Abstract
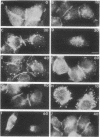
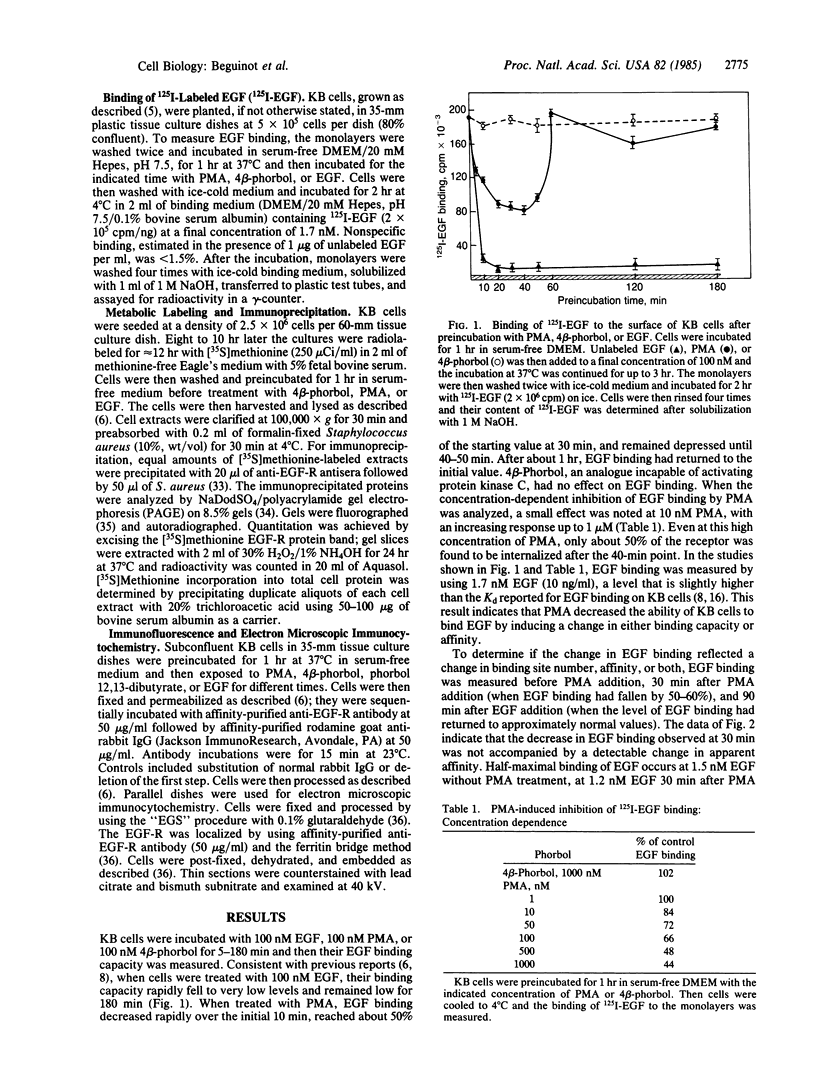
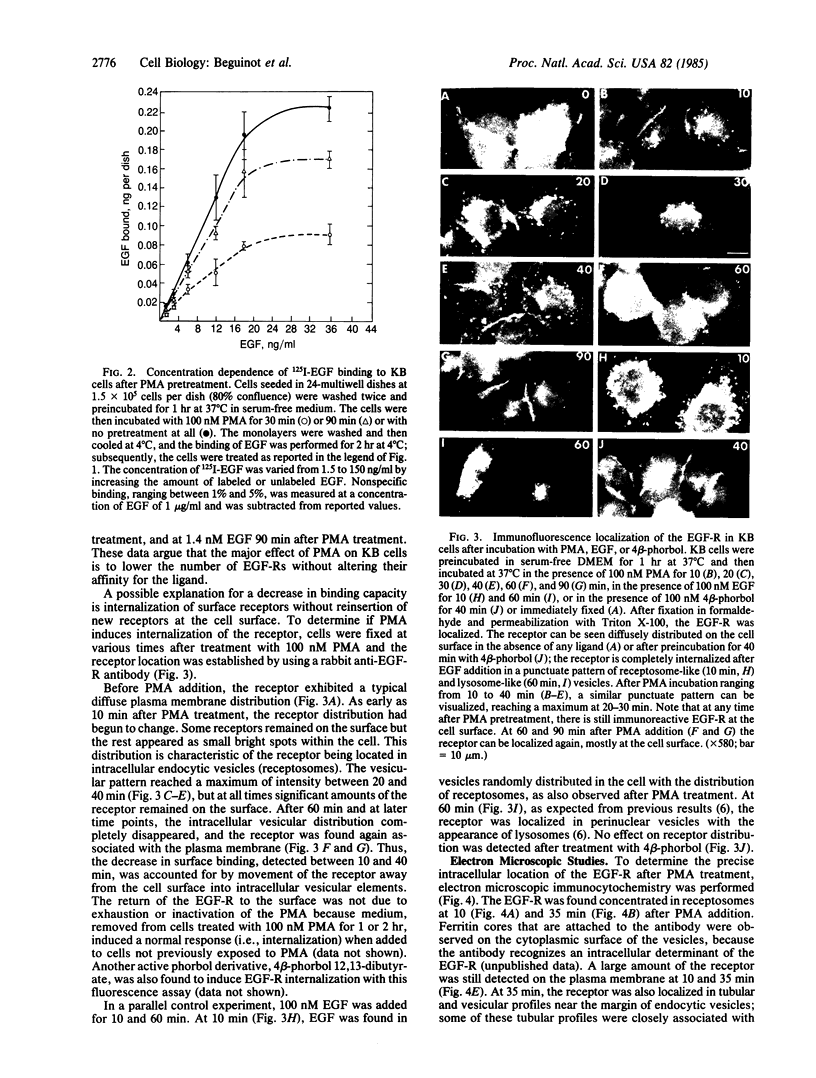
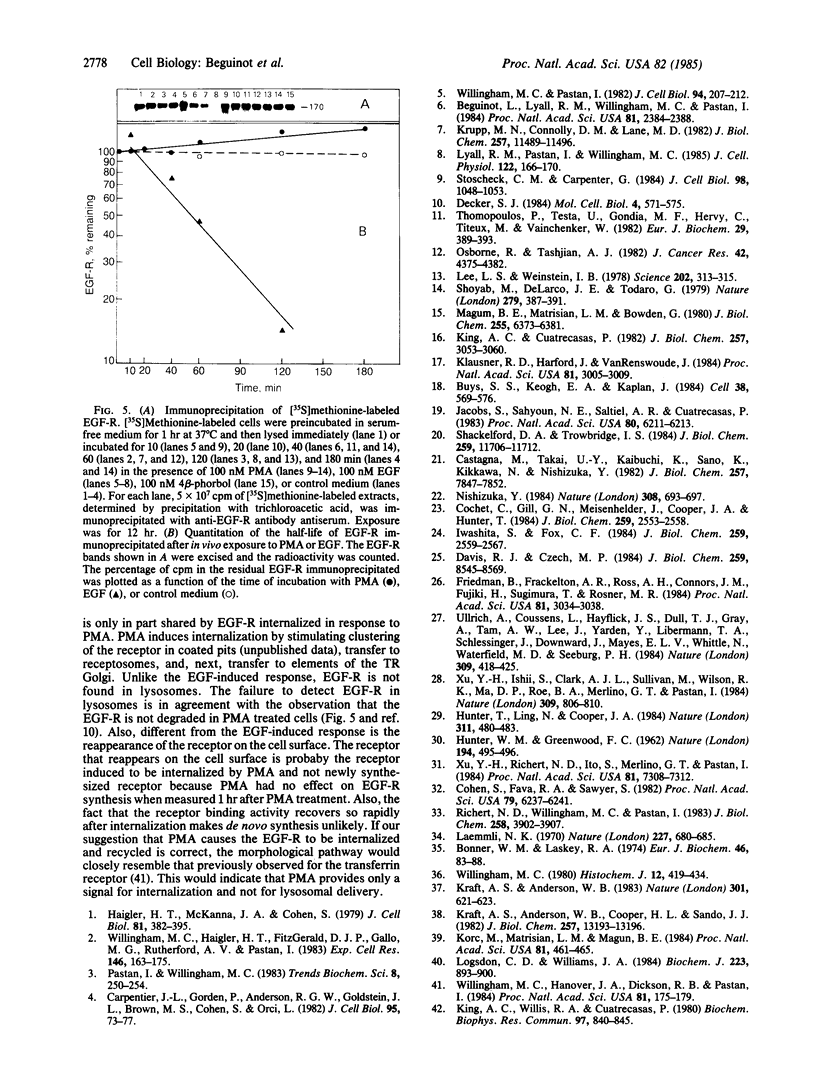
4 beta-Phorbol 12-myristate 13-acetate (PMA) treatment of KB cells at 37 degrees C rapidly induces a 50% reduction in epidermal growth factor (EGF) binding that is maximal by 30 min. EGF binding activity returns to the original value by 1 hr and remains constant for 2 hr thereafter. Using a polyclonal antibody directed against the cytoplasmic domain of the EGF receptor (EGF-R), we examined the fate of the receptor after PMA treatment. Immunofluorescent and electron microscopic localization of the EGF-R after PMA treatment demonstrated that about 50% of the receptor became internalized into endocytic vesicles (receptosomes) and Golgi-associated structures. Unlike EGF-induced internalization, PMA-induced internalization did not cause delivery of EGF-R to lysosomes or receptor degradation. Rather, receptor reappeared on the cell surface. No stimulation of EGF-R synthesis was observed after 1 hr of PMA treatment. Loss of cell surface binding correlated with the internalization of the EGF-R observed morphologically. A possible explanation for these observations is that PMA, an activator of protein kinase C, confers a signal sufficient for EGF-R clustering and internalization but not for transport to lysosomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguinot L., Lyall R. M., Willingham M. C., Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buys S. S., Keogh E. A., Kaplan J. Fusion of intracellular membrane pools with cell surfaces of macrophages stimulated by phorbol esters and calcium ionophores. Cell. 1984 Sep;38(2):569–576. doi: 10.1016/0092-8674(84)90511-7. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cohen S., Fava R. A., Sawyer S. T. Purification and characterization of epidermal growth factor receptor/protein kinase from normal mouse liver. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6237–6241. doi: 10.1073/pnas.79.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters mediate phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984 Jul 10;259(13):8545–8549. [PubMed] [Google Scholar]
