Abstract
The last five years have witnessed a remarkable renaissance in vitamin D research and a complete re-evaluation of its benefits to human health. Two key factors have catalyzed these changes. First, it now seems likely that localized, tissue-specific, conversion of 25-hydroxyvitamin D (25OHD) to 1,25-dihydroxyvitamin D (1,25(OH)2D) drives many of the newly recognized effects of vitamin D on human health. The second key factor concerns the ongoing discussion as to what constitutes adequate or optimal serum vitamin D (25OHD) status, with the possibility that vitamin D-deficiency is common to communities across the globe. These two concepts appear to be directly linked when low serum concentrations of 25OHD compromise intracrine generation of 1,25(OH)2D within target tissues. But, is this an over-simplification? Pro-hormone 25OHD is a lipophilic molecule that is transported in the circulation bound primarily to vitamin D binding protein (DBP). While the association between 25OHD and DBP is pivotal for renal handling of 25OHD and endocrine synthesis of 1,25(OH)2D, what is the role of DBP for extra-renal synthesis of 1,25(OH)2D? We hypothesize that binding to DBP impairs delivery of 25OHD to the vitamin D-activating enzyme 1α-hydroxylase in some target cells. Specifically, it is unbound, ‘free’ 25OHD that drives many of the non-classical actions of vitamin D. Levels of ‘free’ 25OHD are dependent on the concentration of DBP and alternative serum binding proteins such as albumin, but will also be influenced by variations in DBP binding affinity for specific vitamin D metabolites. The aim of this review will be to discuss the merits of ‘free 25OHD’ as an alternative marker of vitamin D status, particularly in the context of non-classical responses to vitamin D.
1.1 Introduction
Steroid hormones and sterols such as vitamin D are highly lipophillic, and therefore share a common requirement for serum carrier proteins to ensure effective delivery to target cells. Given the abundance of proteins in serum, some of this transport will be non-specific. Nevertheless, there are many ligand-specific serum carriers of steroid hormones and sterols including corticosteroid-binding globulin (CBG) (glucocorticoids, mineralocorticoids), vitamin A (retinol)-binding protein, vitamin D-binding protein (DBP), sex hormone-binding globulin (SHBG) (estrogens, androgens), and thyroid hormone-binding globulin. Although these proteins have been studied primarily in the context of their impact on serum assays for their respective ligands, it is now clear that they may also fulfill alternative functions. For example, CBG and SHBG not only act as high affinity serum transporters, but are also able to bind to cell membranes in their liganded forms (1, 2), suggesting alternative actions as signal transducers (3–5). In a similar fashion, DBP can function as a macrophage-activating factor (MAF) (6) and actin-binder (7), actions independent of its vitamin D metabolite binding functions. Despite the pluripotent properties of steroid binding globulins, many recent studies of these proteins have focused only on their ability to deliver hormone ligands to target cells.
The mechanisms by which ligands are released from binding globulins and acquired by target cells are crucial to steroid hormone signaling pathways. This is particularly important for vitamin D where there is increasing evidence for extra-renal, intracrine, conversion of pro-hormone 25-hydroxyvitamin D (25OHD) to active 1,25-dihydroxyvitamin D (1,25(OH)2D) (8–10). In this setting, the impact of vitamin D will be very much dependent on tissue-specific expression of the vitamin D-activating enzyme 25-hydroxyvitamin D-1α-hydroxylase (1α-hydroxylase) and the nuclear receptor for 1,25(OH)2D, the vitamin D receptor (VDR). Another crucial factor influencing this mechanism will be the availability of substrate 25OHD for activation by 1α-hydroxylase. As serum concentrations of 25OHD are usually considered to be the principal marker of vitamin D ‘status’ for any given individual, the intracrine model has been proposed as a potential explanation for studies linking vitamin D-deficiency with various human health parameters (11). However, in proposing a model for vitamin D function centered on serum 25OHD (rather than the endocrine 1,25(OH)2D model), it is important to recognize that 25OHD circulates bound to its cognate binding globulin, DBP (12). Furthermore, because 25OHD is bound by DBP with much higher affinity than 1,25(OH)2D, it is likely that DBP will have a much greater impact on 25OHD-mediated intracrine responses. In this way, DBP and its interaction with 25OHD may be an important consideration in our interpretation of the physiological impact of vitamin D and this will be discussed in greater detail in the following review.
