Abstract
Background
Scientific enthusiasm about gene x environment interactions, spurred by the 5-HTTLPR (serotonin transporter polymorphic-region) x SLEs (stressful life events) interaction predicting depression, have recently been tempered by sober realizations of small effects and meta-analyses reaching opposing conclusions. These mixed findings highlight the need for further research. Converging evidence suggests that the effects of 5-HTTLPR genotype may be neurodevelopmental in origin but we are not aware of empirical studies that have investigated whether the 5-HTTLPR genotype x SLE interaction predicts preschool-onset depression (PO-MDD), the earliest validated form of depression.
Methods
Children (n = 234) aged 3–5 were recruited for a longitudinal study designed to examine PO-MDD. In a comprehensive baseline assessment, the child’s primary caregivers completed questionnaires and were interviewed about their child’s behaviors, psychiatric symptoms, and exposure to SLEs.
Results
A 5-HTTLPR x SLEs interaction emerged, such that children homozygous for the short allele were more susceptible to depression in the context of elevated SLE than long allele carriers. In contrast, at low SLE exposure, short allele homozygotes had fewer depressive symptoms. The data were best fit by a plasticity model with a substantial reduction in fit by diathesis-stress models.
Conclusions
Extending studies in adult and adolescent populations, these data suggest that 5-HTTLPR genotype may provide plasticity to environmental influence, for better or worse. Specifically, children homozygous for the short allele were more susceptible to the depressogenic effects of SLEs but benefitted, in the form of reduced depressive symptoms, in the context of relatively benign environmental conditions (i.e., relatively low SLE exposure). These data highlight the importance of examining gene x environment interactions across development, environment, and outcome but should be interpreted cautiously given the small sample size.
Keywords: Depression, stress, 5-HTTLPR, serotonin, gene*, interaction, plasticity, childhood, development, gene x environment
Introduction
A small but growing body of literature suggests that clinically significant depressive symptoms can arise in children as young as 3 years old (Luby et al., 2002). Such early-onset depression occurs in 1–2% of children but relatively little is known about risk factors or whether there is etiologic convergence across depression based on age of onset (Egger & Angold, 2006; Wichstrom et al., 2012). Because preschool depression may reflect a more severe and chronic form, and treatment and prevention efforts may be more successful when applied early, it is important to identify individual differences that predict its development (Luby, 2010; Zisook et al., 2007). A logical starting place is to evaluate whether factors predicting adult- and adolescent-onset depression, also predict preschool-onset childhood depression.
In addition to genetic variation (Sullivan, Neale, & Kendler, 2000), little doubt remains that stress, particularly when occurring early in life, is amongst the strongest predictors of MDD (Hammen, 2005). Vulnerability to depression has predominantly been conceptualized within a diathesis-stress framework in which diatheses, or individual differences such as cognitive biases or genetic background, promote the development of depression in the context of stress. One of the earliest and arguably most controversial illustrations of the diathesis-stress model showed that serotonin transporter-linked-polymorphic-region (5-HTTLPR) genotype moderates the depressogenic effects of stressful life events (SLEs) (Caspi et al., 2003). Specifically, this report demonstrated that the short allele of the 5-HTTLPR (which has been linked to relatively reduced serotonin transporter expression as well as putative intermediate depressive phenotypes (Hariri et al., 2002; Lesch et al., 1996) confers vulnerability to stress-related MDD relative to the long allele. The intuitive appeal of this hypothesis led to multiple replication attempts producing conflicting findings (Gillespie, Whitfield, Williams, Heath, & Martin, 2005; Kendler, Kuhn, Vittum, Prescott, & Riley, 2005). Recent meta-analyses have weighed heavily against evidence for the 5-HTTLPR x SLEs interaction (Duncan & Keller, 2011; Munafo, Durrant, Lewis, & Flint, 2009; Risch et al., 2009), although opinions continue to diverge (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Karg, Burmeister, Shedden, & Sen, 2011), urging further research.
As the center of a maelstrom of controversy, the overwhelming majority of depression studies have sought to replicate rather than extend the 5-HTTLPR x SLEs interaction. For example, despite emerging evidence that the effects of 5-HTTLPR genotype on depression may be driven by its influence on neurodevelopment (Caspi et al., 2010; Hariri & Holmes, 2006), to our knowledge, no human research has evaluated the 5-HTTLPR x SLEs interaction outside of adolescent- or adult-onset depression. The importance of examining the developmental trajectory of this relationship is further underscored in light of evidence that SLEs are a strong predictor of early depressive episodes, but are less predictive of its future recurrence (Kendler, Thornton, & Gardner, 2000; Stroud, Davila, & Moyer, 2008). This is broadly consistent with the kindling hypothesis which posits that the earliest episodes of depression might be more sensitive to environmental adversity whereas, via kindling, subsequent episodes are more greatly contingent on prior depressive episodes and less reliant on environmental provocation (Kendler et al., 2000; Post, 1992). These studies suggest a developmental rubric underpinning the interface of stress and diathesis for MDD, potentially with a more pronounced impact of their interaction earlier in development.
The goal of the present investigation was to examine whether 5-HTTLPR genotype moderates the association between SLEs and preschool-onset depression. Consistent with the literature, initially, we hypothesized that the short allele would be associated with increased risk for stress-related depression relative to the long allele. However, emergent theoretical work suggesting that diatheses may provide differential susceptibility to the environment, for better or worse, as opposed to vulnerability to negative outcomes in the context of adversity, has refined the diathesis-stress model and begun to accumulate empirical support (Belsky & Pluess, 2009; Belsky, Pluess, & Widaman, 2013; Hankin et al., 2011; Pluess, Belsky, Way, & Taylor, 2010). In light of this evidence, we further hypothesized that the short allele of the 5-HTTLPR polymorphism might produce benefits with regard to depression when SLE exposure was low, providing plasticity to environmental influence, for better or worse, as opposed to vulnerability to adversity soley.
