Abstract
Many features in the mammalian sensory thalamus, such as the types of neurons, their connections, or their neurotransmitters, are conserved in evolution. We found a wide range in the proportion of gamma-aminobutyric acidergic (GABAergic) neurons in the medial geniculate body, from <1% (bat and rat) to 25% or more (cat and monkey). In the bat, some medial geniculate body subdivisions have no GABAergic cells. Species-specific variation also occurs in the somesthetic ventrobasal complex. In contrast, the lateral geniculate body of the visual system has about the same proportion of GABAergic cells in many species. In the central auditory pathway, only the medial geniculate body shows this arrangement; the relative number of GABAergic cells in the inferior colliculus and auditory cortex is similar in each species. The range in the proportion of GABAergic neurons suggests that there are comparative differences in the neural circuitry for thalamic inhibition. We conclude that the number of GABAergic neurons in thalamic sensory nuclei may have evolved independently or divergently in phylogeny. Perhaps these adaptations reflect neurobehavioral requirements for more complex, less stereotyped processing, as in speech-like communication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calford M. B., Aitkin L. M. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983 Nov;3(11):2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford M. B., Webster W. R. Auditory representation within principal division of cat medial geniculate body: an electrophysiology study. J Neurophysiol. 1981 Jun;45(6):1013–1028. doi: 10.1152/jn.1981.45.6.1013. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I. T., Jones E. G., Powell T. P. The projection of the auditory cortex upon the diencephalon and brain stem in the cat. Brain Res. 1969 Oct;15(2):305–340. doi: 10.1016/0006-8993(69)90160-7. [DOI] [PubMed] [Google Scholar]
- Finlay B. L., Darlington R. B. Linked regularities in the development and evolution of mammalian brains. Science. 1995 Jun 16;268(5217):1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Frisina R. D., O'Neill W. E., Zettel M. L. Functional organization of mustached bat inferior colliculus: II. Connections of the FM2 region. J Comp Neurol. 1989 Jun 1;284(1):85–107. doi: 10.1002/cne.902840107. [DOI] [PubMed] [Google Scholar]
- Gerren R. A., Weinberger N. M. Long term potentiation in the magnocellular medial geniculate nucleus of the anesthetized cat. Brain Res. 1983 Apr 11;265(1):138–142. doi: 10.1016/0006-8993(83)91344-6. [DOI] [PubMed] [Google Scholar]
