Abstract
Objective
The purpose of this case series is to report the effects of manipulation under anesthesia (MUA) for patients with lumbopelvic (lumbar spine, sacroiliac and/or pelvic, hip) pain in an outpatient ambulatory/hospital-based setting.
Methods
A retrospective chart review of cases treated at an outpatient ambulatory surgical center in New York and a general hospital in New York was performed. Patients with pre- and postintervention Oswestry Low Back Pain Disability Index (ODI) scores and lumbopelvic and hip complaints were included (N = 18). No intervention other than MUA was administered between the initial and follow-up ODI scoring. Scores on the ODI were assessed within 1 week prior to MUA and again within 2 weeks postprocedure.
Results
Patients underwent 2 to 4 chiropractic MUA procedures over the course of 7 to 8 days as per National Academy of Manipulation Under Anesthesia physicians' protocols. Preprocedure ODI scores ranged from 38 to 76, with an average score of 53.4. Postprocedure scores ranged from 0 to 66, with an average score of 32.8. For each patient, ODI scores were lower after MUA, with an average decrease of 20.6. Sixteen of 18 patients experienced a clinically meaningful improvement in ODI score. No adverse reactions were reported.
Conclusions
For 16 of the 18 patients with chronic lumbopelvic pain reported in this study, MUA showed clinically meaningful reduction in low back pain disability.
Key indexing terms: Manipulation, Chiropractic, Lumbar vertebrae, Back pain, Anesthesia
Introduction
Low back pain (LBP) is a common, costly problem that afflicts 60% to 90% of the population at some point during adulthood,1,2 with 1 in 4 people experiencing back pain at any given time. Thirty percent of people who experience acute LBP, which is defined as pain lasting less than 4 weeks,3 will transition to a chronic LBP, a painful state lasting longer than 12 weeks.4 Although prevalence estimates vary across studies from 5% to 90%, careful estimates suggest that 15% of all cases of LBP are facetogenic, that is, arising from zygapophyseal (Z) joints.5 Roughly 25% of patients with LBP have pain due to sacroiliac joint pathology.6 Despite these estimates, the cause of nonspecific LBP remains unknown in a majority of cases.
The use of high-velocity, low-amplitude (HVLA) manipulation of the spine has been shown to reduce pain and improve function in both acute and chronic back pain when compared to sham manipulation.7-9 By directing the manual thrust past the physiologic barrier, direct HVLA manipulation differs from indirect nonthrust manual therapy by the associated audible release of the articular surface during HVLA manipulation, referred to as cavitation. The effects of cavitation at the spinal Z-joint have been studied extensively.10-13 The audible separation of these joints is associated with release of tissue adhesions, stimulation of the afferent nerve to the Z-joint and spinal muscles, and reflex neurologic and possibly immunologic sequelae and inflammatory chemical down-regulation.10-14
There are a percentage of patients in whom cavitation of the spinal facet joint is not possible because of spasm, guarding, and inhibitory mechanisms15-17 despite meeting clinical criteria for its use.18-20 Manipulation under anesthesia (MUA) is a pain management procedure using passive stretches combined with spinal manipulation under conscious sedation or general anesthesia with the goal of relieving musculoskeletal pain. The MUA procedure generally consists of sedation, mobilization/stretching/traction, manipulation, and post-MUA care.15-17,21-29 Anesthesia minimizes pain, muscle spasm, and protective guarding that may occur during manipulation. Manipulation on a sedated patient is purported to enhance the practitioner’s ability to break apart adhesions and repair segmental dysfunction,11,15,17,30 leading to increased ligament, tendon, muscle, and articular flexibility.22
Manipulation under anesthesia has been used as an intervention for back pain in some form for more than 80 years.31 Although it was the mainstay of orthopedists prior to the mid-1960s,24,32 today, MUA is most commonly performed by doctors of chiropractic and medical pain management specialists33,34 and is reported to have improved techniques and safer, rapid-acting anesthetics with short half-lives.17,23
Manipulation under anesthesia is routinely used for pain of various etiologies. Indications include disk herniation/prolapse/protrusion/bulge, joint or spinal ankylosis, failed low back surgery, nonresponsive muscle contraction, compression syndromes with nonosteophytic entrapment, and whiplash-associated disorders.26 The procedure is generally reserved for patients who are responsive to noninvasive spinal manipulation techniques but who continue to experience pain and/or reduced mobility. A clinical justification for MUA has been suggested by the National Academy of Manipulation Under Anesthesia Physicians,28 as well as the International Academy of MUA Physicians35 and the National Association of MUA Physicians.36
The purpose of this case series is to study the effects of chiropractic MUA for patients with lumbopelvic (lumbar spine, sacroiliac and/or pelvic, hip) pain in an outpatient ambulatory/hospital-based setting.