Methods
Participants
Children between the ages of 3.0 and 5.11 were recruited from community daycare, preschool. and primary care sites throughout the Saint Louis area for participation in a longitudinal study designed to examine preschool-onset depression. Recruitment sites were chosen at random using a geographically stratified method to best approximate the composition of the Saint Louis community. To recruit a large group of depressed children (see Luby et al., 2009 for more detail regarding the recruitment of this sample), as well as smaller groups of healthy children and those with other psychiatric disorders for comparison, a validated screening checklist, The Preschool Feelings Checklist (PFC) (Luby, Heffelfinger, Koenig-McNaught, Brown, & Spitznagel, 2004) was completed by caregivers. Children with a PFC score of ≥ 3 were oversampled because a PFC score ≥ 3 has high sensitivity and specificity for the diagnosis of depression. Approximately 6,000 checklists were distributed to sites between May 2003 and March 2005; 1,474 were returned. Of these returned checklists, 899 children met all inclusion criteria and were contacted by phone for further screening. Preschoolers with chronic medical illness, marked speech and language delays. and/or neurologic or Autistic Spectrum Disorders were excluded. Those without exclusions (N=416) were invited for study participation and N=305 (i.e., 73%) agreed and completed the baseline wave of the study. The longitudinal study is currently ongoing and the data in the present manuscript reflect baseline data. Genetic data, via saliva samples, was collected at later study waves from 234 participants whom make up the sample of the present report (Table 1); additional genotyping for more subjects within the PDS longitudinal sample is not presently planned. The study was approved by the Institutional Review Board at Washington University in St. Louis School of Medicine. Caregivers provided written informed consent and children provided verbal consent.
Table 1.
Demographic and diagnostic characteristics of the sample
| S/S (N=56)
|
S/L (N=117)
|
L/L (N=61)
|
χ2 | p | ||||
|---|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | |||
| T1 age | 5.47 | 0.2426 | ||||||
| 3 years | 39.3 | 22 | 23.9 | 28 | 29.5 | 18 | ||
| 4 years | 37.5 | 21 | 47.9 | 56 | 37.7 | 23 | ||
| 5 years | 23.2 | 13 | 28.2 | 33 | 32.8 | 20 | ||
| Gender | 3.17 | 0.2048 | ||||||
| Male | 44.6 | 25 | 55.6 | 65 | 60.7 | 37 | ||
| Female | 55.4 | 31 | 44.4 | 52 | 39.3 | 24 | ||
| Ethnicity | 4.69 | 0.3202 | ||||||
| Caucasian | 50.0 | 28 | 58.1 | 68 | 57.4 | 35 | ||
| African American | 35.7 | 20 | 26.5 | 31 | 36.1 | 22 | ||
| Other | 14.3 | 8 | 15.4 | 18 | 6.6 | 4 | ||
| T1 total family income | 4.59 | 0.5974 | ||||||
| <$20,000 | 24.5 | 14 | 20.6 | 22 | 19.6 | 11 | ||
| $20,001–$40,000 | 16.4 | 9 | 19.6 | 21 | 26.8 | 15 | ||
| $40,001–$60,000 | 23.6 | 13 | 15.9 | 17 | 14.3 | 8 | ||
| >$60,000 | 34.5 | 19 | 43.9 | 47 | 39.3 | 22 | ||
| T1 parental education | 4.24 | 0.6445 | ||||||
| High school or less | 21.4 | 12 | 11.1 | 13 | 18.0 | 11 | ||
| Some college | 37.5 | 21 | 43.6 | 51 | 37.7 | 23 | ||
| College degree | 14.3 | 8 | 19.7 | 23 | 19.7 | 12 | ||
| Graduate education | 26.8 | 15 | 25.6 | 30 | 24.6 | 15 | ||
| T1 psychiatric disorders | ||||||||
| MDD | 17.9 | 10 | 30.8 | 36 | 26.2 | 16 | 3.25 | 0.1974 |
| ADHD | 21.4 | 12 | 14.5 | 17 | 13.1 | 8 | 1.80 | 0.4056 |
| Oppositional defiant | 26.8 | 15 | 25.6 | 30 | 23.0 | 14 | 0.25 | 0.8823 |
| Conduct | 12.5 | 7 | 12.8 | 15 | 9.8 | 6 | 0.36 | 0.8357 |
| Generalized anxiety | 7.1 | 4 | 6.8 | 8 | 8.2 | 5 | F.E. | 0.9460 |
| Separation anxiety | 10.7 | 6 | 19.7 | 23 | 19.7 | 12 | 2.36 | 0.3072 |
| PTSD | 1.8 | 1 | 1.7 | 2 | 3.3 | 2 | F.E. | 0.8413 |
| Mania | 7.1 | 4 | 9.4 | 11 | 11.5 | 7 | 0.64 | 0.7249 |
| Mean | SD | Mean | SD | Mean | SD | F | p | |
|
|
||||||||
| N T1core MDD symptoms | 2.29 | 1.71 | 2.66 | 1.89 | 2.48 | 1.70 | 0.84 | 0.4344 |
ADHD = Attention-deficit/hyperactivity disorder; F.E. = Fisher’s Exact test; MDD = Major depressive disorder; PTSD = Post-traumatic stress disorder
Assessment of Preschool-Onset Childhood Depression and Stressful Life Events
At baseline, children and their caregivers participated in a 3–4 hour comprehensive laboratory assessment during which primary caregivers (92% biological mothers) were interviewed about their child’s behaviors, emotions, and psychiatric symptoms, as well as the child’s exposure to stressful and traumatic life events using the Preschool Age Psychiatric Assessment (PAPA). The PAPA is an interviewer based diagnostic assessment with empirically established test re-test reliability designed for use in caregivers of children aged 2.0–6.0 (Egger et al., 2006). Interviewers were trained to reliability administer the PAPA and audio was recorded for quality control. Details of reliability and quality control have been previously reported (Luby, Si, Belden, Tandon, & Spitznagel, 2009). In addition to generating categorical PO-MDD diagnosis using a DSM-IV computer algorithm which sets aside the duration criterion based on empirical data suggesting it may not be applicable in young children (Gaffrey, Belden, & Luby, 2011), the number of depressive symptoms was also summed to provide a continuous measure of depression severity (Gaffrey, Luby, et al., 2011; Luby, Mrakotsky, Heffelfinger, Brown, & Spitznagel, 2004). Traumatic and stressful life events were also measured by the PAPA which has established reliability for these assessments (Costello, Angold, March, & Fairbank, 1998). At baseline assessment primary caregivers were asked about the preschoolers experience of stressful and traumatic events over their lifetime. Life events were categorized into 15 categories and the total frequency of events was used for analyses (Table 2).
