circuit (see below).
These anatomical studies were of some interest, but the possible physiological actions of NKB in ewes remained largely unknown. The sex differences in expression raised the possibility that the ARC population might be involved in the preovulatory LH surge, which occurs in female, but not male (or androgenized female) sheep [10]. However, expression of mRNA levels for the NKB precursor did not increase at the time of the estrogen-induced surge [4] and was suppressed 4 hrs after initiation of a surge-inducing estradiol treatment in ovariectomized (OVX) ewes [11]. In our initial attempt to assess the action of NKB in ewes, we observed a dramatic increase in LH concentrations following icv injection of senktide, an agonist for the NKB receptor (NK3R) [12]. However, we did not actively pursue this observation because we had no funding for this work and because it conflicted with reported inhibitory effects of senktide in rats [13]. Moreover, during this period, the discovery of the critical role of kisspeptin in human reproduction [14, 15] attracted the attention of virtually all reproductive neuroendocrinologists, so what little interest there was in NKB waned even further.
The lack of interest in NKB was dramatically altered by two reports. First, because of the high expression of ERα in ovine ARC kisspeptin-positive cells [16], we tested them for co-localization with NKB and dynorphin and concluded that in ewes there was a single population of neurons in the ARC that contained all three peptides [17]. This population, which we named KNDy (for kisspeptin, NKB, and dynorphin, and derived from the origin of “kisspeptin”) neurons [18] has since been described in rats [19, 20], mice [21], and goats [22]. As discussed at the end of this review, there is also considerable evidence for their existence in women [23], but their role in men remains under debate [24]. The second critical observation was the report that loss-of-function mutations in the genes for NKB or its receptor caused infertility in humans [25]. Thus, although the reproductive deficits resulting from loss of NKB-signaling may not be as severe as those seen in Kiss1R-deficent individuals [26], both NKB and kisspeptin appear to have actions necessary for normal secretion of GnRH in humans. As was the case with the earlier reports on Kiss1R mutations, the report by Topaloglu et al. focused the attention of reproductive neuroendocrinologists on the role of NKB. Moreover, several characteristics of these KNDy neurons (discussed in detail below) soon led four different groups to propose that they play a key role in synchronizing the secretory activity of GnRH neurons to produce episodic secretion of this neuropeptide [21, 27-29].
This article will focus primarily on the role of NKB in the generation of GnRH pulses in the ewe. Specifically, we will: 1) summarize the neuroanatomy of NKB signaling on the ovine diencephalon, 2) describe the evidence from a number of species that led to the development of the initial model for the GnRH pulse generator, 3) discuss experiments in the ewe and goat designed to test the proposed roles of NKB (and other KNDy peptides) in this model that resulted in a modification of it, and 4) close with a more general discussion of the possible roles of NKB.
Neuroanatomy of NKB signaling
Cell bodies
The two reports that examined distribution of NKB neurons in the ovine diencephalon agree that the vast majority of NKB-containing cell bodies are located in the ARC (Fig 1). One report observed scattered (<2 cells/section) NKB-immunoreactive (ir) cell bodies in the POA [4], but these were not seen in the other study [9], possibly because an immunofluorescent, not immunoperoxidase, protocol was used [9]. It should be noted that animals were not pre-treated with colchicine in either study, so NKB-producing cells that rapidly transport this peptide to terminals may have been missed. As discussed below, the presence of NKB-containing fibers that do not contain dynorphin or kisspeptin are consistent with this possibility or that some NKB fibers in the hypothalamus arise from other brain regions. Thus further studies using in situ hybridization are needed before any definite conclusions can be drawn on the distribution of NKB-producing neurons outside the ARC in the ewe.
Fig 1.
Confocal images (1.5 μm thickness) of the same section through the ovine ARC stained for dynorphin (green) and NKB (red). Panel C is a computer overlay of these two images so that cells containing both peptides appear as yellow. Magnification bar: 50 μm. Reprinted from Foradori et al. [9].
Because almost all ARC NKB neurons in female sheep also contain kisspeptin and dynorphin, any one of these peptides can be used as a marker for these cells. Regardless of which antigen has been labeled there is general agreement that NKB-ir cell bodies are concentrated in the middle and caudal regions of this nucleus. It is, however, unclear whether there are more NKB cells in the middle or caudal ARC as there are reports of more cells in the caudal [4, 17, 30], more cells in the middle [18, 31], and similar NKB cell numbers in both regions [5, 17, 30, 32]. This variability cannot be explained by technical differences because the same groups have reported slightly different distributions in different papers (for example see [17, 18] and [17, 32]). Instead these variations most likely reflect three factors: 1) there is probably a peak in distribution of KNDy neurons near the transition between the middle and caudal ARC, 2) most studies examined a very limited number (1-3) of coronal sections in each area, and 3) there are differences in the definitions of which sections are in the middle or caudal ARC. Thus sections taken from the anterior portions of the middle and caudal ARC might well indicate more cells in the caudal ARC, while two sections taken from the posterior portions of each would indicate more in the middle ARC. A description of the distribution of KNDy neurons in a complete series of sections through the ovine ARC would thus be useful in clarifying the reasons for these discrepancies. It should also be noted that because expression of the three KNDy peptides is differentially regulated, with kisspeptin and NKB increasing [33, 34], and dynorphin decreasing [35], after OVX the high degree of co-localization may vary with endocrine status. For example, there are large seasonal differences in the number of kisspeptin-ir neurons in estradiol-treated OVX ewes [30], that are not seen if dynorphin is used as marker for these cells [31]. However, these differences in expression are generally not region-specific so that the relative distribution of KNDy neurons within the ARC does not change. The one exception to the latter statement is in intact male rams. As was first reported for NKB [4], there are far fewer kisspeptin and dynorphin-ir cells in male than female sheep [18, 33], and this sex difference occurs primarily in the more posterior regions of the ARC [4, 18] so that in rams there are only slightly fewer KNDy cells in the rostral ARC.