Methods
Data Collection
This case series based upon a retrospective chart review was deemed exempt under 45 CFR 46.101(b)(4) from the United Health Services Hospitals Institutional Review Board. Patient charts from visits between June 23, 2010, and August 29, 2011, were reviewed by an independent reviewer invited by the authors to the practice. Charts were reviewed for patients treated at United Health Services Outpatient Ambulatory Surgical Center, Johnson City, NY, and Binghamton General Hospital, Binghamton, NY.
Thirty randomly selected case files were included, 18 of which met criteria for the review. Patients with concomitant cervical, thoracic, or shoulder complaints were excluded, as well as charts wherein Oswestry Low Back Pain Disability Indexes (ODIs) were incomplete or outside the 2-week postprocedure window. Data collected from the charts included sex, age, insurance type, diagnoses, and ODI scores. The decision to use MUA was based on established clinical prediction rules.19 The criteria used to determine the medical necessity of the MUA procedure were derived from the National Academy of Manipulation Under Anesthesia Physicians and the International Academy of MUA Physicians. This includes a favorable response to conservative, noninvasive chiropractic and medical treatments, with continued intractable pain and/or biomechanical dysfunction. In all cases, there was documentation that patient's pain threshold had inhibited the effectiveness of conservative manipulation, delaying or prolonging treatment effects.
Adverse Events
A serious adverse event was defined as disk herniation; increased radicular pain; or neurological sequelae such as cauda equina syndrome, sensory or reflex loss, or motor weakness.
Oswestry Disability Index
The ODI Questionnaire37 is a self-reported, 10-item questionnaire that quantifies a patient’s subjective perception of his or her disability as it relates to pain in the lower back. The ODI has 10 sections: pain, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling. The ODI is widely used by clinicians who treat LBP for determining functional disability due to LBP and is simple, quick, and inexpensive to administer. The instrument has been validated in various patient populations and across languages.38 Moreover, the ODI is reliable and has a good internal consistency with a Cronbach α between 0.71 and 0.86.39,40 Researchers have recommended the use of ODI or similarly robust instrument in MUA studies.29 Notably, a change of between 5 and 9 points is considered by patients to be clinically important.41
In each included case, subjects filled out an ODI questionnaire within 1 week prior to their MUA procedure and again within 2 weeks of their final MUA treatment. The ODI scores were recorded by the clinician in the office notes, along with the dated and patient-signed index documents, as part of the patient’s chart. No intervention (eg, physical therapy, injections, chiropractic) was administered between the initial ODI assessment and follow-up other than MUA.
MUA Procedure
All MUA procedures were performed by DT at United Health Services, Outpatient Ambulatory Surgical Center in Johnson City, NY, or Binghamton General Hospital, Binghamton, NY. A board-certified anesthesiologist induced and maintained conscious sedation; and patients were monitored throughout the procedure by electrocardiography, blood pressure, and pulse oximetry.
From the supine position, the following were performed on the left then right lower extremities. While in the supine position, the patient’s knee was flexed to 90°, with a contact on the popliteal fossa. In this position, the patient then underwent passive right external hip flexion stretching, followed by neutral and then internal hip flexion. This was followed by the traditional Patrick/Fabere hip stretch slightly beyond the elastic barrier. Then, the hip was flexed and internally rotated with a slight impulse posterolaterally as a piriformis bow stretch. The lower extremity was then returned to a neutral position; and then passive abduction of the leg was performed with myofascial stripping of the semimembranosus, gracilis, and adductor muscles. With a contact distally at the ankle mortice joint, the patient then underwent external long axis lower extremity traction manipulation to the hip with 30° to 35° of external rotation with caudal traction. In many cases, a second manipulative impulse with internal hip rotation was performed. At that point, the patient was rotated into a lateral decubitus position for passive stretching of the contralateral quadratus lumborum followed by myofascial stripping of the tensor fascia latae on that side. While still in the lateral decubitus position, manipulation of the hemipelvis was performed. Segmental localization of the involved vertebral motor units was achieved via palpation. Then, the elastic barrier of resistance was found; and a low-velocity manipulative impulse was performed to the involved lumbar vertebral motor units into rotation. Both legs were alternately subjected to straight leg raise hamstring stretch (sciatic nerve release maneuver) using linear force to gradually increase hip flexion and dorsiflexion at end range to release the gastrocnemius and soleus muscles.