Table 2.
Stressful life events and their occurrence in the sample
| Stressful life events | % | N |
|---|---|---|
| Change in daycare/school | 43.6 | 102 |
| Death of a pet | 22.2 | 52 |
| Lives/attends daycare/school in unsafe environment | 1.3 | 3 |
| Loss of home without family separation | 1.7 | 4 |
| Lost significant person through moving | 14.5 | 34 |
| Marital family conflict | 3.0 | 7 |
| Moving house | 56.4 | 132 |
| New child in home | 56.4 | 132 |
| New parental figure | 15.8 | 37 |
| Parental arrest | 8.1 | 19 |
| Parental divorce | 6.8 | 16 |
| Parental hospitalization | 42.7 | 100 |
| Parental separation | 23.5 | 55 |
| Reduction in standard of living | 6.0 | 14 |
| Separation from parent (1 week or more) | 32.9 | 77 |
Genotyping
5-HTTLPR and the 5-HTT SNP at rs25531 were genotyped according to established procedures. Saliva was collected using oragene collection kits from which DNA was extracted using the manufacturer’s protocol (www.dnagenotek.com). A PCR protocol (Forward primer: 5′-TGAATGCCAGCACCTAACCC-3′, Reverse primer: 5′GTGCCACCTAGACGCCAGG-3′) was used to amplify a 400 (S) or 444bp (L) region. The amplification was followed by a restriction enzyme digestion with MspI (New England Biolabs, Beverly, MA) to determine the Sa, Sg, La and Lg alleles. The digestion products were electrophoresed on a 2.5% agarose gel, stained with ethidium bromide and visualized under UV light. Participants were grouped as S homozygotes (i.e., SS), heterozygotes (i.e., SL), or L homozygotes (i.e., LL; Table 1). When the L allele was present alongside the G allele of the rs25531 SNP, it was re-categorized as an S allele due to functional and clinical evidence that the presence of the G allele at the SNP within the long allele results in S-like function.(Wendland, Martin, Kruse, Lesch, & Murphy, 2006; Zalsman et al., 2006). Sixty individuals in the present sample were recategorized in this manner. Alleles were within HWE for the 5-HTTLPR and rs25531 within long alleles (χ2 < 2.27; ps > .13).
Statistical Analysis
Prior to all data analyses, SLE outliers were winsorized; specifically, values that were greater than 3 standard deviations (SDs) above or below the mean were set to the closest observed value within 3 SDs of the mean. In an effort to diminish potential artifactual scaling effects (Eaves, 2006), the number of MDD symptoms was natural log transformed (after adding 1 to all subjects to account for those who endorsed 0 symptoms) because of its positively skewed distribution. A linear and logistic regression were used to assess the main effects of 5-HTTLPR genotype and stressful life events, as well as the 5-HTTLPR genotype x stressful life event interaction, on the number of log transformed depressive symptoms and a diagnosis of preschool-onset depression, respectively. The PROCESS macro was used for analyses and predictor variables were mean centered prior to the computation of interaction terms and analyses (Hayes, in press). Age, gender, and ethnicity (i.e., European American/not European American; African American/not African American) were included as covariates in analyses. Post-hoc testing for significant interactions was conducted by using simple slope testing and Johnson-Neyman tests, which identify the values on the continuum of a moderator (in this case, stressful life events) at which point the effect of a predictor variable (i.e., in this case, 5-HTTLPR genotype) transitions between statistical significance in either direction along this continuum (Roisman et al., 2012).