It should also be noted that dual-immunofluorescent procedures do identify some NKB-ir neurons in the ovine ARC that do not contain dynorphin [9] or kisspeptin [17]. This could reflect technical limitations in the ability of this technique because fluoresecent probes (which are required for co-localization studies) may not detect low levels of dynorphin or kisspeptin expression. Alternatively it could represent a unique population of NKB-ir neurons; if so, this population is not observed in rats because 99% of NKB-ir neurons in the ARC also contain dynorphin in this species [19]. Because there is no evidence at this time for functional or anatomical sub-populations of KNDy neurons in the ovine ARC, we think the simplest explanation for these observations is that expression of dynorphin and/or kisspeptin was too low to be detected in some neurons in these experiments.
Projections of ARC NKB neurons
Because only ARC NKB cell bodies also contain kisspeptin and dynorphin, the existence of fibers and varicosities containing both NKB and kisspeptin, or NKB and dynorphin can be used as indices of projections from ARC NKB neurons. One study has described fibers containing either NKB alone, or both NKB and dynorphin in the POA and hypothalamus [9]. The latter very likely arise from KNDy neurons, but whether fibers containing only NKB come from the few ARC NKB cells that do not contain dynorphin or from NKB cells found in other areas remains to be determined. There has been considerably more work analyzing KNDy inputs onto cell bodies or dendrites using triple-label ICC to identify these inputs. The most dramatic example of such innervation is onto KNDy neurons themselves (Fig. 2), with >90% of NKB/dynorphin-ir cell bodies receiving close contacts that also contained these two peptides [9]. There is thus a high level of reciprocal innervation among this ARC population. This reciprocal innervation has also been observed in goats [36] and rats [19]. Tract tracing studies have demonstrated that it extends to bilateral connections between ARC nuclei on each side of the third ventricle [36, 37], most likely via fibers that pass under the ventricle in the internal zone of the median eminence that have been observed in a number of species including primates [38]. As discussed below, the discovery of this neural network among KNDy neurons had important implications for the possible physiological role of these neurons.
Fig 2.
Reciprocal connections among dynorphin (red)/NKB (green) cells in the ovine arcuate nucleus. Confocal optical section (1.5 μm thickness) through the ARC showing individual channels (Panels A and B) and overlay (Panel C) illustrating close contacts (e.g., arrow) that contain both peptides onto a KNDy cell body. Magnification bar: 10 μm. Reprinted from Foradori et al. [9].
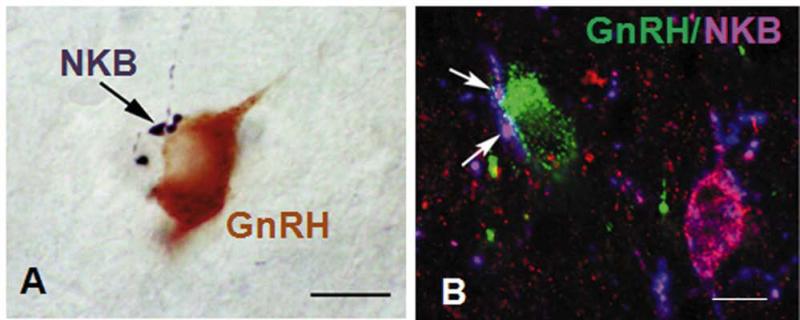
The other target of KNDy neurons of particular interest for this discussion is obviously GnRH neurons. The first evidence for such input came from Goubillon et al. who reported that 39% of POA GnRH neurons (Fig. 3A) and 23% of GnRH neurons in the anterior hypothalamic area (AHA) received close contacts from NKB-containing varicosities [4]. They inferred that this input came from the ARC NKB-ir cells, but could not rule out other possible sources. More recently, using confocal analysis of kisspeptin/dynorphin-positive close contacts, we observed that GnRH neurons in the POA (59%), AHA (62%) and mediobasal hypothalamus (MBH, Fig 3B) (78%) received input from KNDy cell bodies [39]. There is also strong evidence supporting projections from KNDy neurons to GnRH axons and terminals in the ovine median eminence [9, 40]. Retrograde tracing studies in sheep have provided evidence that ERα-positive neurons in the ARC and VMH [19], and more specifically KNDy neurons [41], project to the regions in the POA containing the most GnRH neurons. Studies using anterograde tracers, on the other hand, have yielded conflicting data; one reported almost no input to GnRH neurons from the ventromedial nucleus (VMN) [42], while the other observed approximately 50% of GnRH neurons received input from this area, probably because larger volumes of tract tracer were injected [43]. The former study also concluded that there was no direct input from the ARC to POA GnRH neurons, but tracer injections appeared to be in the rostral ARC where few KNDy neurons are located. Moreover, conventional tracing studies frequently provide a somewhat limited view of efferent projections because only a small percentage of neurons in the injection site actually take up the tracer. Thus we think that the use of dual-ICC to identify KNDy efferents provides a more global analysis of their projections, although it provides no information on the percentage of KNDy cells projecting to a specific target. Finally, although there is limited information on the projections from ovine KNDy neurons to other areas, or cell types, there is evidence that these neurons project to other non-KNDy cells within the ARC (e.g., Fig. 6), including to about 7% of POMC-ir and 20 % of NPY-ir cell bodies [44].
Fig. 3.
Panel A: NKB-containing close contact immunostained using nickel-enhanced DAB (blue-black) onto a GnRH neuron immunostained using DAB (brown) in the POA. Magnification bar: 10 μm. Reprinted from Goubillon et al. [4]. Panel B: MBH GnRH neuron (green) with two close contacts (arrows) containing both dynorphin (red) and NKB (blue); note nearby KNDy neuron (magenta) also contains both NKB and dynorphin. Magnification bar: 10 μm. Reprinted from Lehman et al. [39].
Fig. 6.
Two sections through the ovine ARC stained for NK3R (red: panels A,D) and NKB (green, panels B & E). Panel C is overlay of Panels A and B, illustrating extensive NK3R in this NKB neuron. Panel F is overlay of Panels D and E, illustrating a non-NKB containing cell with NK3R. Note NKB-ir terminals in close contact with this neuron (e.g., arrow). Magnification bar: 10 μm. Reprinted from Amstalden et al. [49].