After the MUA procedure, patients were transferred to the recovery room and monitored according to postanesthesia protocol.
Statistics
The ODI scores before and after treatment were compared using a paired Student t test, whereas sex and insurance-type comparisons were made using independent t tests with Pearson product-moment correlations. P values of less than .05 were considered significant. Statistical analyses were done using SPSS 18 (IBM Corporation, Armonk, NY).
Results
Manipulation under anesthesia was used to treat chronic LBP in 30 reviewed cases. Twelve cases were excluded because of concomitant musculoskeletal pain complaints or insufficient ODI data. Of the 18 included patient cases, 8 were male and 10 were female. Ages ranged from 29 to 60 years, with a mean age of 45. Diagnosed conditions fell within the recognized categories of conditions responsive to MUA as listed in Fig 1. The majority of patients (13 of 18; 72%) had 3 MUA treatments. Three patients in the sample had 2 treatments, and 2 of 18 patients had 4 treatments. Pre-MUA ODI scores were consistent with moderate to severe patient disability, and the level of pain interfered with lifestyle and activities of daily living. These initial scores did not vary by sex, age, or type of payment (insurance type).
Fig 1.
Primary and secondary diagnoses of patients treated with MUA.
| Cervical sprain strain |
| Cervicalgia |
| Cervicobrachial syndrome/brachial neuritis |
| Disc degeneration |
| Displacement/disc displacement |
| Failed back syndrome |
| Hip pain |
| Hip sprain |
| Leg length inequality |
| Lumbalgia |
| Lumbar disc degeneration |
| Lumbar disc displacement |
| Lumbar disc space narrowing |
| Lumbar post-laminectomy syndrome |
| Lumbar sprain strain |
| Lumbar subluxation |
| Lumbosacral radicular syndrome/radiculitis |
| Pelvic somatic dysfunction |
| Radiculopathy |
| Reflex sympathetic dystrophy sacroiliac |
| Somatic dysfunction |
| Sciatic neuralgia |
| Sciatica |
| Somatic dysfunction |
| Thoracic pain |
In all 18 cases, ODI scores were lower after MUA than before treatment (Fig 2). The mean ± SD ODI score was 53.5 ± 11.2 before and 32.8 ± 18.3 after MUA, which was a statistically significant decrease (P < .001). In 16 of 18 cases, ODI scores were at least 8 points lower after MUA, an amount consistent with a clinically meaningful reduction in LBP disability. Insurance type, sex, or age did not influence the degree to which MUA affected ODI scores. None of the 18 patients reported a serious adverse event from MUA treatments.
Fig 2.
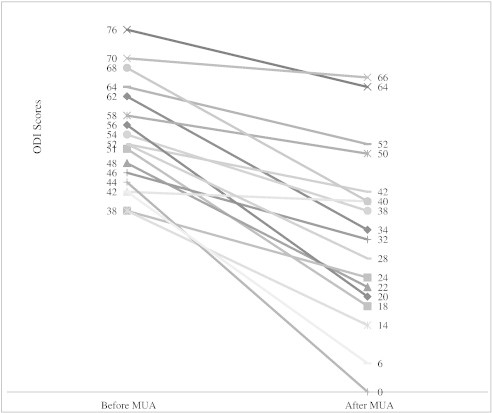
The ODI scores before and after MUA. The mean ODI score decreased from 53.5 ± 11.2 to 32.8 ± 18.3 after MUA (P < .001).
Discussion
This study described the results from 18 patients who underwent single or multiple MUAs to treat chronic lumbar back pain. No patient reported any complications. All patients had improvements in ODI scores, and the mean ODI score decreased significantly after MUA. In 16 of 18 cases, ODI scores were at least 8 points lower after MUA. Because a change of 5 to 9 points on the ODI is considered clinically meaningful,41 all but 2 patients in this sample had a true reduction in LBP disability after MUA.