Results
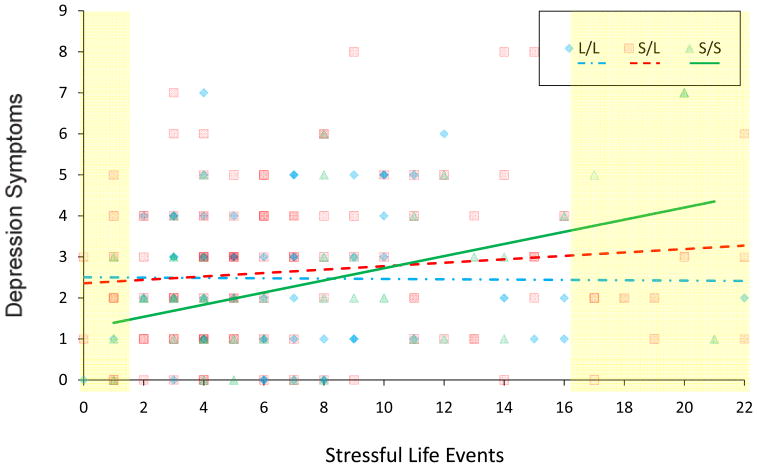
There was no evidence for main effects of 5-HTTLPR genotype on number of depression symptoms (B= −0.03, SE= 0.05 p = 0.63), MDD diagnosis (B= −0.03, SE=0.41 p=.41) or the number of stressful life events (B=0.13, SE=0.34 p=.69). Number of stressful life events was associated with depressive symptoms at a trend level (B=0.02, SE=0.01 p=0.09), but only in the absence of the interaction term. Including the 5-HTTLPR genotype x SLE interaction in the models produced significant interactions for both the number of depression symptoms, ΔF(1,226) = 4.80, Δr2 = .02 p < 0.03, and MDD diagnosis, Z = 2.56, p < 0.02 (Figure 1).
Figure 1.
Number of MDD Core Symptoms by 5HTT Genotype and Stressful Life Events Frequency
Note: Depression symptoms represent raw, untransformed data. SLEs represent winsorized data. Yellow highlighted areas represent regions of significance.
Post-hoc simple-slope tests revealed a positive association between number of stressful life events and number of depressive symptoms in short (SS) homozygotes (t = 2.79, P < .006) while this association was absent in (SL) heterozygotes (t = 1.56, p < .12) and long (LL) homozygotes (t = −0.61, p > .53). Moreover, Johnson-Neyman tests revealed that the short allele was associated with increased depressive symptoms with increasing exposure to SLE (i.e., > 16.15 SLEs, all ps < .05) but decreased depressive symptoms with decreasing exposure to stressful life events (i.e., < 1.35 SEs; all ps < .05). Post hoc analyses revealed the same pattern for PO-MDD diagnosis; there was a positive association between stressful life events in short homozygotes (z = 2.51, p < .02) that was absent in heterozygotes (z = 0.56; p < .57) and long homozygotes (z = −1.55, p < .13). Moreover Johnson-Neyman tests revealed a similar pattern; at high levels of stressful life events (i.e., > 11.54 SLEs; all ps < .05), the short allele was associated with increased depression; however, at low levels of stressful life events (i.e., < 3.37 SLEs; all ps < .05), it was associated with decreased depression. Additional analyses across different genotype groupings (i.e., S carriers vs LL and L carriers vs. SS) and within European American and African American subsamples are reported on in the online appendix. Briefly, these results suggest that the present effects are driven by S homozygotes (see also Figure 1). Analyses in ethnic subsamples produce significant or trending results for each in a direction consistent with the entire sample.
To empirically test whether a diathesis stress or plasticity (differential susceptibility) model better explained the observed data, we employed recently developed model fitting procedures (Widaman et al., 2012). Specifically, we compared strong and weak versions of plasticity and diathesis-stress (Belsky et al., 2013). The strong version of each model presumes that L carrier children are not affected by early life stressful events (thus constraining their slope to 0), whereas the weaker models propose that L carriers are affected by these events (hence their slope parameter is estimated), but to a lesser degree than S homozygotes. Both models assume that S homozygotes are affected by SLEs. In the diathesis stress model, the cross-over point (i.e., the point at which the slope of L carriers and S homozygotes intersects) was constrained to be the lowest observed frequency of stressful life events in the dataset, testing the assumption that increasing stressful events would be associated with a greater number of depressive symptoms in S homozygotes and that group lines would intersect at the lowest observed SLE value. Conversely, in the plasticity model, this cross-over point parameter was allowed to vary freely, testing the assumption that SLEs would be significantly related at both ends of the continuum.
Model fitting analyses suggest these data best fit plasticity (i.e., differential susceptibility) models (r2 = 0.49 – 0.53; AIC = −272.11 – −270.94); diathesis stress models resulted in a substantial reduction of fit (r2 = 0.007 – 0.02; AIC = −263.98 – −266.05; all ps < .009; Table 3). Thus, the best fitting model was a strong plasticity model, indicating that S homozygotes were susceptible to increased and decreased depressive symptoms at both the high and low ends of SLE exposure, respectively and that L carriers were relatively unaffected by SLE.
Table 3.