Inputs to ARC NKB neurons
The most obvious inputs to these cell bodies are the reciprocal projections from other KNDy neurons described above. It should be noted, that although almost all KNDy neurons receive close contacts containing KNDy peptides, we do not know whether this input derives from the same neuron (i.e. are autosynapses), or from other KNDy cell bodies. The other major input that has been described to ovine KNDy neurons is glutamatergic in nature. Specifically, we observed that virtually all KNDy neurons receive vGlut2-containing varicosities and these inputs represented the majority of synaptic terminals in contact with KNDy neurons [45]. Depending on stage of the estrous cycle, approximately one-half to one-third of these vGlut2-ir vesicles also contained KNDy peptides, indicating that there are at least two sources of glutamatergic input to these neurons.
The other afferents that have been examined contact a more limited number of ARC NKB cells. Input from POA kisspeptin neurons (based on varicosities containing kisspeptin and synaptophysin, but not other products of KNDy cells) is virtually non-existent (Merkley et al., unpublished data), and only about 17% of KNDy cell bodies are contacted by GnRH-ir vesicles [46]. Input from NPY (13-30%) and POMC (32-44%) [44] neurons is higher than that from GnRH neurons; it has been suggested [47] that this input may allow metabolic signals, such as leptin, to modulate activity of ovine KNDy neurons and exogenous leptin did increase Kiss1 mRNA (grains/cell) in the ARC of food-restricted ewes [44]. There is one report, based on single-cell RT-PCR analysis of laser-captured cells, that ovine KNDy neurons contain mRNA for the leptin receptor [44], but they apparently do not contain active leptin receptors because leptin did not induce pSTAT3 in any KNDY neurons [48]. The input from GnRH, NPY, and POMC neurons could be part of a reciprocal circuit, but since only a small percentage of each population is involved, whether true reciprocal connections occur at the cellular level remains unclear. It is also important to keep in mind that if the KNDy population functions as an integrated network, then input to a subset of these neurons could affect the whole population. Thus it is important to test the physiological roles of these inputs despite the somewhat limited number of anatomical contacts.
NK3R expression
There is currently only one detailed description of NK3R expression in the ovine diencephalon, but it provides important information relevant to the possible physiological actions of ARC NKB neurons [49]. There were relatively abundant cells expressing NK3R in the paraventricular nucleus (PVN), ARC and premammillary region (PMR), with slightly lower expression in the POA (Fig 4). Moderate numbers of cells containing NK3R were also observed in the retrochiasmatic area (RCh), bed nucleus of the stria terminalis (BNST), and dorsal medial hypothalamus (DMH). NKB-containing close contacts onto NK3R-ir cells were evident in the ARC (see below) and more rarely seen in other areas, including the PVN, RCh, DMH, and PMR, but not in the POA. Extensive NK3R-ir fibers were observed in the ARC, median eminence, and lateral septum (LS). Considerable fiber staining was also observed in the BNST, POA, PVN, RCh, DMH, and PMR.
Fig. 4.
Sites of NK3R in the ovine diencephalon depicted on a schematic of a parasagittal section of the ovine hypothalamus. Insets are examples of ICC staining for NK3R next to coronal schematics containing camera lucida drawings of NK3R-ir cells (solid circles). Abbreviations: ac: anterior commissure; AHA: anterior hypothalamic area; ARC: arcuate nucleus; cp: cerebral peduncle; DMH: dorsomedial hypothalamus; fx: fornix; mARC: middle arcuate nucleus; MB: mammillary body; ME: median eminence; OC: optic chiasm; OVLT: organum vasculosum of lamina terminalis; POA: preoptic area; pt: pars tuberalis; PVN: paraventricular nucleus; RCh: retrochiasmatic area; VMH: ventromedial hypothalamus; vmPOA: ventromedial POA; 3v: third ventricle. Redrawn from Amstalden et al. [49] .
Although an earlier study had found NK3R expression in some GnRH neurons in the rat [43], we found no evidence for this in the ewe [49]. Despite clear signal for NK3R elsewhere in the same sections (Fig. 5), none of the 517 GnRH-ir cells examined also contained NK3R. Similarly although NK3R- and GnRH-containing fibers were intermingled in the median eminence, no colocalization could be detected. This absence of NK3R expression in GnRH neurons is unlikely to reflect the steroidal milieu because tissue from both follicular (n=3) and luteal phase (n=3) ewes was examined. In contrast to GnRH neurons, many NKB-ir cells in the ARC contained NK3R and these were interspersed among non-NKB-ir cells that also contained this receptor (Fig 6). Approximately 65% of NKB-ir cells contained NK3R, but these cells were a minority (39%) of NK3R-positive cells in this region. Not surprisingly, NKB cells that contained NK3R were contacted by NKB-ir close contacts (from other KNDy neurons), but interestingly so were non-identified NK3R-ir cells (Fig 6D-F). Thus one can conclude from these data that, in the ewe, NKB can act on KNDy cells and other neurons in the ARC but has no direct effect on GnRH neurons. These observations, together with the evidence that KNDy neurons also contain kisspeptin laid the foundation for a new model to explain synchronization of GnRH neural activity.
Fig. 5.
The same section in the POA stained for NK3R (red) and GnRH (green). Note in overlay (Panel C) that the GnRH neuron contains no NK3R-ir. Magnification bar: 10 μm Reprinted from Amstalden et al. [49].
A model of KNDy neurons as key components of the GnRH pulse generator
Development of model
Several lines of evidence reported primarily between 2007 and 2009, taken together, raised the possibility that KNDy neurons are integral to the synchronous activation of GnRH neurons needed for episodic GnRH secretion. These included: 1) the presence of kisspeptin, NKB, and dynorphin in the same population of neurons in several species [17, 19-22], 2) evidence that NKB [25], like kisspeptin [14, 15], was critical for normal GnRH secretion in humans, 3) the use of Kiss1r antagonists demonstrated that endogenous kisspeptin was required for episodic LH secretion [50] and push-pull data indicated that kisspeptin and GnRH pulses were correlated in the monkey median eminence [51], 4) the presence of NK3R in KNDy neurons [43, 49] and of reciprocal connections between them [9, 19] provided a simple explanation for coordinated release of kisspeptin, and 5) work in goats indicated that bursts of multi-unit activity (MUA) associated with LH pulses were recorded from the vicinity of KNDy neurons [52]. This confluence of information led four groups [21, 27-29], working largely independently of each other, to propose in late 2009 and early 2010 that KNDy neurons represented the long-sought GnRH pulse generator.