The 18 patients in our sample had slightly higher disability than LBP patients on average. In a review conducted by the original creators of the ODI after 20 years in use, people with chronic back pain have weighted mean ODI scores that average 43.3 with a standard deviation of 10 to 21.38 The average starting score in the current sample was 10 points higher. Normal populations, on the other hand, have ODI scores of 10.38 It is important to note that not all patients in this sample returned to this level of function.
Although the author uses other methods to evaluate response to the procedure (including computerized digital inclinometry range of motion, visual analog scale, straight leg raise incline changes, etc), this is the first study to use the ODI to assess disability in LBP patients after MUA. In 1999, West and coauthors17 published the results of a relatively large MUA study of 177 patients, a portion of whom had lumbopelvic symptoms. The authors reported that visual analog scale scores, a measure of patient-reported pain, improved by 60% in lumbar pain patients after MUA, with similar improvements in the ability to work and perform activities of daily living and decreased reliance on pain medication.17 In a prospective study, Palmieri et al29 reported a 50% and 51% decrease on a numeric pain scale and Roland Morris Questionnaire, respectively, in 38 MUA-treated patients. The control arm, those who did not undergo the MUA procedure, saw their numeric scale drop only 26% and their Roland Morris Questionnaire score drop 38%. In 2003, Davis et al16 used orthopedic and neurologic examination findings, medication use, and functional capacity as markers of improvement in 2 patients with MUA. Both patients had failed to achieve pain relief after multiple back surgeries. Manipulation under anesthesia performed on 3 consecutive days, with adjunct physical and outpatient therapy, resulted in a marked improvement in pain in both cases. Dougherty and colleagues34 used an empirical rating system to assess MUA efficacy. In 2005, Cremata et al21 used passive ranges of motion, decreases in visual analog scale rating, and diminishment of subsequent visit frequency to assess progress after MUA in 4 patients. In general, MUA was deemed to be effective in restoring articular and myofascial movements. Although these objective parameters are effective in evaluating progress, it has been well documented in spinal procedure literature that a patient’s own estimates of his or her function may be the most important outcome measure.42 The ODI is reliable and well scaled to the measurement of clinical changes in LBP disability compared to other instruments.41
Limitations
There were inherent limitations in this study. A referral bias could lead to referral of patients with more chronic pain, which could affect results. Referral of patients who were highly motivated could also influence results. The small study size contributes to a selection or referral bias. Reviews of case series are the least methodologically robust epidemiological study because they are at risk for several types of bias such as case selection bias.43 All past cases were considered, and only those with insufficient data features (eg, missing ODI scores) were excluded. Patients with successful treatments were more likely to submit a complete second ODI questionnaire, which would have the effect of overrating the benefit of MUA. Finally, retrospective studies are limited. A larger prospective study would need to be performed to formally evaluate benefit.
Alternatively approaching this study as a case-control series brought a separate set of problems, most important of which was the issue of an adequate control group. Offering MUA to patients usually occurs in the most difficult cases, namely, the cases in which pain continues to be intractable after other forms of treatment. It was likely that any control series in this case would be composed of less complex cases with more easily treated LBP. Moreover, pre- and posttreatment ODI scores for other forms of manipulation were not readily available as a comparator group. It is also important to note that, by publishing this cases series, we may be adding to publication bias because most other articles on MUA are case reports and case series.23 Prospective study of MUA would allow proper control (sham) group comparisons. Dr. Taber is currently conducting a prospective trial of this type in patients with lumbopelvic pain.
It has been suggested that effectiveness studies of spinal manipulation (without anesthesia) are no longer needed because results from more than 200 published studies support the intervention’s beneficial effect.44 On the other hand, large-scale, carefully controlled, prospective studies on the effect of MUA on LBP are still needed. These studies should include MUA-treated patients compared to sham controls and in head-to-head trials with related treatments such as extensive traditional spinal manipulation, exercise therapy, spinal injections, or surgery.
Conclusions
This report describes the treatment of 18 patients with chronic lumbopelvic back pain using single or multiple MUAs. Sixteen of the 18 patients had clinically meaningful reduction in LBP disability, and the mean ODI score decreased after MUA for all patients. No complications were reported.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Acknowledgment
The authors thank Dr. Michael T. Sapko for his editorial assistance with this manuscript.