Model fitting of plasticity and diathesis stress models
| Strong Plasticity (Model 1A) | Weak Plasticity (Model 1B) | Stong DS (Model 2A) | Weak DS (Model 2B) | |
|---|---|---|---|---|
| rSS | 70.635 | 70.358 | 73.783 | 72.487 |
| Parameters | 3 | 4 | 2 | 3 |
| B0 | 1.135 (0.041) | 1.148 (0.052) | 1.094 (0.040) | 1.002 (0.061) |
| B1 | 0.00 (−) | 0.010 (0.011) | 0.00 (−) | 0.021 (0.010) |
| C | 1.146 (1.206) | 1.316 (1.438) | −4.79 (−) | −4.79 (−) |
| B2 | 0.073 (0.022) | −0.073 (0.022) | 0.031 (0.015) | |
| R2 | 0.049 | 0.053 | 0.007 | 0.024 |
| F vs. 1A | 0.91 | 10.30 | ||
| F vs. 1A df | 1,230 | 1,231 | ||
| F vs. 1A p | 0.342 | 0.002 | ||
| F vs. 1B | 5.60 | 6.96 | ||
| F vs. 1B df | 2,230 | 1,230 | ||
| F vs. 1B p | 0.0042 | 0.0089 | ||
| F vs. 2A | 4.13 | |||
| F vs. 2A df | 1,231 | |||
| F vs. 2A p | 0.0433 | |||
| AICc | −272.11 | −270.94 | −263.98 | −266.05 |
rSS = residual sum of squares
Parameters = # of parameters estimated in the model
B0 = the estimated y value (i.e., log-transformed depressive symptoms) at which point the two groups cross-over or converge
B1 = the slope for the x value (i.e., mean-centered stressful life event frequency) for the long allele carrier group
C = the estimated x value (i.e., mean-centered stressful life event frequency) at which point the two groups cross-over or converge
B2 = the slope for the x value (i.e., mean-centered stressful life event frequency) for the short homozygote group
R2 = estimated amount of variance in log-transformed depressive symptoms explained by the model
F vs. 1A = an F test of the difference in R2 for the specified model in contrast to Model 1A (i.e., Strong Plasticity)
F vs. 1B = an F test of the difference in R2 for the specified model in contrast to Model 1B (i.e., Weak Plasticity)
F vs. 2A = an F test of the difference in R2 for the specified model in contrast to Model 2A (i.e., Strong Diathesis-Stress)
Values in () represent SE. (−) in SE represents a fixed parameter
AICc = Akaike’s Information Criteria with a correction for sample size
Discussion
That SLEs promote onset and exacerbate course of depression is unequivocal (Hammen, 2005). The mechanism linking SLEs to depression, however, remains an area of active research. One etiologic mechanism that is frequently explored is the extent to which genetic liability to depression modifies this relationship. For instance, in an early seminal study using data on identical and fraternal twins, Kendler and colleagues (Kendler et al., 1995) found that the risk for depression onset was elevated in individuals whose identical co-twin also had a history of depression, but only if they had themselves been exposed to a severe SLE. Almost a decade later, an initial report demonstrated that 5-HTTLPR genotype moderated the well-documented depressogenic effects of stressful life events. This influential study by Caspi and colleagues (Caspi et al., 2003) has generated numerous replication attempts that have yielded conflicting findings. Indeed, even meta-analyses have reached opposing conclusions; one recent meta-analysis that adopted an inclusive approach suggests that this effect is replicable when broadly conceptualizing SLEs (Karg et al., 2011), while another using more conservative inclusion criteria for SLE measurement, concludes it is a false positive that has been propagated by publication bias (Duncan & Keller, 2011).
Much like the original report (Caspi et al., 2003) and several replication studies in adults (Karg et al., 2011), we found that preschool-aged children homozygous for the 5-HTTLPR short allele are more susceptible to the depressogenic effects of stress relative to individuals with the long allele. Consistent with emergent data (Hankin et al., 2011; Pluess et al., 2010), post hoc and model fitting analyses provided support for a plasticity model wherein short homozygotes might benefit from benign environments and experience less depression, relative to long allele carriers, in the context of low SLE exposure. These data allude to the significance of considering the developmental framework in which this GxE interplay may operate and suggest that some non-replications may potentially arise from attenuation of the 5-HTTLPR x SLE interaction effects in later episodes of depression, possibly due to kindling effects observed for main effects of SLEs.
Similarities and Differences with the Extant Literature
The results of this study are broadly consistent with literature suggesting that the short 5-HTTLPR allele provides plasticity to the environment; however, there are some key differences in the results reported here. First, studies have predominantly reported a graded relationship in which heterozygotes (i.e. ‘SL’ genotypes) are intermediate in their vulnerability to depression upon exposure to SLEs between homozygote groups (SS and LL) or a relationship in which short allele carriers (SS and SL) are more susceptible to depression than long homozygotes (LL) in the context of stress. The results of our study are driven by differences between short homozygotes (SS) and long allele carriers (SL and LL) (see also online appendix). While this direction of effect has been previously reported (Hankin et al., 2011), it is not the predominant pattern noted in the literature. Importantly, however, when analyzing the data across 3 genotype groups the graded pattern, wherein heterozygotes are intermediate is observed, though not at a statistically significant level, perhaps due to the relatively small sample that comprises this study. Second, there was a main effect of genotype (when comparing long allele carriers against short allele homozygotes) in our sample wherein long allele carriers were more susceptible to depression diagnosis regardless of SLE exposure. Of note, there was no significant main effect when analyzing the data as three different genotype groups (SS, SL and LL) or considering depressive symptoms. Critically, this sample has a large African American population that may contribute to these differences. For example, Gelernter and colleagues (Gelernter, Kranzler, Coccaro, Siever, & New, 1998) have found that, contrary to the relationship observed in Caucasian individuals (Lesch et al., 1996), African Americans with the long allele have elevated neuroticism. Despite accounting for ethnicity as a covariate, the ethnic diversity of the sample may have contributed to the genotype main effect. However, secondary analyses conducted separately in Caucasian and African-American subjects revealed interaction effects in the same direction as the entire sample.