- Harris R. M., Hendrickson A. E. Local circuit neurons in the rat ventrobasal thalamus--a GABA immunocytochemical study. Neuroscience. 1987 Apr;21(1):229–236. doi: 10.1016/0306-4522(87)90335-6. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Ogren M. P., Vaughn J. E., Barber R. P., Wu J. Y. Light and electron microscopic immunocytochemical localization of glutamic acid decarboxylase in monkey geniculate complex: evidence for gabaergic neurons and synapses. J Neurosci. 1983 Jun;3(6):1245–1262. doi: 10.1523/JNEUROSCI.03-06-01245.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig T. J., Morel A. Organization of the thalamocortical auditory system in the cat. Annu Rev Neurosci. 1983;6:95–120. doi: 10.1146/annurev.ne.06.030183.000523. [DOI] [PubMed] [Google Scholar]
- Imig T. J., Reale R. A. Ipsilateral corticocortical projections related to binaural columns in cat primary auditory cortex. J Comp Neurol. 1981 Nov 20;203(1):1–14. doi: 10.1002/cne.902030102. [DOI] [PubMed] [Google Scholar]
- Ledoux J. E., Ruggiero D. A., Forest R., Stornetta R., Reis D. J. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987 Oct 1;264(1):123–146. doi: 10.1002/cne.902640110. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A., Nobin A., Stenevi U. The adrenergic innervation of the rat thalamus as revealed by the glyoxylic acid fluorescence method. J Comp Neurol. 1974 Apr 1;154(3):317–347. doi: 10.1002/cne.901540307. [DOI] [PubMed] [Google Scholar]
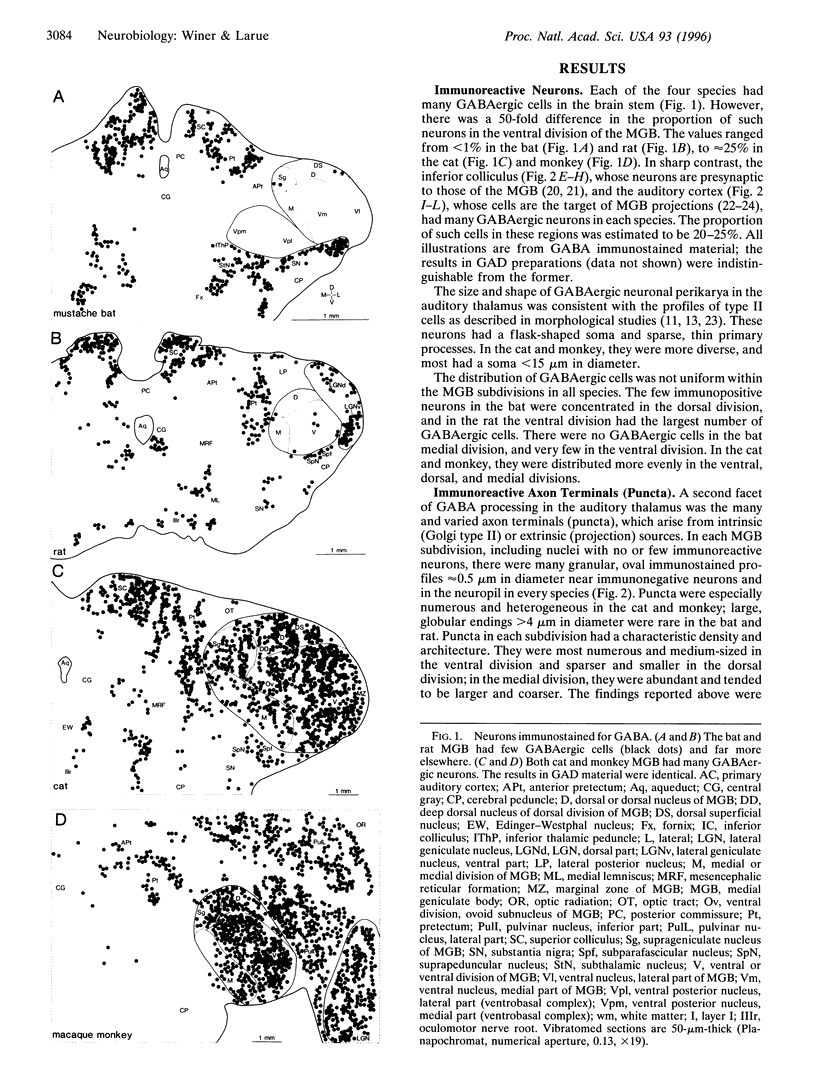
- MOREST D. K. THE NEURONAL ARCHITECTURE OF THE MEDIAL GENICULATE BODY OF THE CAT. J Anat. 1964 Oct;98:611–630. [PMC free article] [PubMed] [Google Scholar]
- Montero V. M. The interneuronal nature of GABAergic neurons in the lateral geniculate nucleus of the rhesus monkey: a combined HRP and GABA-immunocytochemical study. Exp Brain Res. 1986;64(3):615–622. doi: 10.1007/BF00340502. [DOI] [PubMed] [Google Scholar]
- Morest D. K. Dendrodendritic synapses of cells that have axons: the fine structure of the Golgi type II cell in the medial geniculate body of the cat. Z Anat Entwicklungsgesch. 1971;133(2):216–246. doi: 10.1007/BF00528025. [DOI] [PubMed] [Google Scholar]
- Morest D. K. Synaptic relationships of Golgi type II cells in the medial geniculate body of the cat. J Comp Neurol. 1975 Jul 15;162(2):157–193. doi: 10.1002/cne.901620202. [DOI] [PubMed] [Google Scholar]
- Morest D. K., Winer J. A. The comparative anatomy of neurons: homologous neurons in the medial geniculate body of the opossum and the cat. Adv Anat Embryol Cell Biol. 1986;97:1–94. doi: 10.1007/978-3-642-70652-3. [DOI] [PubMed] [Google Scholar]