1.2 DBP, megalin and the free hormone hypothesis
Although 99.9% of 25OHD circulates bound to DBP or other serum proteins, the general assumption for lipid soluble molecules such as vitamin D is that biological activity involves unbound or free fractions even though this component in serum is very small (13, 14). Indeed the ‘free-hormone hypothesis’ has been proposed as a universal mechanism for cellular uptake of steroid hormones (15, 16), largely because these molecules are highly lipophillic and therefore have the potential to rapidly and passively diffuse across cell membranes. Nevertheless, in recent years the ‘free-hormone hypothesis’ has come under increased scrutiny due, in part, to disparity between the likely amounts of free hormone available for passive diffusion and the levels required to efficiently occupy intracellular target receptors. For example, it has been estimated that concentrations of free 1,25(OH)2D in serum are approximately 10−13M (13, 17), which is much less than the apparent concentrations normally quoted for binding to the VDR (dissociation constant (Kd) = approximately 10−10M).
Other reservations concerning the free hormone hypothesis stem from studies which have assessed the mechanisms by which steroid binding proteins such as DBP interact with target cells. The most important of these concern megalin, a large transmembrane protein which acts as a multi-ligand receptor within several tissue, notably the apical surface of renal proximal tubule cells (18, 19). In the kidney megalin acts as a cell surface receptor for DBP, with the resulting complex being internalized through endocytosis in proximal tubule cells (20) (Figure 1). The presence of 1α-hydroxylase in proximal tubules, coupled with DBP’s relatively high affinity for 25OHD, means that the acquisition and internalization of DBP via its megalin receptor is a pivotal component of renal vitamin D metabolism. Consistent with this, megalin knockout mice are unable to recover DBP from the glomerular filtrate, and thus lose it and its vitamin D cargo in urine. As a consequence, megalin knockout mice are unable to adequately metabolize 25OHD to 1,25(OH)2D resulting in a bone phenotype that resembles vitamin D-deficient rickets (20). Since then, other studies have shown that cubulin (21) and disabled-2 (22) work in conjunction with megalin to facilitate the renal processing of DBP.
Figure 1. Megalin and the receptor-mediated uptake of 25-hydroxyvitamin D in the kidney.
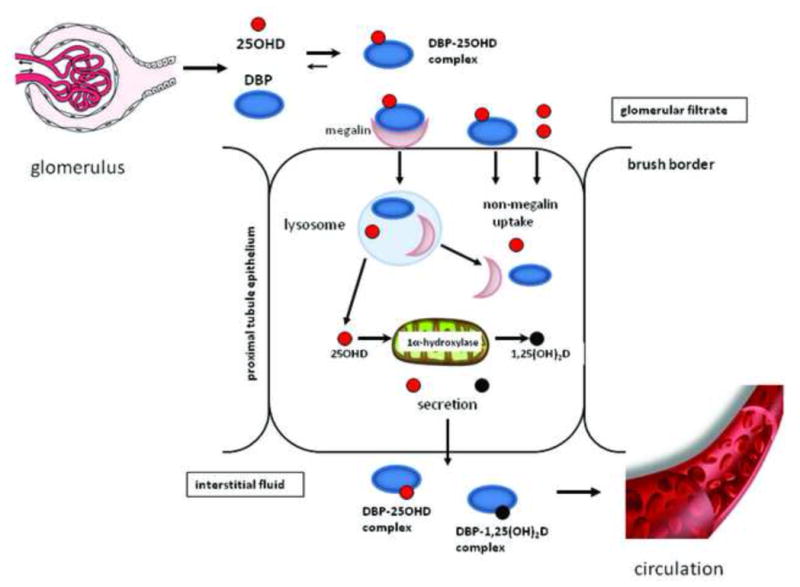
DBP-bound 25OHD in the glomerular filtrate is recovered via expression of the multi-ligand membrane receptor megalin which endocytically internalizes DBP in proximal tubule epithelial cells. The resulting release of 25OHD provides substrate for the mitochondrial 1α-hydroxylase and leads to renal synthesis of 1,25(OH)2D to facilitate circulating levels of this hormone and support endocrine actions of this hormone, whilst also maintaining circulating levels of 25OHD.