- Decker S. J. Aspects of the metabolism of the epidermal growth factor receptor in A431 human epidermoid carcinoma cells. Mol Cell Biol. 1984 Apr;4(4):571–575. doi: 10.1128/mcb.4.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B., Frackelton A. R., Jr, Ross A. H., Connors J. M., Fujiki H., Sugimura T., Rosner M. R. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 May;81(10):3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Resolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol esters. J Biol Chem. 1982 Mar 25;257(6):3053–3060. [PubMed] [Google Scholar]
- King A. C., Willis R. A., Cuatrecasas P. Accumulation of epidermal growth factor within cells does not depend on receptor recycling. Biochem Biophys Res Commun. 1980 Dec 16;97(3):840–845. doi: 10.1016/0006-291x(80)91453-9. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M., Matrisian L. M., Magun B. E. Cytosolic calcium regulates epidermal growth factor endocytosis in rat pancreas and cultured fibroblasts. Proc Natl Acad Sci U S A. 1984 Jan;81(2):461–465. doi: 10.1073/pnas.81.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Connolly D. T., Lane M. D. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11489–11496. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., Williams J. A. Intracellular Ca2+ and phorbol esters synergistically inhibit internalization of epidermal growth factor in pancreatic acini. Biochem J. 1984 Nov 1;223(3):893–900. doi: 10.1042/bj2230893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall R. M., Pastan I., Willingham M. C. EGF induces receptor down-regulation with no receptor recycling in KB cells. J Cell Physiol. 1985 Jan;122(1):166–170. doi: 10.1002/jcp.1041220125. [DOI] [PubMed] [Google Scholar]
- Magun B. E., Matrisian L. M., Bowden G. T. Epidermal growth factor. Ability of tumor promoter to alter its degradation, receptor affinity and receptor number. J Biol Chem. 1980 Jul 10;255(13):6373–6381. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Osborne R., Tashjian A. H., Jr Modulation of peptide binding to specific receptors on rat pituitary cells by tumor-promoting phorbol esters: decreased binding of thyrotropin-releasing hormone and somatostatin as well as epidermal growth factor. Cancer Res. 1982 Nov;42(11):4375–4381. [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Induction of expression and phosphorylation of the human interleukin 2 receptor by a phorbol diester. J Biol Chem. 1984 Oct 10;259(19):11706–11712. [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos P., Testa U., Gourdin M. F., Hervy C., Titeux M., Vainchenker W. Inhibition of insulin receptor binding by phorbol esters. Eur J Biochem. 1982 Dec 15;129(2):389–393. doi: 10.1111/j.1432-1033.1982.tb07062.x. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C. Electron microscopic immunocytochemical localization of intracellular antigens in cultured cells: the EGS and ferritin bridge procedures. Histochem J. 1980 Jul;12(4):419–434. doi: 10.1007/BF01011958. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Haigler H. T., Fitzgerald D. J., Gallo M. G., Rutherford A. V., Pastan I. H. The morphologic pathway of binding and internalization of epidermal growth factor in cultured cells. Studies on A431, KB, and 3T3 cells, using multiple methods of labelling. Exp Cell Res. 1983 Jun;146(1):163–175. doi: 10.1016/0014-4827(83)90334-8. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Hanover J. A., Dickson R. B., Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. H. Transit of epidermal growth factor through coated pits of the Golgi system. J Cell Biol. 1982 Jul;94(1):207–212. doi: 10.1083/jcb.94.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. H., Ishii S., Clark A. J., Sullivan M., Wilson R. K., Ma D. P., Roe B. A., Merlino G. T., Pastan I. Human epidermal growth factor receptor cDNA is homologous to a variety of RNAs overproduced in A431 carcinoma cells. 1984 Jun 28-Jul 4Nature. 309(5971):806–810. doi: 10.1038/309806a0. [DOI] [PubMed] [Google Scholar]
- Xu Y. H., Richert N., Ito S., Merlino G. T., Pastan I. Characterization of epidermal growth factor receptor gene expression in malignant and normal human cell lines. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7308–7312. doi: 10.1073/pnas.81.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]