Roles of KNDy peptides
All four groups proposed the same roles for kisspeptin, NKB, and dynorphin in their models (Fig 7). First, kisspeptin was proposed to be the output signal that drives GnRH secretion during each pulse. This role was based on evidence cited above that kisspeptin was critical for LH pulses and the presence of Kiss1r in GnRH neurons in several species [32, 53, 54]. In contrast, GnRH neurons do not contain the κ-opioid receptor (KOR) that mediates dynorphin action, and few [55], or none [49], of them contain NK3R. Because KNDy neurons do not contain Kiss1r, at least in sheep [40], and exogenous kisspeptin failed to alter MUA in the ARC [52, 56], the original model proposed no role for kisspeptin in controlling activity of KNDy neurons. Second, because many KNDy neurons contain NK3R [43, 49], it was hypothesized that a small increase in NKB would act on these neurons to initiate a positive feedback loop within their network at the start of a GnRH pulse. Because of the explosive nature of positive feedback loops, the resulting kisspeptin release could produce the rapid increase in GnRH secretion that is seen at the beginning of each pulse [57]. Finally, dynorphin release would be stimulated from these same neurons by NKB and, after a short delay, would inhibit activity of the KNDy network and thus terminate GnRH release at the end of a pulse. The role of dynorphin was largely speculative because it was unclear whether KNDy neurons contained KOR. However, earlier evidence demonstrated that iv administration of naloxone, an antagonist for KOR and μ-opioid receptors, increased the amplitude and prolonged GnRH pulses in ewes [58], and a contemporaneous study reported that icv infusion of a specific KOR antagonist increased the frequency of ARC MUA in goats [22]. This proposal generated considerable interest, and a fair amount of controversy, and like all useful hypotheses has led to considerable work to test its viability. The rest of this review will be limited largely to subsequent work in sheep and goats, with a focus on testing the role of NKB in the generation of GnRH pulses, using agonists and antagonist to NK3R.
Fig. 7.
Model for actions of KNDy peptides in initiation and termination of each GnRH pulse. Each GnRH pulse is initiated by NKB (purple) acting within the KNDy network (within dashed oval), which stimulates kisspeptin (green) release to drive GnRH (blue) secretion. GnRH release is then terminated by dynorphin (red) release from KNDy neurons acting directly on KNDy neurons. Note that the color in each terminal indicates the biologically active transmitter (possibly due to selective expression of post-synaptic receptors), not selective transport of that peptide to the terminal. Abbreviations: Rdyn: kappa opioid receptor; RKs: Kiss1r; RNKB: NK3R. Redrawn from Lehman et al. [27].
Role of NKB in control of episodic GnRH secretion
Effects of NK3R agonists
Two agonists have been used to investigate the role of NKB (or more accurately NK3R): senktide, which is highly selectively for NK3R [59], and NKB, which acts predominantly via NK3R but can potentially act via the other two tachykinin receptors [60]. Two studies in ewes have reported stimulatory effects of senktide given either peripherally [33] or into the third ventricle [61], but the relevance of these observations to control of episodic GnRH secretion is unclear because the site of action of senktide in these studies is not known. In fact, the LH pattern following icv administration of senktide more closely resembled the LH surge, than episodic LH secretion, and this effect could be produced with local administration in the RCh [61], which does not contain KNDy cell bodies.
Three studies using NKB have indicated that this agonist can stimulate KNDy neurons. First, icv injection of NKB in OVX goats increased the frequency of bursts of MUA [22], which presumably originates from KNDy neurons. Second, icv infusion of NKB stimulated LH secretion in anestrous ewes and dramatically increased Fos expression in KNDy neurons [62]. Although these two studies provide strong evidence that NKB can stimulate KNDy neural activity, it is still unclear where this effect occurs because NKB was given icv. Therefore we examined the effects of local administration of NKB to the ARC of OVX ewes [46]. As illustrated in Fig. 8, microimplants containing crystalline NKB consistently increased LH pulse frequency, while control (empty) microimplants had no effect. These results are consistent with the increase in bursts of MUA with icv injections of NKB in OVX goats [22]. However, that study also reported that NKB induced a transitory decrease in LH concentrations that was not observed with ARC microimplants in our work (pre: 10.5 ± 0.8 ng/ml vs post: 10.6 ± 1.4 ng/ml. n=9). Taken together these two studies indicate that NKB acts in the ARC to stimulate the GnRH pulse generator, but also acts elsewhere in the hypothalamus to inhibit GnRH secretion. The local effect of NKB microimplants could reflect an action on non-KNDy neurons that contain NK3R (Fig. 6) in the ARC [49], but the simplest explanation is a direct effect on the KNDy neurons that also contain this receptor.
Fig. 8.
Effects of empty (open bars, top panels), or NKB-containing (shaded bars, bottom panels), microimplants placed in the ARC on LH pulse patterns in two individual OVX ewes. Solid circles depict peaks of identified LH pulses. Data from Goodman et al. [46].