Although megalin-dependent uptake of DBP has a clear role to play in renal vitamin D endocrinology, it is not yet clear whether a similar mechanism is utilized by other vitamin D target tissues. Outside the kidney, megalin is expressed by several tissues including the placenta, mammary gland and parathyroid glands, which are known to exhibit extra-renal 1α-hydroxylase activity, suggesting a similar DBP-megalin interaction to that described for the kidney (23). However, the functional significance of this is still far from clear. Megalin-mediated uptake of DBP has been described for T47D breast cancer cells, which co-express megalin and cubulin, as well as 1α-hydroxylase and the vitamin D receptor (VDR) (24, 25). Notably, endocytic uptake of DBP by these cells promoted responses to 25OHD but not 1,25(OH)2D, suggesting that the principal function of megalin in this model is to support intracrine conversion of 25OHD to 1,25(OH)2D. A similar mechanism has also been proposed for intracrine metabolism of vitamin D in osteoblasts (26, 27).
1.3 Vitamin D responses in DBP knockout mice
In common with megalin knockout animals, analysis of mice with ablation of the gene for DBP has provided further insight into the role of this protein in vitamin D physiology. DBP knockout mice are healthy and fertile despite having lower circulating levels of 25OHD and 1,25(OH)2D (28). In the absence of DBP, vitamin D metabolites are more likely to bind to albumin, which has a lower affinity for both 25OHD and 1,25(OH)2D relative to DBP and will therefore be less effective in preventing urinary loss of vitamin D metabolites. Significantly, when DBP knockout mice were placed on a vitamin D deficient diet, they succumbed to bone mineralization abnormalities more rapidly than their wild type counterparts, underlining the importance of DBP in maintaining serum vitamin D concentrations under conditions of dietary restriction. Conversely, loss of the DBP gene protects mice against potential toxic effects of vitamin D and attenuates the timing of vitamin D-induced responses in peripheral tissues. Collectively, these observations suggest that a key function of DBP is to maintain stable levels of 25OHD and/or 1,25(OH)2D in serum whilst modulating their bioavailability to peripheral tissues.
More recent studies have confirmed the detrimental effect of DBP knockout on circulating levels of 1,25(OH)2D but, paradoxically, showed that target tissue levels of 1,25(OH)2D in DBP knockout mice were no different to those from heterozygous littermates (29). Furthermore, parallel studies in vitro, showed that cells cultured in serum from DBP knockout mice were significantly more sensitive to 1,25(OH)2D compared to cells cultured in serum from control mice (29). These data suggest that for some VDR-expressing tissues, DBP is not an active facilitator of 1,25(OH)2D uptake, but instead functions to limit its tissue bioavailability. Given the higher affinity DBP has for 25OHD compared to 1,25(OH)2D it is likely that similar effects will also be observed for the pro-hormone form of vitamin D. This, in turn, may be a key factor in regulating the magnitude of peripheral synthesis of 1,25(OH)2D via extra-renal 1α-hydroxylase.
Although the uptake of 25OHD by proximal tubule cells is clearly linked to the internalization of DBP, it is possible that different mechanisms apply to other megalin-expressing tissues. Specifically, megalin-binding of DBP may simply act to increase localized concentrations of ‘free’ vitamin D metabolites at the cell membrane, thereby facilitating more effective passive diffusion of these molecules. It is also possible that for some cells internalization of DBP takes place via mechanisms that do not involve megalin. Uptake of DBP has been reported for B-lymphocytes, but this does not appear to involve the same clathrin-coated pits that are characteristic of the megalin-mediated pathway (30). One possibility is that binding of DBP to this type of cell is mediated by the gamma Fc receptors that also associate with immunoglobulins (31). Finally, in most cases, megalin-DBP interaction appears to facilitate transport of 25OHD and its subsequent intracrine activation via localized expression of CYP27B1. By contrast, studies using serum from wild type and DBP knockout mice have shown that DBP attenuates responses to its weaker binding ligand, 1,25(OH)2D, raising the possibility that endocrine responses to 1,25(OH)2D are independent of its binding protein (29).