References
- 1.Deyo R.A., Tsui-Wu Y.J. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976) 1987;12(3):264–268. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Skovron M.L. Epidemiology of low back pain. Baillieres Clin Rheumatol. 1992;6(3):559–573. doi: 10.1016/s0950-3579(05)80127-x. [DOI] [PubMed] [Google Scholar]
- 3.Philadelphia Panel evidence-based clinical practice guidelines on selected rehabilitation interventions for low back pain. Phys Ther. 2001;81(10):1641–1674. [PubMed] [Google Scholar]
- 4.Bowman J.M. The meaning of chronic low back pain. AAOHN J. 1991;39(8):381–384. [PubMed] [Google Scholar]
- 5.Schwarzer A.C., Wang S.C., Bogduk N., McNaught P.J., Laurent R. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54(2):100–106. doi: 10.1136/ard.54.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simopoulos T.T., Manchikanti L., Singh V. A systematic evaluation of prevalence and diagnostic accuracy of sacroiliac joint interventions. Pain Physician. 2012;15(3):E305–E344. [PubMed] [Google Scholar]
- 7.Assendelft W.J., Morton S.C., Yu E.I., Suttorp M.J., Shekelle P.G. Spinal manipulative therapy for low back pain. Cochrane Database Syst Rev. 2004;(1):CD000447. doi: 10.1002/14651858.CD000447.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Bronfort G., Haas M., Evans R., Leininger B., Triano J. Effectiveness of manual therapies: the UK evidence report. Chiropr Osteopat. 2010;18:3. doi: 10.1186/1746-1340-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou R., Huffman L.H. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 10.Brodeur R. The audible release associated with joint manipulation. J Manipulative Physiol Ther. 1995;18(3):155–164. [PubMed] [Google Scholar]
- 11.Cramer G.D., Ross K., Pocius J. Evaluating the relationship among cavitation, zygapophyseal joint gapping, and spinal manipulation: an exploratory case series. J Manipulative Physiol Ther. 2011;34(1):2–14. doi: 10.1016/j.jmpt.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ianuzzi A., Khalsa P.S. Comparison of human lumbar facet joint capsule strains during simulated high-velocity, low-amplitude spinal manipulation versus physiological motions. Spine J. 2005;5(3):277–290. doi: 10.1016/j.spinee.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mooney V., Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976;115:149–156. [PubMed] [Google Scholar]
- 14.Cramer G.D., Gregerson D.M., Knudsen J.T., Hubbard B.B., Ustas L.M., Cantu J.A. The effects of side-posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial with sixty-four subjects. Spine (Phila Pa 1976) 2002;27(22):2459–2466. doi: 10.1097/00007632-200211150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Dagenais S., Mayer J., Wooley J.R., Haldeman S. Evidence-informed management of chronic low back pain with medicine-assisted manipulation. Spine J. 2008;8(1):142–149. doi: 10.1016/j.spinee.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Davis G.D., Fernando C.A., da Motta M.A. Manipulation of the low back under general anesthesia: case studies and discussion. J Neuromusculoskelet Syst. 1993;4(3):7–14. [Google Scholar]
- 17.West D.T., Mathews R.S., Miller M.R., Kent G.M. Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22(5):299–308. doi: 10.1016/s0161-4754(99)70062-x. [DOI] [PubMed] [Google Scholar]
- 18.Koes B.W., Assendelft W.J., van der Heijden G.J., Bouter L.M. Spinal manipulation for low back pain. an updated systematic review of randomized clinical trials. Spine (Phila Pa 1976) 1996;21(24):2860–2871. doi: 10.1097/00007632-199612150-00013. [discussion 72–3] [DOI] [PubMed] [Google Scholar]
- 19.Childs J.D., Fritz J.M., Flynn T.W. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141(12):920–928. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- 20.Flynn T., Fritz J., Whitman J. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine (Phila Pa 1976) 2002;27(24):2835–2843. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]
- 21.Cremata E., Collins S., Clauson W., Solinger A.B., Roberts E.S. Manipulation under anesthesia: a report of four cases. J Manipulative Physiol Ther. 2005;28(7):526–533. doi: 10.1016/j.jmpt.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Gordon R.C. An evaluation of the experimental and investigational status and clinical validity of manipulation of patients under anesthesia: a contemporary opinion. J Manipulative Physiol Ther. 2001;24(9):603–611. doi: 10.1067/mmt.2001.119859. [DOI] [PubMed] [Google Scholar]
- 23.Kohlbeck F.J., Haldeman S. Medication-assisted spinal manipulation. Spine J. 2002;2(4):288–302. doi: 10.1016/s1529-9430(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 24.Siehl D., Bradford W.G. Manipulation of the low back under general anesthesia. J Am Osteopath Assoc. 1952;52(4):239–242. [PubMed] [Google Scholar]
- 25.Greenman PE. Manipulation with the Patient under Anesthesia. J Am Osteopath Assoc. 1992;92(9):1159–60, 67–70. [PubMed]
- 26.Morningstar M.W., Strauchman M.N. Management of a 59-year-old female patient with adult degenerative scoliosis using manipulation under anesthesia. J Chiropr Med. 2010;9(2):77–83. doi: 10.1016/j.jcm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morningstar M.W., Strauchman M.N. Manipulation under anesthesia for patients with failed back surgery: retrospective report of 3 cases with 1-year follow-up. J Chiropr Med. 2012;11(1):30–35. doi: 10.1016/j.jcm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Academy of Manipulation Under Anesthesia Physicians. Manipulation under anesthesia. [Internet] [cited 9/11/2013]. Available from: http://www.fcghealth.com/pages/mua_phys_corn_national_namua.htm.