Effects of NK3R antagonist
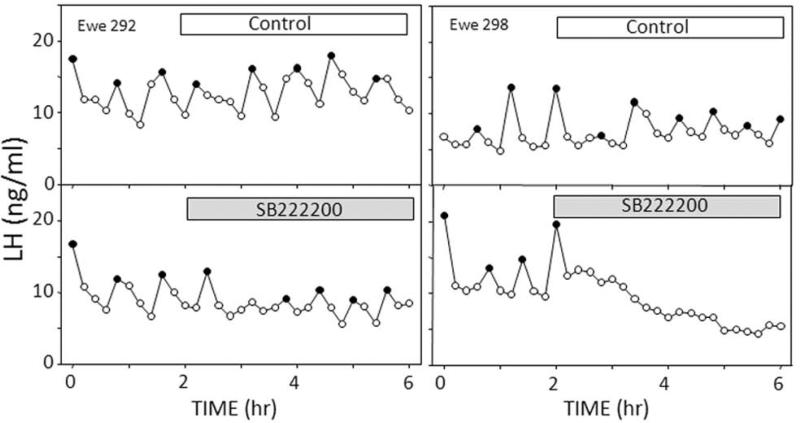
While NK3R agonists demonstrate possible effects of NKB, they provide no information on the role of endogenous NKB. The simplest approach to address the latter question in large animals is with the use of receptor antagonists, but this technique is complicated because the available NK3R antagonists, such as SB222200, are insoluble in water, so DMSO is usually used as a solvent. On the other hand, the insolubility of SB222200 is not a problem if microimplants containing crystalline drug are used. Therefore, we repeated the previous experiment with local NKB treatment except we used microimplants containing SB222200 [46] and observed that this NK3R antagonist disrupted episodic LH secretion when placed in the ARC of OVX ewes (Fig. 9). The duration of this effect was variable, ranging from 84 min to at least 240 min, the duration of sampling after the start of treatment (Fig. 9). The reason for this variability in effectiveness is not clear as there was no obvious correlation with location of microimplantation site.
Fig. 9.
Effects of empty microimplants (open bars, top panels), or microimplants containing the NK3R antagonist, SB222200 (shaded bars, bottom panels) placed in the ARC on LH pulse patterns in two individual OVX ewes. Solid circles depict peaks of identified LH pulses. Individual animals selected to illustrate variation in duration of effects of the antagonist. Reprinted from Goodman et al. [46].
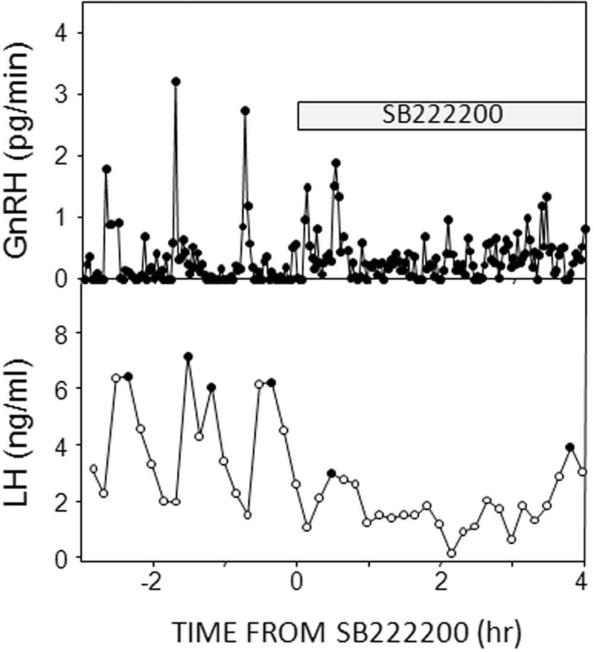
We have also begun to examine the effects of SB222200 on GnRH secretion into the hypophysial portal circulation. This is technically challenging because the ewes have to be heparinized for portal blood collection so placement of microimplants into tissue is likely to cause a hemorrhagic lesion in the site of interest. However, by placing microimplants just to the tip of the guide tube we can overcome this problem. In preliminary work, we have so far examined the effects of SB222200 by sampling hypophysial portal blood every 2 min from 3 hrs before to 4 hrs after insertion of microimplants in one ewe (Fig. 10). Assessment of LH pulses in jugular samples collected simultaneously at 10 min intervals, confirmed that SB222200 disrupted episodic secretion of this hormone as in previous work. Similarly, three clear GnRH pulses were evident before start of SB222200 treatment, but after treatment a more erratic pattern of GnRH release occurred. The initial GnRH pulse after the start of SB222200 appeared to be biphasic, and after that synchronous GnRH release was no longer evident. These preliminary results, if confirmed by future work, will provide strong evidence that the actions of endogenous NKB in the ovine ARC are critical for the synchronization of GnRH secretion during episodic secretion.
Fig. 10.
Effects of ARC microimplants containing the NK3R antagonist, SB222200 (shaded bar), on GnRH secretion rates into hypophysial portal blood collected every 2 min (top panel), and LH concentrations in jugular blood collected every 10 min (bottom panel).
Interactions of NKB and other KNDy peptide
Interactions with kisspeptin
The absence of NK3R in ovine GnRH neurons [49] indicates that any effects of NKB must be mediated by another transmitter, and the initial model (Fig. 7) proposed that NKB stimulated GnRH release via kisspeptin. Although there is no direct evidence in sheep that this mediator is kisspeptin, the induction of Fos in KNDy neurons by an icv infusion of NKB that stimulated LH release [62] is consistent with this hypothesis. Moreover, there is strong evidence that genetic [63] or pharmacological [64] disruption of kisspeptin signaling blocks the stimulatory actions of NKB in mice and monkeys, respectively. It thus seems reasonable to assume this is also the case in sheep, although this should be tested with a Kiss1r antagonist.
The stimulatory actions of kisspeptin on GnRH release most likely occur at both GnRH cell bodies and terminals. As noted above, there is anatomical support for KNDy projections to cell bodies in the POA, and AHA, but the highest level of innervation is to MBH GnRH neurons [39] that are also selectively activated (based on Fos expression) when episodic LH secretion is stimulated in sheep [65]. Similarly, kisspeptin projections to the median eminence [9, 40] may also be involved because kisspeptin can stimulate GnRH release from explants of ovine median eminence tissue [40].
Although it was initially proposed that kisspeptin has no effect on ovine KNDy neurons because they do not contain Kiss1r [40], we observed that local administration of a Kiss1r antagonist to the ARC consistently inhibits LH pulse frequency [46]. Thus it appears that endogenous kisspeptin does play a role in stimulating the activity of the KNDy neural network, but this must be an indirect effect via non-KNDy, Kiss1r-positive neurons found in the ovine ARC. Consequently, we have added a kisspeptin-responsive interneuron to our model (Fig. 11) and propose that these neurons are stimulated by kisspeptin released from KNDy neurons and act in concert with NKB to stimulate KNDy release of kisspeptin onto GnRH neurons. Interestingly, a similar inhibitory effect of a Kiss1r antagonist was observed in OVX rats [66] so that endogenous kisspeptin appears to have similar effects in the ARC of sheep and rats.