1.4 Extra-renal 1α-hydroxylase, the free hormone hypothesis revisited
The recent upsurge of interest in vitamin D and human health has stemmed from two key developments in the vitamin D field. The first is the ongoing debate as to what constitutes optimal vitamin D status and how to achieve this safely through normal sunlight exposure and/or dietary supplementation. The second concerns the more widespread acceptance of extra-renal synthesis of 1,25(OH)2D as a mechanism for mediating potential beneficial effects of enhanced serum 25OHD levels. As outlined above, the efficacy of the localized intracrine mechanism will be highly dependent on the ability of 1α-hydroxylase-expressing target cells to acquire substrate 25OHD. It is possible that some extra-renal tissues achieve this by employing megalin-mediated receptor uptake of DBP-bound 25OHD similar to that found in the proximal tubules of the kidney. However, most extra-renal tissues do not appear to express megalin or its associated co-receptors, suggesting that these tissues are more likely to acquire 25OHD that is not bound to DBP, in other words free, or bioavailable, 25OHD.
The potential importance of the free hormone hypothesis with respect to vitamin D is best illustrated by the extra-renal 1α-hydroxylase activity in immune cells such as monocytes, macrophages and dendritic cells. Studies in vitro by our group have shown that monocytes exposed to increasing doses of 25OHD in the presence of DBP show dose-dependent induction of antibacterial proteins such as cathelicidin (32, 33). However, in the absence of DBP (using monocytes cultured with serum from DBP knockout mice), this response was much more potent (34). In a similar fashion, 25OHD-mediated induction of cathelicidin in monocytes cultured without DBP could be rheostatically suppressed with the addition of increasing doses of purified DBP (34). The inference from these studies is that monocyte responses to 25OHD are attenuated in the presence of DBP. Importantly, further studies suggested that this effect of DBP is not solely dependent on changes in DBP concentration but may also be influenced by variations in DBP binding affinity for 25OHD. Monocytes exposed to 25OHD showed much more potent induction of antibacterial cathelicidin when cultured with human serum containing lower affinity forms of DBP as defined by Gc SNPs (34). The over-arching conclusion from these observations is that the ability of 25OHD to promote monocyte antibacterial activity is dependent on both the serum concentration and genotype of DBP. Similar observations have also been made for antigen presentation by dendritic cells, where the ability of 25OHD to promote tolerogenic regulatory T cell activity was enhanced with either lower serum concentrations of DBP, or with lower affinity genetic variants of DBP (35).
1.5 Bound, free and bioavailable 25OHD in the circulation
In serum, the vast majority of vitamin D metabolites bind preferentially to DBP, but they are also known to associate with serum albumin. The affinity of 25OHD (Ka = 6 × 105 M−1) and 1,25(OH)2D (Ka = 5.4 × 104 M−1) for albumin is substantially lower than that observed with 25OHD (Ka = 7 × 108 M−1) and 1,25(OH)2D (Ka = 4 × 107 M−1) for DBP (13, 36). However, because of the relative abundance of albumin in serum (650 μM) compared to DBP (5 μM) the potential remains for some vitamin D metabolites to be transported in the circulation by albumin. Additionally, in keeping with other steroid hormones, the vast majority of the DBP in serum is empty because of its molar abundance relative to the concentrations of vitamin D metabolites found in the circulation. In view of these observations, it seems likely that most circulating vitamin D metabolites are bound to a carrier protein of some sort. Nevertheless, at any given time, a small proportion of vitamin D metabolites will not be bound to DBP or albumin, but will instead be unbound or ‘free’. In the case of 25OHD it is estimated that less than 0.1% of the total circulating levels of this metabolite are ‘free’. Thus, an alternative interpretation of unbound 25OHD has been proposed termed ‘bioavailable’ 25OHD. Bioavailable 25OHD refers to all the circulating 25OHD that is not bound to DBP, in other words that which is free plus that which is bound to albumin. Bioavailable 25OHD represents approximately 10% of all circulating 25OHD and has been used as an alternative to free 25OHD in some clinical studies (37, 38).
It is important to recognize that although physical measurement of free serum 25OHD has been described previously (36, 39), this process is generally labor-intensive and time-consuming. Proposed ELISA strategies for measurement of free 25OHD in serum have recently been reported (40) but are, as yet, not commercially available. As a consequence, most studies of free vitamin D have utilized alternative strategies for estimating this component of vitamin D based on mathematical models that incorporate binding coefficients for DBP and albumin, coupled to measurement of total serum 25OHD and DBP (41, 42). This strategy has recently been used to estimate both free and bioavailable fractions of circulating 25OHD, and their relationship with biomarkers of vitamin D status (37, 38). To date, mathematical estimations of free 25OHD have relied on equations that utilize average binding coefficients for DBP and albumin. However, it is important to recognize that genotypic variations in these proteins may be associated with significant changes in binding affinity and/or serum concentrations.