Interestingly, the results of this study suggest a crossover interaction wherein individuals with the short allele appear more sensitive to SLE exposure, for better or worse. Specifically, at high SLE exposure, short homozygotes (SS) are more vulnerable to depression but at low SLE exposure they appear to benefit from a benign environment and experience relatively reduced rates of depression. These findings are consistent with theoretical work, supported by empirical data, suggesting that many genotypes, including the short allele of the 5-HTTLPR polymorphism, originally conceptualized as conferring vulnerability to adversity may be better conceptualized as conferring variability in susceptibility to environmental influence, in both positive and negative directions (Belsky & Pluess, 2009). Critically, the cross-over interaction we observed was present for a continuous measure of depressive symptoms as well as a dichotomous diagnosis of depression. The convergence of findings across a continuous and dichotomous phenotype suggests that the observed interaction may not be a spurious finding resulting from scaling effects as has been observed with simulated data (Eaves, 2006). However, some caution is required with interpreting the crossover interaction – while intuitively appealing, simulation data suggest that smaller samples, like the present one, are more likely to produce false-positive crossover interaction effects (Sher, personal communication).
Possible Contributors to Mixed Results in the Literature
SLEs are amongst the strongest predictors of initial depression onset, but their predictive power is diminished in subsequent recurrent depressive episodes (Kendler et al., 2000; Post, 1992; Stroud et al., 2008). This is consistent with the kindling theory of depression which posits that SLEs are associated with early episodes of depression while later episodes of depression are predicted by previous episodes but not SLEs (Kendler et al., 2000; Post, 1992). Implicit in this hypothesis is that depressive episodes induce psychobiological changes that set the stage for subsequent episodes (i.e. these individuals are kindled) which are then less tightly linked to environmental experiences. Importantly, however, research suggests that while SLEs are less linked to recurrent episodes, minor stressors, such as daily hassles, which would not be detected as an SLE, may provoke a depressive episode in a kindled individual (Monroe & Harkness, 2005). As such it may be that conflicting findings associating the 5-HTTLPR genotype x SLE interaction with depression are affected by kindling. Some data suggesting this interaction is driven by more low-level stressors in adults is consistent with this possible interpretation (Kendler et al., 2005). Critically, however, while the present data demonstrate that 5-HTTLPR x SLE predicts preschool-onset depression, they do not directly assess the kindling hypothesis. It will be important for future longitudinal studies to assess whether the 5-HTTLPR x SLE interaction is diminished or disappears in recurrent depressive episodes.
Another feature of the present study worth noting is the assessment of SLEs. A wealth of literature suggests that mode of measurement of SLEs can have a profound impact on their reported occurrence and predictive utility (Monroe & Reid, 2008). Collectively, research suggests that interview-based assessments provide more accurate data relative to self-report checklists. However, in many studies, particularly those involving large samples, self-report checklists are used (Monroe & Reid, 2008). In this study we used an interview-based assessment of SLEs, which may provide a better phenotyping of SLEs than the self-report measures used in many gene x environment interaction studies. Additionally, because children were relatively young during this assessment, the retrospective recall was for a short duration and concerned early childhood, a period in which parents are highly aware and attentive to stress.
The Importance of Neural Development
In light of the present results and meta-analytic data suggesting that early life stress as opposed to stress that occurs later in life, is a particularly potent predictor of depression in short allele carriers, it is important to consider emerging neuroscience data suggesting that 5-HTTLPR genotype and early life stress may act through shared neurodevelopmental consequences to influence depression vulnerability (Caspi et al., 2010; Hariri & Holmes, 2006; Karg et al., 2011). First, supporting the role of 5-HTT disruption in the etiology of depressed mood, pharmacologic blockade of the 5-HTT in early life has been shown to result in anxiety- and depression- like behaviors in rodents (Ansorge, Zhou, Lira, Hen, & Gingrich, 2004; Homberg, Schubert, & Gaspar, 2010). Second, there is also evidence from human studies indicating that the effects of the 5-HTTLPR polymorphism on amygdala function is driven by brain structure, not current 5-HTT availability; this suggests that differences conferred by 5-HTT genotype arise from indirect neurodevelopmental consequences on brain structure as opposed to its direct effects on serotonin transporter function (Kobiella et al., 2011). Perhaps most importantly, there is growing support for the idea that both 5-HTTLPR and early life stress may have downstream consequences on serotonin receptor function. Research across species suggests that the short allele is associated with diminished 5-HT1A binding, which may reflect downstream consequences of elevated serotonergic tone (Christian et al., 2013; David et al., 2005). A similar effect is seen as a consequence of early life stress exposure in rodents, non-human primates, and humans (Goodfellow, Benekareddy, Vaidya, & Lambe, 2009; Jovanovic, Perski, Berglund, & Savic, 2011; Spinelli et al., 2010), clearly implicating shared neurobiological pathways. In fact, this reduced 5-HT1A binding is associated with differences in cortical neurodevelopment that may confer vulnerability to depression and anxiety through disrupted prefrontal regulation of subcortical circuits, including the amygdala (Goodfellow et al., 2009; Jovanovic et al., 2011; Pezawas et al., 2005). Collectively, these results suggest that 5-HTTLPR genotype and early life stress may confer depression vulnerability through potentially additive effects on serotonergic receptor regulation during early life.
Limitations and Future Directions
While this study has several notable strengths including a sample of preschool aged children with depression (aged 3–5), and an interview-based assessment of SLEs, it is not without limitations. First and foremost, while the sample size is relatively large for an investigation of preschool-onset depression, it is relatively small for a study of gene x environment interaction; it is clearly underpowered to detect the small effect that meta-analyses suggest this interaction may confer (Duncan & Keller, 2011). Moreover, given the unique population (i.e., preschool-aged children with depression) we were unable to obtain a replication sample as has been suggested for GxE in light of false positive concerns (Duncan & Keller, 2011). As such, it will be important for further research to replicate this interaction at this developmental stage. We are only aware of one study (the Generation R study) in a similarly aged sample. This recent report showed that a relatively large sample (n = 2,136) of similarly aged young children with the short 5-HTTLPR allele are more susceptible to emotional problems if their mother experienced post-natal anxiety or depression symptoms (Tiemeier et al., 2012). However, this study did not report on SLEs or depression more specifically. Perhaps, most importantly, it is unclear exactly how maternal postnatal depression and anxiety symptoms translate to risk for the young child. These maternal symptoms likely reflect both a genetic loading for mood and anxiety disorders as well as a potentially stressful environment. As such, the present study and the Generation R study have broadly consistent and potentially generalizable effects but may not represent replication in a more direct sense. Nevertheless, the generalizable and convergent result can provide more confidence in the findings reported here (Karg et al., 2011).