- 29.Palmieri N.F., Smoyak S. Chronic low back pain: a study of the effects of manipulation under anesthesia. J Manipulative Physiol Ther. 2002;25(8):E8–E17. doi: 10.1067/mmt.2002.127072. [DOI] [PubMed] [Google Scholar]
- 30.Cramer G.D., Henderson C.N., Little J.W., Daley C., Grieve T.J. Zygapophyseal joint adhesions after induced hypomobility. J Manipulative Physiol Ther. 2010;33(7):508–518. doi: 10.1016/j.jmpt.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Riches E.W. End-results of manipulation of the back. Lancet. 1930;215(5566):957–960. [Google Scholar]
- 32.Mensor M.C. Non-operative treatment, including manipulation, for lumbar intervertebral-disc syndrome. J Bone Joint Surg Am. 1965;47:1073–1074. [PubMed] [Google Scholar]
- 33.Shekelle P.G., Adams A.H., Chassin M.R., Hurwitz E.L., Brook R.H. Spinal manipulation for low-back pain. Ann Intern Med. 1992;117(7):590–598. doi: 10.7326/0003-4819-117-7-590. [DOI] [PubMed] [Google Scholar]
- 34.Dougherty P., Bajwa S., Burke J., Dishman J.D. Spinal manipulation postepidural injection for lumbar and cervical radiculopathy: a retrospective case series. J Manipulative Physiol Ther. 2004;27(7):449–456. doi: 10.1016/j.jmpt.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 35.International Academy of MUA Physicians. Manipulation under anesthesia didactic and clinical proctoring protocol for MUA certification courses. [Internet] [cited 9/11/2013]; Available from: http://www.muaphysicians.com/protocols.html
- 36.Gordon R.C., editor. Manipulation under anesthesia: concepts in theory and application. CRC Press Taylor & Francis Group; Boca Raton, FL: 2005. [Google Scholar]
- 37.Fairbank J.C., Couper J., Davies J.B., O'Brien J.P. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 38.Fairbank J.C., Pynsent P.B. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. [discussion 52] [DOI] [PubMed] [Google Scholar]
- 39.Wittink H., Turk D.C., Carr D.B., Sukiennik A., Rogers W. Comparison of the redundancy, reliability, and responsiveness to change among SF-36, Oswestry Disability Index, and Multidimensional Pain Inventory. Clin J Pain. 2004;20(3):133–142. doi: 10.1097/00002508-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Gronblad M., Hupli M., Wennerstrand P. Intercorrelation and test-retest reliability of the Pain Disability Index (PDI) and the Oswestry Disability Questionnaire (ODQ) and their correlation with pain intensity in low back pain patients. Clin J Pain. 1993;9(3):189–195. doi: 10.1097/00002508-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Davidson M., Keating J.L. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther. 2002;82(1):8–24. doi: 10.1093/ptj/82.1.8. [DOI] [PubMed] [Google Scholar]
- 42.Chou R., Qaseem A., Snow V. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 43.Kempen J.H. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol. 2011;151(1):7e1–10. doi: 10.1016/j.ajo.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurwitz E.L. Commentary: exercise and spinal manipulative therapy for chronic low back pain: time to call for a moratorium on future randomized trials? Spine J. 2011;11(7):599–600. doi: 10.1016/j.spinee.2011.04.021. [DOI] [PubMed] [Google Scholar]