Phenotypic variations in DBP were first described more than 50 years ago based on isoelectric focusing migrations patterns of a then unnamed serum protein (43). These different proteins were originally referred to as Group-Specific Component (Gc)1F, Gc1S and Gc2, but are now known to be polymorphisms in the gene for DBP (also referred to as Gc). The resulting variations in DBP amino acid sequence (Gc1S = a D416E amino switch from Gc1F, and Gc2 = a T420K amino acid change from Gc1F) appears to alter the binding affinity of vitamin D ligands for DBP, with Gc1F having the highest avidity for vitamin D metabolites and Gc2 the lowest (44, 45) though another study indicated little affinity differences (46). Gc alleles have also been linked to serum levels of DBP with Gc2 being the least abundant and Gc1F the most abundant (46). Notably Gc alleles show distinct racial distribution patterns. Black and Asian populations are far more likely to carry the Gc1F form of DBP, while Whites more frequently exhibit the Gc1S form of DBP. Likewise, the lower affinity Gc2 form is far more likely to be found in Whites and rarely found in Blacks. It has been speculated that these affinity differences reflect the physiological consequences of human evolution from darker pigmented skin to lighter complexions and the concomitant changes in epidermal synthesis of parental vitamin D that accompanied this (47). With these observations in mind, studies from our group have adapted the original mathematical algorithms for calculating free and bioavailable 25OHD (41, 42), to incorporate Gc polymorphic variations in DBP binding affinity (48).
In the absence of specific assays for measuring free 25OHD, studies incorporating this parameter have been restricted to mathematical estimates and have reported variable findings. Initial association studies reported that free or bioavailable 25OHD are better correlates of bone mineral density in a cohort of healthy adults (37). Estimated free 25OHD was also shown to be a better correlate of circulating parathyroid hormone (PTH) levels in chronic kidney disease patients receiving hemodialysis (38). In a more recent study using a larger cohort of healthy adults, the authors described good correlation between the serum concentrations of 25OHD and DBP, but found that free or bioavailable 25OHD did not provide a better correlate of vitamin D-responsive response markers at the endocrine level such as serum parathyroid hormone (PTH) concentrations (49). However, in the study, Dastani, et al acknowledged that the cohort was relatively vitamin D sufficient, with limited variations in DBP and free 25OHD levels (49). Much greater differences in DBP and free/bioavailable 25OHD were observed in a study of pediatric chronic kidney disease patients (50), suggesting that this parameter may be more important in some clinical settings where DBP concentrations are elevated or suppressed. It is also important to recognize that, to date, none of the studies where free 25OHD has been estimated have incorporated genotypic variations in DBP binding affinity, which may modify existing association data. However, it seems likely that this strategy will be circumvented by the advent of assays to physically measure serum concentrations of free 25OHD.
1.6 Future studies on DBP and free vitamin D
Further studies of free and bioavailable 25OHD in humans are required, notably in the setting of vitamin D supplementation trials and under conditions of enhanced or suppressed expression of DBP. Recent publications have shown a correlation between serum concentrations of 25OHD and DBP (51, 52). Also, recent reports have described changes in total serum concentrations of 25OHD, 1,25(OH)2D and DBP in subjects following supplementation with either cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2) (53). For both treatment arms, total serum levels of 25OHD were similar at baseline, but at the end of the study serum levels of 25OHD3 increased significantly more than serum 25OHD2. For both forms of vitamin D serum DBP concentrations also increased following supplementation in line with the raised serum 25OHD levels. Despite this, the calculated free or bioavailable 25OHD was similar for subjects with either vitamin D2 or vitamin D3 supplementation. The relative inefficiency of vitamin D2 to raise total serum levels of 25OHD relative to vitamin D3 has been attributed in part to the lower binding affinity of DBP for 25OHD2 relative to 25OHD3. As such, megalin-mediated renal recovery of DBP-25OHD2 yielded slightly lower 25OHD2 recovery resulting in a net lower increase of total 25D after supplementation (Figure 2). Conversely this apparent inefficiency of 25OHD2 binding to DBP may be advantageous in the setting of extra-renal metabolism of 25OHD where decreased binding to DBP may aid uptake of 25OHD2 by target cells such as monocytes. In future studies it will be important to incorporate separate measures for 25OHD2 and 25OHD3 in either estimating or physically measuring the free concentrations of these metabolites. Finally, it will also be important to better define non-vitamin D molecules that may influence DBP-25OHD binding. Previous studies have shown that polyunsaturated fatty acids can decrease the binding affinity of vitamin D metabolites for DBP (54), and may thus have the potential to influence their bioavailability.