Duncan & Keller (Duncan & Keller, 2011), as well as others, have also argued that low confidence in priors (i.e. the biological rationale for the selection of 5-HTT as a candidate gene) and subsequent lack of identification of this genotype at genomewide levels of statistical significance (5E-8) in genomewide association studies (GWAS), coupled with low power (discussed above) contribute to an inordinately high false discovery rate. Despite replicating the broad results of the Generation R study, we cannot exclude the possibility that our finding constitutes a false positive. However, it is also worth noting that there is a robust literature that implicates 5-HTT in the etiology of depression (Caspi et al., 2010). Whether investigators consider this burden of evidence to sufficiently categorize 5-HTT as a putative candidate is, largely, a matter of opinion. There are also several reasons why GWAS may not implicate the 5-HTTLPR polymorphism. The 5-HTTLPR may be imputed with accuracy (~93–95%) in Caucasians using multiple SNPs contained on some GWAS arrays (Knodt, 2012; Lu et al., 2012). However, we are unaware of any GWAS reporting use of such methodology for imputation. Furthermore, the utility of such imputation procedures in ethnicities other than Caucasians requires additional attention (Knodt, 2012). Moreover, GWAS have largely examined the main effects of genotype on depression and related constructs. Given that main effects of 5-HTTLPR on psychopathology are uncommon (although noted in the present study), it would not be entirely surprisingly that they are not found. In the absence of systematic genomewide GxE efforts (which are emerging) with careful assessment of SLEs, as well as imputation allowing for 5-HTTLPR typing, this criticism of the putative role of 5-HTTLPR may be premature.
While we speculate that kindling effects may diminish 5-HTTLPR x SLE interaction effects in later depressive episodes, we were unable to directly test this hypothesis with the current baseline data. It will be important for longitudinal studies (including longitudinal data from the current study that is now being collected) to directly test this hypothesis as has been done for main effects of SLEs on depression (Kendler et al., 2000). In this sample, the 5-HTTLPR x SLEs interaction predicted depression. However, this study did not probe the neurobiological mechanisms through which this effect may emerge. Human neuroimaging and non-human animal models suggest several pathways including individual differences in threat-related amygdala reactivity, 5-HT1A availability, HPA axis function, reward processing, conflict monitoring, and neurodevelopment (Caspi et al., 2010). It will be important for future multimodal research to interrogate this interaction while simultaneously collecting measures of biological function to assess potentially mediating roles.
Lastly, PO-MDD is just beginning to be characterized and its clinical significance is not yet well understood (Luby et al., 2009). However, it has shown longitudinal stability with MDD in later childhood and has been detected in numerous independent study samples (Bufferd, Dougherty, Carlson, Rose, & Klein, 2012; Luby et al., 2009; Wichstrom et al., 2012). Further, there is a rapidly emerging literature demonstrating biological correlates similar to those known in adult MDD (Barch, Gaffrey, Botteron, Belden, & Luby, 2012; Gaffrey, Luby, et al., 2011; Gaffrey, Luby, Botteron, Repovs, & Barch, 2012; Pagliaccio et al., 2012). Collectively, these new findings are beginning to provide evidence for the validity of PO-MDD.
Summary
The present study suggests that short homozygotes with high exposure to early life stressful life events are vulnerable to the development of preschool-onset depression. These data are consistent with the original report of this association in adults as well as recent meta-analytic data supporting the association between early adversity and adult-onset depression (Caspi et al., 2003; Karg et al., 2011). These data critically extend this previous research by showing that this interaction may be present even for extremely early-onset preschool depression. Further research is needed to: replicate this relationship at this developmental period, examine empirically whether this relationship is diminished in recurrent episodes consistent with the kindling model of depression, and test candidate neurodevelopmental mechanisms.
Supplementary Material
Key points.
5-HTTLPR and early life stressful life events interact to predict preschool-onset depression.
This interaction suggests differential susceptibility; short homozygotes have increased rates of depression in the context of elevated early adversity but reduced rates in the context of low adversity.
These results converge with recent meta-analytic data and neuroscience research suggesting that the 5-HTTLPR x SLEs interaction may be driven by neurodevelopmental influences.
We speculate that kindling, may explain some mixed findings within the 5-HTTLPR x SLEs literature.
Acknowledgments
We are thankful to the Preschool Depression Study staff and participant families. We are also grateful to Lindsay Michalski for her review of drafts of this manuscript. NARSAD and NIMH (MH090786) grants to JL funded this research.
The authors have declared that they have no competing or potential conflicts of interest.
Abbreviations
- 5-HTTLPR
serotonin transporter linked polymorphic-region
- SLE
stressful life event
- SLEs
stressful life events
- MDD
major depressive disorder
Footnotes
Conflict of interest statement: No conflict of interests declared.
Additional supporting information is provided along with the online version of this article.
Online appendix: Serotonin-transporter-linked polymorphic region (5-HTTLPR) genotype and stressful life events interact to predict preschool onset depression: A replication and developmental extension (Word document).