Fig. 11.
Modified model for control of KNDy neural activity proposed to drive episodic GnRH secretion in sheep and goats. Each GnRH pulse is initiated by NKB (purple) acting within the KNDy network (within dashed oval), which stimulates kisspeptin (green) release to drive GnRH (blue) secretion and activates unidentified Kiss1r-containing ARC neurons (grey) that reinforce the stimulatory actions of NKB on KNDy neurons. GnRH release is then terminated by dynorphin (red) release from KNDy neurons acting either directly on KNDy neurons and/or the unidentified Kiss1r-containing neurons. Note that the color in each terminal indicates the biologically active transmitter (possibly due to selective expression of post-synaptic receptors), not selective transport of that peptide to the terminal.
Interactions with dynorphin
The interactions of NKB and dynorphin in control of GnRH secretion remain largely unexplored, particularly when compared to the variety of studies that have explored the interactions of NKB and kisspeptin. As noted above, there is strong evidence that endogenous opioid tone terminates each GnRH pulse and limits the amount of GnRH released during the secretory phase of the pulse [58], but the opioid involved remains to be definitely determined. The ability of icv administration of a KOR antagonist to increase bursts of MUA in goats [22] is consistent with an important role for dynorphin and the same antagonist increased LH pulse frequency when given into the ARC of OVX ewes [46]. Thus endogenous dynorphin acts in the ARC to hold MUA and GnRH pulse frequency in check. However, whether dynorphin acts directly on KNDy neurons or via the Kiss1r-containing interneurons cannot be determined until we are able to identify KOR at the cellular level in ewes. Moreover, the mechanisms responsible for controlling dynorphin release from KNDy neurons, including whether it is released simultaneously with, or slightly after, NKB remain unknown. This is a difficult issue to address, although determining whether NKB and dynorphin are present in the same or different secretory vesicles within presynaptic terminals would help differentiate between these possibilities.
Conclusion and future directions
There is now strong evidence that the actions of NKB in the ARC of the ewe are important for episodic GnRH secretion and that, specifically, an increase in endogenous NKB initiates each GnRH pulse by stimulating ARC KNDy neurons to release kisspeptin onto GnRH neurons. Whether NKB plays similar roles in other species is less clear.
The infertility induced by mutations that disrupt NKB signaling [25] indicates a critical role for this neuropeptide in humans, but the concept of KNDy neurons has been questioned because men have few dynorphin-ir cells [24]. However, there are alternate explanations for this observation that are consistent with the presence of KNDy neurons in men. First, loss of antigen during the post-mortem period from this tissue could explain both the low expression of dynorphin and that only a third of NKB-ir cells also contained kisspeptin. In contrast, 95% of NKB-ir somata contained kisspeptin in another study from this group [67], and all NKB-positive neurons in freshly-fixed ARC tissue from male monkeys contained kisspeptin [38]. Second, there is a marked sex difference this population, with men having far fewer kisspeptin-containing cells than women [67]. As described earlier, a similar sexual dimorphism is observed in sheep and has been described for dynorphin as well as kisspeptin and NKB [18] so it is likely that this too contributes to the low number of dynorphin-ir cells in men. In this regard, it should be noted that dynorphin-producing cells are readily detectable in the ARC of young women using in situ hybridization [68], and these cells also likely produce both kisspeptin and NKB [23, 69]. In light of the strong evidence for the presence of KNDy neurons in women, we think it is premature to rule out their existence in men at this time.
Although there is strong evidence that NKB is critical for GnRH secretion in humans and sheep, its role in rodents is less clear because inactivating mutations of NK3R do not produce infertility in mice [70], like they do in humans [25]. Moreover, in contrast to their effects in sheep (Fig. 9), NK3R antagonists have no effect on episodic LH secretion in rats [71, 72], or on the ability of NKB to stimulate electrical activity in murine KNDy neurons in slice preparations [73]. Interestingly, recent data raise the possibility that these species differences may reflect more redundancy in tachykinin signaling in rodents. Thus LH pulses were inhibited by blockade of all three tachykinin receptors (NK1R, NK2R, and NK3R) in rats [72], as were the stimulatory effects of NKB on electrical activity of murine KNDy neurons [73]. Thus NKB appears to be able to act via all three tachykinin receptors in rodents. In addition, the other two tachykinins (substance P and neurokinin A) may be able to replace NKB because they stimulated the electrical activity of KNDy neurons in vitro [73].
While considerable work in ewes, and other species, has focused on the possible role of NKB and KNDy neurons in pulse generation, there is less information on other physiological roles of NKB. However, mounting evidence supports a role for KNDy neurons in mediating the ability of exposure to males to stimulate episodic LH secretion in female sheep and goats [74]. This effect, which is primarily pheromonal in nature [75], has been known for many years and is often employed to induce breeding out of season [76]. Elegant work in goats reviewed by Okamura et al. has demonstrated that brief exposure to male odors induces a burst in MUA (presumably from KNDy neurons) within seconds [77]. Moreover, ram exposure increased Fos expression in KNDy neurons in the ARC of ewes and a Kiss1r antagonist inhibited the ram-induced increase in LH pulses [78]. Thus ARC kisspeptin has been implicated in the increased GnRH release caused by male sociosexual stimuli, but whether NKB is also involved remains to be determined.
There is less evidence for other possible reproductive functions for NKB. For example, NKB may be involved in the initiation of puberty [33], and the inhibitory effects of estradiol on NKB expression [11] raise the possibility that it may be involved in steroid negative feedback in adult ewes. Similarly, decreased activity of KNDy neurons has been implicated in the inhibitory effects of photoperiod [30], lactation [20], undernutrition [47], and stress [79] on reproductive function. Determining whether these proposed roles simply reflect the importance of episodic GnRH secretion to these processes, and assessing the relative roles and interactions of NKB with kisspeptin requires further work. Similarly, because most work has focused on the actions of NKB in the ARC, the roles (if any) of NK3R expression elsewhere in the hypothalamus and POA (Fig. 4) in control of LH secretion remain unknown, although we have evidence that activation of NK3R in the RCh stimulates LH secretion in the ewe [61]. Finally the biological significance of the sexual dimorphism in NKB expression remains an unanswered question, although this characteristic was identified in the first study on the NKB system in the ewe [4]. It is interesting to note that a prenatal androgen treatment that masculinizes many neuroendocrine systems induces this sexual dimorphism in NKB cell number, but has no effect on the number of ARC neurons expressing kisspeptin [18]. Thus, although ARC kisspeptin and NKB are often tightly coupled, there are instances when the two can be readily distinguished, suggesting that they may well have distinct functions in the control of GnRH secretion.