Figure 2. A role for DBP in tissue discrimination of 25OHD2 and 25OHD3.
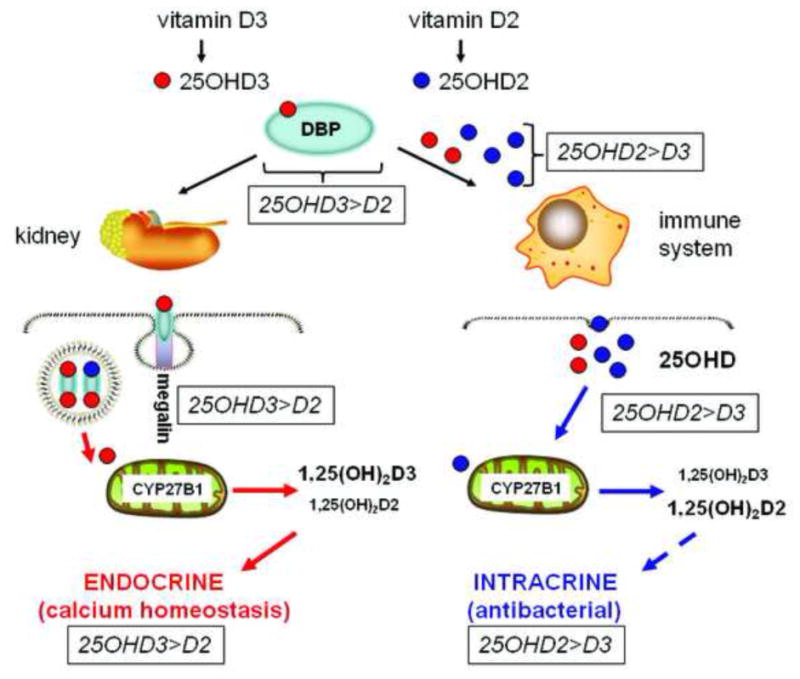
25-hydroxyvitamin D2 (25OHD2) binds to DBP with lower affinity than 25OHD3. This will impair renal handling of 25OHD2 relative to 25OHD3, providing a potential explanation for the relative inefficiency of supplementary vitamin D2 to achieve optimal serum levels of total 25OHD relative to supplementary vitamin D3. By contrast, for extra-renal tissues such as immune cells, impaired binding of 25OHD2 to DBP may facilitate enhanced bioavailability to target cells relative to 25OHD3. In this way, a lower total serum concentration of 25OHD2 may be as effective as a higher total serum concentration of 25OHD3 in promoting intracrine synthesis of 1,25(OH)2D and associated innate immune responses.
1.7 Conclusions
In the last five years there has been revived interest in the role of DBP in vitamin D and human health, with the target levels of serum 25OHD required for vitamin D sufficiency being subject to much scrutiny. Based on recent studies, it is possible that free or bioavailable 25OHD will provide a more meaningful marker of vitamin D function. For example, an individual with low serum 25OHD according to current parameters (less than 50 nM 25OHD), might nevertheless have adequate levels of free 25OHD if serum levels of DBP are low or if the person has a Gc genotype associated with a form of DBP for which vitamin D metabolites exhibit a lower affinity. These considerations are likely to be extremely important as the number of randomized placebo control vitamin D supplementation trials increase, particularly when considering the relative merits of supplementation with vitamin D2 or vitamin D3.
Highlights.