Please note that Wiley-Blackwell Publishing are not responsible for the content or functionality of any supporting materials supplied by the authors (although this material was peer reviewed by JCPP referees and Editors along with the main article). Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Barch DM, Gaffrey MS, Botteron KN, Belden AC, Luby JL. Functional brain activation to emotionally valenced faces in school-aged children with a history of preschool-onset major depression. Biol Psychiatry. 2012;72(12):1035–1042. doi: 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M, Widaman KF. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. J Child Psychol Psychiatry. 2013 doi: 10.1111/jcpp.12075. [DOI] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: continuity from ages 3 to 6. Am J Psychiatry. 2012;169:1157–1164. doi: 10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Christian BT, Wooten DW, Hillmer AT, Tudorascu DL, Converse AK, Moore CF, Schneider ML. Serotonin transporter genotype affects serotonin 5-HT1A binding in primates. J Neurosci. 2013;33:2512–2516. doi: 10.1523/JNEUROSCI.4182-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Angold A, March J, Fairbank J. Life events and post-traumatic stress: the development of a new measure for children and adolescents. Psychol Med. 1998;28:1275–1288. doi: 10.1017/s0033291798007569. [DOI] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci. 2005;25(10):2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ. Genotype x Environment interaction in psychopathology: fact or artifact? Twin Res Hum Genet. 2006;9:1–8. doi: 10.1375/183242706776403073. [DOI] [PubMed] [Google Scholar]
- Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. J Child Psychol Psychiatry. 2006;47:313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Belden AC, Luby JL. The 2-week duration criterion and severity and course of early childhood depression: implications for nosology. J Affect Disord. 2011;133(3):537–545. doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch J, Barch DM. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. J Affect Disord. 2011;129:364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53:964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Coccaro EF, Siever LJ, New AS. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155:1332–1338. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Goodfellow NM, Benekareddy M, Vaidya VA, Lambe EK. Layer II/III of the prefrontal cortex: Inhibition by the serotonin 5-HT1A receptor in development and stress. J Neurosci. 2009;29:10094–10103. doi: 10.1523/JNEUROSCI.1960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. [Review] Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JR, Oldehinkel AJ. Differential susceptibility in youth: evidence that 5-HTTLPR x positive parenting is associated with positive affect ‘for better and worse’. Transl Psychiatry. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010;31:60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Perski A, Berglund H, Savic I. Chronic stress is linked to 5-HT(1A) receptor changes and functional disintegration of the limbic networks. Neuroimage. 2011;55:1178–1188. doi: 10.1016/j.neuroimage.2010.12.060. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Knodt A. Imputation of Microsatellite Markers with Tag SNPs. Duke University; Durham, NC: 2012. M.S. [Google Scholar]
- Kobiella A, Reimold M, Ulshofer DE, Ikonomidou VN, Vollmert C, Vollstadt-Klein S, Smolka MN. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry. 2011;1:e37. doi: 10.1038/tp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lu AT, Bakker S, Janson E, Cichon S, Cantor RM, Ophoff RA. Prediction of serotonin transporter promoter polymorphism genotypes from single nucleotide polymorphism arrays using machine learning methods. Psychiatr Genet. 2012;22:182–188. doi: 10.1097/YPG.0b013e328353ae23. [DOI] [PubMed] [Google Scholar]
- Luby JL. Preschool Depression: The Importance of Identification of Depression Early in Development. Curr Dir Psychol Sci. 2010;19:91–95. doi: 10.1177/0963721410364493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Koenig-McNaught AL, Brown K, Spitznagel E. ThePreschool Feelings Checklist: a brief and sensitive screening measure for depression in young children. J Am Acad Child Adolesc Psychiatry. 2004;43:708–717. doi: 10.1097/01.chi.0000121066.29744.08. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, Hildebrand T. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry. 2002;41:928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Gene-environment interactions in depression research: genetic polymorphisms and life-stress polyprocedures. Psychol Sci. 2008;19:947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby J, Gaffrey M, Belden A, Botteron K, Gotlib IH, Barch DM. Anomalous functional brain activation following negative mood induction in children with pre-school onset major depression. Dev Cogn Neurosci. 2012;2:256–267. doi: 10.1016/j.dcn.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J, Way BM, Taylor SE. 5-HTTLPR moderates effects of current life events on neuroticism: differential susceptibility to environmental influences. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1070–1074. doi: 10.1016/j.pnpbp.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis-stress: recommendations for evaluating interaction effects. Dev Psychopathol. 2012;24(2):389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Stein EA. Effects of early-life stress on serotonin(1A) receptors in juvenile Rhesus monkeys measured by positron emission tomography. Biol Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Moyer A. The relationship between stress and depression in first onsets versus recurrences: a meta-analytic review. J Abnorm Psychol. 2008;117:206–213. doi: 10.1037/0021-843X.117.1.206. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Velders FP, Szekely E, Roza SJ, Dieleman G, Jaddoe VW, Verhulst FC. The Generation R Study: A review of design, findings to date, and a study of the 5-HTTLPR by environmental interaction from fetal life onward. J Am Acad Child Adolesc Psychiatry. 2012;51:1119–1135. doi: 10.1016/j.jaac.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wichstrom L, Berg-Nielsen TS, Angold A, Egger HL, Solheim E, Sveen TH. Prevalence of psychiatric disorders in preschoolers. J Child Psychol Psychiatry. 2012;53:695–705. doi: 10.1111/j.1469-7610.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, Belsky J. Distinguishing ordinal and disordinal interactions. Psychol Methods. 2012;17:615–622. doi: 10.1037/a0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, Rush AJ. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.