Finally, workers are just beginning to explore non-reproductive functions of NKB and KNDy neurons. Recent work suggests an important role for these neurons in mediating the inhibitory actions of estradiol on body weight in rats [80], but whether this applies to sheep or other species is unknown. Perhaps more interestingly, the effects of senktide on temperature regulatory systems in the POA of rats [81] have led to the hypothesis that NKB from KNDy neurons may explain the well-established temporal link between hot flushes and episodic LH secretion in post-menopausal women [82]. If this proves to be the case, one could infer that KNDy neurons are very likely part of the GnRH pulse generator in women.
Acknowledgements
We thank our many students and colleagues who are responsible for much of the work described in this review (see original citations). This work was supported by grants from NIH (R01-HD039916, RO1HD017854).
Footnotes
The authors have nothing to declare
References
- 1.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-b and substance-p messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 2.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. The Journal of clinical endocrinology and metabolism. 1999;84:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 3.Rance NE, Bruce TR. Neurokinin b gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 4.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin b-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141:4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- 5.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- 6.Dufourny L, Skinner DC. Progesterone receptor, estrogen receptor alpha, and the type ii glucocorticoid receptor are coexpressed in the same neurons of the ovine preoptic area and arcuate nucleus: A triple immunolabeling study. Biology of reproduction. 2002;67:1605–1612. doi: 10.1095/biolreprod.102.005066. [DOI] [PubMed] [Google Scholar]
- 7.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta- endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the suffolk ewe. Endocrinology. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- 8.Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide y, and beta-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology. 1997;138:2585–2595. doi: 10.1210/endo.138.6.5208. [DOI] [PubMed] [Google Scholar]
- 9.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin b immunoreactivity in the arcuate nucleus and median eminence of the sheep. Journal of neuroendocrinology. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 10.Foster DL, Padmanabhan V, Wood RI, Robinson JE. Sexual differentiation of the neuroendocrine control of gonadotrophin secretion: Concepts derived from sheep models. Reprod Suppl. 2002;59:83–99. [PubMed] [Google Scholar]
- 11.Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin b mrna expression in the infundibular nucleus of ewes. Journal of neuroendocrinology. 2003;15:749–753. doi: 10.1046/j.1365-2826.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- 12.McManus CJ, Valent M, Connors JM, Goodman RL, Lehman MN. A neurokinin b agonist stimulates lh secretion in follicular, but not luteal phase. Ewes. Program, Annual meeting of the Society for Neuroscinence. 2005 Abtr 760.768. [Google Scholar]
- 13.Sandoval-Guzman T, Rance NE. Central injection of senktide, an nk3 receptor agonist, or neuropeptide y inhibits lh secretion and induces different patterns of fos expression in the rat hypothalamus. Brain research. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The gpr54 gene as a regulator of puberty. The New England journal of medicine. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 15.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the kiss1-derived peptide receptor gpr54. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neuroscience letters. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin a and neurokinin b. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 18.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin b/dynorphin (kndy) cell population of the arcuate nucleus: Sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin b immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. The Journal of comparative neurology. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 20.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin b neuronal projections and regulation during lactation in the rat. Journal of neuroendocrinology. 2011;23:52–64. doi: 10.1111/j.1365-2826.2010.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin b neurons in the arcuate nucleus of the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin b and dynorphin a in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rance NE. Menopause and the human hypothalamus: Evidence for the role of kisspeptin/neurokinin b neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hrabovszky E, Sipos MT, Molnar CS, Ciofi P, Borsay BA, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin b, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the kndy neuron concept. Endocrinology. 2012;153:4978–4989. doi: 10.1210/en.2012-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. Tac3 and tacr3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin b in the central control of reproduction. Nature genetics. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonca BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. Tac3/tacr3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin b in neonatal life followed by reversal in adulthood. The Journal of clinical endocrinology and metabolism. 2010;95:2857–2867. doi: 10.1210/jc.2009-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/neurokinin b/dynorphin (kndy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying gnrh pulse generation by the hypothalamus. Brain research. 2010;1364:103–115. doi: 10.1016/j.brainres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin b and the hypothalamic regulation of reproduction. Brain research. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and rfamide-related peptide (rfrp) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: A novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman RL, Maltby MJ, Millar RP, Hileman SM, Nestor CC, Whited B, Tseng AS, Coolen LM, Lehman MN. Evidence that dopamine acts via kisspeptin to hold gnrh pulse frequency in check in anestrous ewes. Endocrinology. 2012;153:5918–5927. doi: 10.1210/en.2012-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150:5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- 33.Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin b in puberty of female sheep. Endocrinology. 2012;153:2756–2765. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JT, Clay CM, Caraty A, Clarke IJ. Kiss-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 35.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized gnrh pulse generator activity among kisspeptin/neurokinin b/dynorphin a (kndy) neurons in goats. The Journal of reproduction and development. 2013;59:40–48. doi: 10.1262/jrd.2012-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin b neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin b stimulates gnrh release in the male monkey (macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: Comparative and developmental aspects. Advances in experimental medicine and biology. 2013;784:27–62. doi: 10.1007/978-1-4614-6199-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory lh surge and stimulates gnrh release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 41.Dufourny L, Caraty A, Clarke IJ, Robinson JE, Skinner DC. Progesterone-receptive beta-endorphin and dynorphin b neurons in the arcuate nucleus project to regions of high gonadotropin-releasing hormone neuron density in the ovine preoptic area. Neuroendocrinology. 2005;81:139–149. doi: 10.1159/000086527. [DOI] [PubMed] [Google Scholar]