Vitamin D metabolites circulate bound to DBP and, to a lesser extent, albumin
25OHD binds to DBP with high affinity and is thus strongly influenced by DBP
For some cells unbound or ‘free’ 25OHD may be the most bioavailable form of 25OHD
DBP may play a pivotal role in the intracrine synthesis of 1,25(OH)2D by immune cells
Free or bioavailable 25OHD is influenced by DBP concentration and binding affinity
Acknowledgments
Grant support: Funded by grants UL1TR000124 and 1R01AR063910
Footnotes
Conflict of Interest: The authors have no disclosures to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orchinik M, Hastings N, Witt D, McEwen BS. High-affinity binding of corticosterone to mammalian neuronal membranes: possible role of corticosteroid binding globulin. The Journal of steroid biochemistry and molecular biology. 1997;60(3–4):229–236. doi: 10.1016/s0960-0760(96)00191-4. [DOI] [PubMed] [Google Scholar]
- 2.Khan MS, Hryb DJ, Hashim GA, Romas NA, Rosner W. Delineation and synthesis of the membrane receptor-binding domain of sex hormone-binding globulin. The Journal of biological chemistry. 1990;265(30):18362–18365. [PubMed] [Google Scholar]
- 3.Hammond GL. Potential functions of plasma steroid-binding proteins. Trends in endocrinology and metabolism: TEM. 1995;6(9–10):298–304. doi: 10.1016/1043-2760(95)00162-x. [DOI] [PubMed] [Google Scholar]
- 4.Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Androgen and estrogen signaling at the cell membrane via G-proteins and cyclic adenosine monophosphate. Steroids. 1999;64(1–2):100–106. doi: 10.1016/s0039-128x(98)00108-1. [DOI] [PubMed] [Google Scholar]
- 5.Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. The Journal of steroid biochemistry and molecular biology. 1999;69(1–6):481–485. doi: 10.1016/s0960-0760(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto N, Naraparaju VR. Role of vitamin D3-binding protein in activation of mouse macrophages. Journal of immunology. 1996;157(4):1744–1749. [PubMed] [Google Scholar]
- 7.Sanger JM, et al. Disruption of microfilament organization in living nonmuscle cells by microinjection of plasma vitamin D-binding protein or DNase I. Proc Natl Acad Sci U S A. 1990;87(14):5474–5478. doi: 10.1073/pnas.87.14.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zehnder D, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. The Journal of clinical endocrinology and metabolism. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 9.Hewison M. Vitamin D and the intracrinology of innate immunity. Molecular and cellular endocrinology. 2010;321(2):103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewison M, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. The Journal of steroid biochemistry and molecular biology. 2007;103(3–5):316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 11.Adams JS, Hewison M. Update in vitamin D. The Journal of clinical endocrinology and metabolism. 2010;95(2):471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocrine reviews. 1989;10(3):294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 13.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. The Journal of clinical investigation. 1984;74(6):1966–1971. doi: 10.1172/JCI111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. The Journal of clinical investigation. 1981;67(3):589–596. doi: 10.1172/JCI110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocrine reviews. 1989;10(3):232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 16.Hammond GL. Access of reproductive steroids to target tissues. Obstet Gynecol Clin North Am. 2002;29(3):411–423. doi: 10.1016/s0889-8545(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 17.Vieth R. Simple method for determining specific binding capacity of vitamin D-binding protein and its use to calculate the concentration of “free” 1,25-dihydroxyvitamin D. Clinical chemistry. 1994;40(3):435–441. [PubMed] [Google Scholar]
- 18.Moestrup SK, Verroust PJ. Megalin- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia. Annual review of nutrition. 2001;21:407–428. doi: 10.1146/annurev.nutr.21.1.407. [DOI] [PubMed] [Google Scholar]
- 19.Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. American journal of physiology Renal physiology. 2001;280(4):F562–573. doi: 10.1152/ajprenal.2001.280.4.F562. [DOI] [PubMed] [Google Scholar]
- 20.Nykjaer A, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 21.Nykjaer A, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad Sci U S A. 2001;98(24):13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. The EMBO journal. 2002;21(7):1555–1564. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundgren S, et al. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem. 1997;45(3):383–392. doi: 10.1177/002215549704500306. [DOI] [PubMed] [Google Scholar]