- 42.Pompolo S, Rawson JA, Clarke IJ. Projections from the arcuate/ventromedial region of the hypothalamus to the preoptic area and bed nucleus of stria terminalis in the brain of the ewe; lack of direct input to gonadotropin-releasing hormone neurons. Brain research. 2001;904:1–12. doi: 10.1016/s0006-8993(01)02372-1. [DOI] [PubMed] [Google Scholar]
- 43.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin b modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. The Journal of comparative neurology. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 44.Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- 45.Merkley CM, Coolen LM, Padmanabhan V, Jackson LM, Goodman RL, Lehman MN. Evidence for transcriptional activation of arcuate kisspeptin neurons, and glutamatergic input to kisspeptin during the preovulatory gnrh surge of the sheep. 91st Annual meeting of the Endocrine Society. 2009:P3–220. [Google Scholar]
- 46.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy S, Millar R, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin b, and dynorphin act in the arcuate nucleus to control activity of the gnrh pulse generator in ewes. Endocrinology. 2013 doi: 10.1210/en.2013-1331. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tena-Sempere M. Kiss-1 and reproduction: Focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83:275–281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- 48.Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG., Jr. Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology. 2011;152:2302–2310. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: Colocalisation in neurokinin b cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. Journal of neuroendocrinology. 2010;22:1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. Journal of neuroendocrinology. 2009;21:813–821. doi: 10.1111/j.1365-2826.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- 53.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of kiss-1 mrna in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 54.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sannella MI, Petersen SL. Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for mu, kappa, or delta opiate receptors. Endocrinology. 1997;138:1667–1672. doi: 10.1210/endo.138.4.5091. [DOI] [PubMed] [Google Scholar]
- 56.Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149:1004–1008. doi: 10.1210/en.2007-1505. [DOI] [PubMed] [Google Scholar]
- 57.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130:503–510. doi: 10.1210/endo.130.1.1727719. [DOI] [PubMed] [Google Scholar]
- 58.Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136:2412–2420. doi: 10.1210/endo.136.6.7750462. [DOI] [PubMed] [Google Scholar]
- 59.Wormser U, Laufer R, Hart Y, Chorev M, Gilon C, Selinger Z. Highly selective agonists for substance p receptor subtypes. The EMBO journal. 1986;5:2805–2808. doi: 10.1002/j.1460-2075.1986.tb04571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regoli D, Boudon A, Fauchere JL. Receptors and antagonists for substance p and related peptides. Pharmacological reviews. 1994;46:551–599. [PubMed] [Google Scholar]
- 61.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin b acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151:3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakamoto K, Murata K, Wakabayashi Y, Yayou KI, Ohkura S, Takeuchi Y, Mori Y, Okamura H. Central administration of neurokinin b activates kisspeptin/nkb neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. The Journal of reproduction and development. 2012;58:700–706. doi: 10.1262/jrd.2011-038. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenrohr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin b, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153:316–328. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 64.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (macaca mulatta) for the view that the action of neurokinin b to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology. 1999;140:5929–5936. doi: 10.1210/endo.140.12.7216. [DOI] [PubMed] [Google Scholar]
- 66.Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O'Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates gnrh pulse generator frequency in the rat. PloS one. 2009;4:e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: Sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin b neurons. The European journal of neuroscience. 2010;31:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- 68.Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. Journal of neuroendocrinology. 2008;20:1376–1381. doi: 10.1111/j.1365-2826.2008.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. The Journal of clinical endocrinology and metabolism. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 70.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin b receptor null mice: Closing the gap between mice and men. Endocrinology. 2012;153:1498–1508. doi: 10.1210/en.2011-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O'Byrne KT. Suppression of the gnrh pulse generator by neurokinin b involves a kappa-opioid receptor-dependent mechanism. Endocrinology. 2012;153:4894–4904. doi: 10.1210/en.2012-1574. [DOI] [PubMed] [Google Scholar]
- 72.Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. The Journal of reproduction and development. 2011;57:409–415. doi: 10.1262/jrd.11-002s. [DOI] [PubMed] [Google Scholar]
- 73.de Croft S, Boehm U, Herbison AE. Neurokinin b activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013 doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- 74.Hawken PA, Martin GB. Sociosexual stimuli and gonadotropin-releasing hormone/luteinizing hormone secretion in sheep and goats. Domestic animal endocrinology. 2012;43:85–94. doi: 10.1016/j.domaniend.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Gelez H, Fabre-Nys C. Role of the olfactory systems and importance of learning in the ewes' response to rams or their odors. Reproduction, nutrition, development. 2006;46:401–415. doi: 10.1051/rnd:2006021. [DOI] [PubMed] [Google Scholar]
- 76.Ungerfeld R, Forsberg M, Rubianes E. Overview of the response of anoestrous ewes to the ram effect. Reproduction, fertility, and development. 2004;16:479–490. doi: 10.10371/RD04039. [DOI] [PubMed] [Google Scholar]
- 77.Okamura H, Murata K, Sakamoto K, Wakabayashi Y, Ohkura S, Takeuchi Y, Mori Y. Male effect pheromone tickles the gonadotrophin-releasing hormone pulse generator. Journal of neuroendocrinology. 2010;22:825–832. doi: 10.1111/j.1365-2826.2010.02037.x. [DOI] [PubMed] [Google Scholar]
- 78.De Bond JA, Li Q, Millar RP, Clarke IJ, Smith JT. Kisspeptin signaling is required for the luteinizing hormone response in anestrous ewes following the introduction of males. PloS one. 2013;8:e57972. doi: 10.1371/journal.pone.0057972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grachev P, Li XF, O'Byrne K. Stress regulation of kisspeptin in the modulation of reproductive function. Advances in experimental medicine and biology. 2013;784:431–454. doi: 10.1007/978-1-4614-6199-9_20. [DOI] [PubMed] [Google Scholar]
- 80.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin b/dynorphin (kndy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152:4894–4905. doi: 10.1210/en.2011-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casper RF, Yen SS, Wilkes MM. Menopausal flushes: A neuroendocrine link with pulsatile luteninizing hormone secreation. Science. 1979;205:823–825. doi: 10.1126/science.462193. [DOI] [PubMed] [Google Scholar]