- 24.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. The Journal of nutrition. 2006;136(11):2754–2759. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chlon TM, Taffany DA, Welsh J, Rowling MJ. Retinoids modulate expression of the endocytic partners megalin, cubilin, and disabled-2 and uptake of vitamin D-binding protein in human mammary cells. The Journal of nutrition. 2008;138(7):1323–1328. doi: 10.1093/jn/138.7.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Driel M, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20(13):2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 27.Atkins GJ, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40(6):1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Safadi FF, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. The Journal of clinical investigation. 1999;103(2):239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149(7):3656–3667. doi: 10.1210/en.2008-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esteban C, et al. Receptor-mediated uptake and processing of vitamin D-binding protein in human B-lymphoid cells. The Journal of biological chemistry. 1992;267(14):10177–10183. [PubMed] [Google Scholar]
- 31.Petrini M, Galbraith RM, Emerson DL, Nel AE, Arnaud P. Structural studies of T lymphocyte Fc receptors. Association of Gc protein with IgG binding to Fc gamma. The Journal of biological chemistry. 1985;260(3):1804–1810. [PubMed] [Google Scholar]
- 32.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 33.Adams JS, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. Journal of immunology. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun RF, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. The Journal of clinical endocrinology and metabolism. 2010;95(7):3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeffery LE, et al. Availability of 25-Hydroxyvitamin D3 to APCs Controls the Balance between Regulatory and Inflammatory T Cell Responses. Journal of immunology. 2012;189(11):5155–5164. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bikle DD, et al. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. The Journal of clinical endocrinology and metabolism. 1986;63(4):954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 37.Powe CE, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(7):1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhan I, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82(1):84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bikle DD, Halloran BP, Gee E, Ryzen E, Haddad JG. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. The Journal of clinical investigation. 1986;78(3):748–752. doi: 10.1172/JCI112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosmalen FMSL, Harbers EG, Mayer GT, Parsons GH. An Immunoassay for free 25-hydroxyvitamin D. Journal of Bone and Mineral Research. 2011;(Suppl):197. [Google Scholar]
- 41.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. The Journal of clinical endocrinology and metabolism. 1985;61(5):969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 42.Dunn JF. Computer simulation of vitamin D transport. Annals of the New York Academy of Sciences. 1988;538:69–76. doi: 10.1111/j.1749-6632.1988.tb48851.x. [DOI] [PubMed] [Google Scholar]
- 43.Hirschfled J, Jonsson B, MR Immune electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol Microbiol. 1959;47:160–168. doi: 10.1111/j.1699-0463.1959.tb04844.x. [DOI] [PubMed] [Google Scholar]
- 44.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Human genetics. 1993;92(2):183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 45.Bouillon R, van Baelen H, de Moor P. Comparative study of the affinity of the serum vitamin D-binding protein. Journal of steroid biochemistry. 1980;13(9):1029–1034. doi: 10.1016/0022-4731(80)90133-8. [DOI] [PubMed] [Google Scholar]
- 46.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clinical chemistry. 2001;47(4):753–756. [PubMed] [Google Scholar]
- 47.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Human genetics. 1986;72(4):281–293. doi: 10.1007/BF00290950. [DOI] [PubMed] [Google Scholar]
- 48.Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by mathematical modeling. PloS one. 2012;7(1):e30773. doi: 10.1371/journal.pone.0030773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dastani Z, et al. In healthy adults, biological activity of vitamin D, as assessed by serum PTH, is largely independent of DBP concentrations. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013 doi: 10.1002/jbmr.2042. [DOI] [PubMed] [Google Scholar]
- 50.Denburg MR, et al. Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28(9):1843–1853. doi: 10.1007/s00467-013-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinstein SJ, et al. Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case-control study. Cancer research. 2012;72(5):1190–1198. doi: 10.1158/0008-5472.CAN-11-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carpenter TO, et al. Vitamin D binding protein is a key determinant of 25-hydroxyvitamin D levels in infants and toddlers. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(1):213–221. doi: 10.1002/jbmr.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glendenning P, Chew GT, Inderjeeth CA, Taranto M, Fraser WD. Calculated free and bioavailable vitamin D metabolite concentrations in vitamin D-deficient hip fracture patients after supplementation with cholecalciferol and ergocalciferol. Bone. 2013;56(2):271–275. doi: 10.1016/j.bone.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Bouillon R, Xiang DZ, Convents R, Van Baelen H. Polyunsaturated fatty acids decrease the apparent affinity of vitamin D metabolites for human vitamin D-binding protein. The Journal of steroid biochemistry and molecular biology. 1992;42(8):855–861. doi: 10.1016/0960-0760(92)90094-y. [DOI] [PubMed] [Google Scholar]