- Mugnaini E., Dahl A. L. Zinc-aldehyde fixation for light-microscopic immunocytochemistry of nervous tissues. J Histochem Cytochem. 1983 Dec;31(12):1435–1438. doi: 10.1177/31.12.6355290. [DOI] [PubMed] [Google Scholar]
- Niimi K., Matsuoka H. Thalamocortical organization of the auditory system in the cat studied by retrograde axonal transport of horseradish peroxidase. Adv Anat Embryol Cell Biol. 1979;57:1–56. doi: 10.1007/978-3-642-67353-5. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Tappaz M. L., Kopin I. J. Production of a specific antiserum to rat brain glutamic acid decarboxylase by injection of an antigen-antibody complex. Neuroscience. 1981;6(12):2689–2700. doi: 10.1016/0306-4522(81)90113-5. [DOI] [PubMed] [Google Scholar]
- Ohara P. T., Chazal G., Ralston H. J., 3rd Ultrastructural analysis of GABA-immunoreactive elements in the monkey thalamic ventrobasal complex. J Comp Neurol. 1989 May 22;283(4):541–558. doi: 10.1002/cne.902830408. [DOI] [PubMed] [Google Scholar]
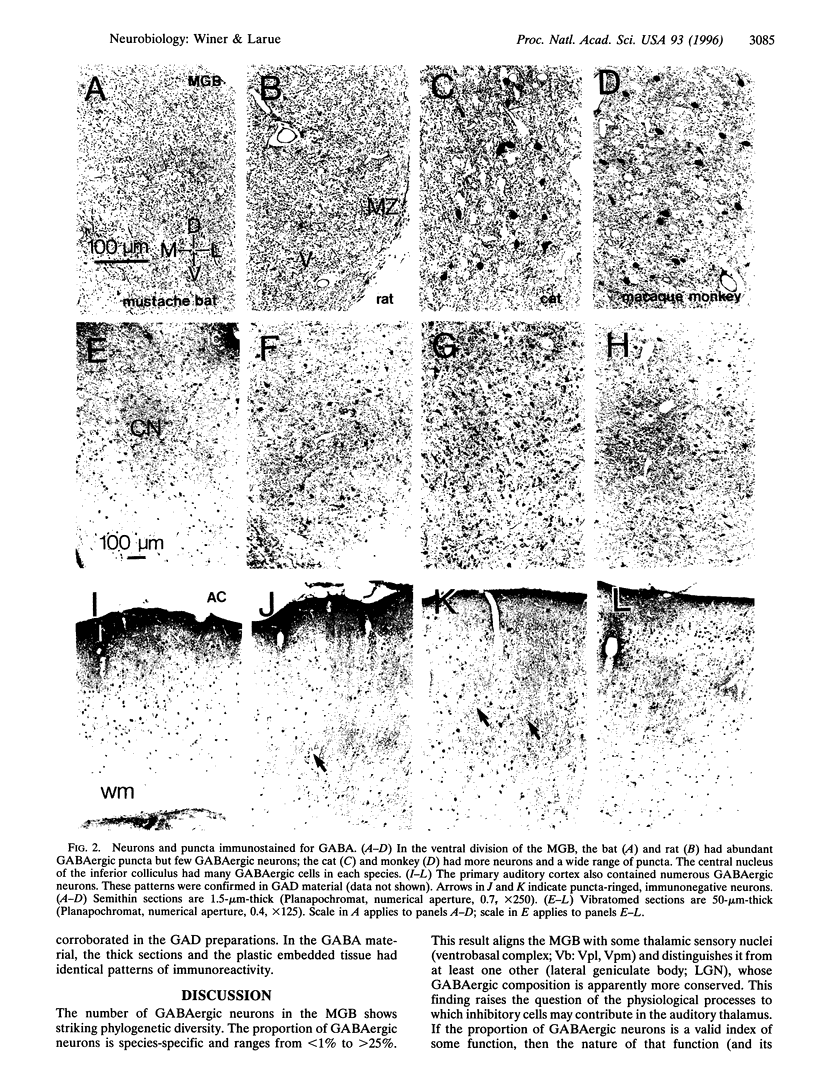
- Ohara P. T., Lieberman A. R., Hunt S. P., Wu J. Y. Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat; immunohistochemical studies by light and electron microscopy. Neuroscience. 1983;8(2):189–211. doi: 10.1016/0306-4522(83)90060-x. [DOI] [PubMed] [Google Scholar]
- Ohara P. T., Lieberman A. R. Some aspects of the synaptic circuitry underlying inhibition in the ventrobasal thalamus. J Neurocytol. 1993 Sep;22(9):815–825. doi: 10.1007/BF01181326. [DOI] [PubMed] [Google Scholar]
- Olsen J. F., Suga N. Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of relative velocity information. J Neurophysiol. 1991 Jun;65(6):1254–1274. doi: 10.1152/jn.1991.65.6.1254. [DOI] [PubMed] [Google Scholar]
- Olsen J. F., Suga N. Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of target range information. J Neurophysiol. 1991 Jun;65(6):1275–1296. doi: 10.1152/jn.1991.65.6.1275. [DOI] [PubMed] [Google Scholar]
- Penny G. R., Conley M., Schmechel D. E., Diamond I. T. The distribution of glutamic acid decarboxylase immunoreactivity in the diencephalon of the opossum and rabbit. J Comp Neurol. 1984 Sep 1;228(1):38–56. doi: 10.1002/cne.902280106. [DOI] [PubMed] [Google Scholar]
- Penny G. R., Fitzpatrick D., Schmechel D. E., Diamond I. T. Glutamic acid decarboxylase-immunoreactive neurons and horseradish peroxidase-labeled projection neurons in the ventral posterior nucleus of the cat and Galago senegalensis. J Neurosci. 1983 Sep;3(9):1868–1887. doi: 10.1523/JNEUROSCI.03-09-01868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
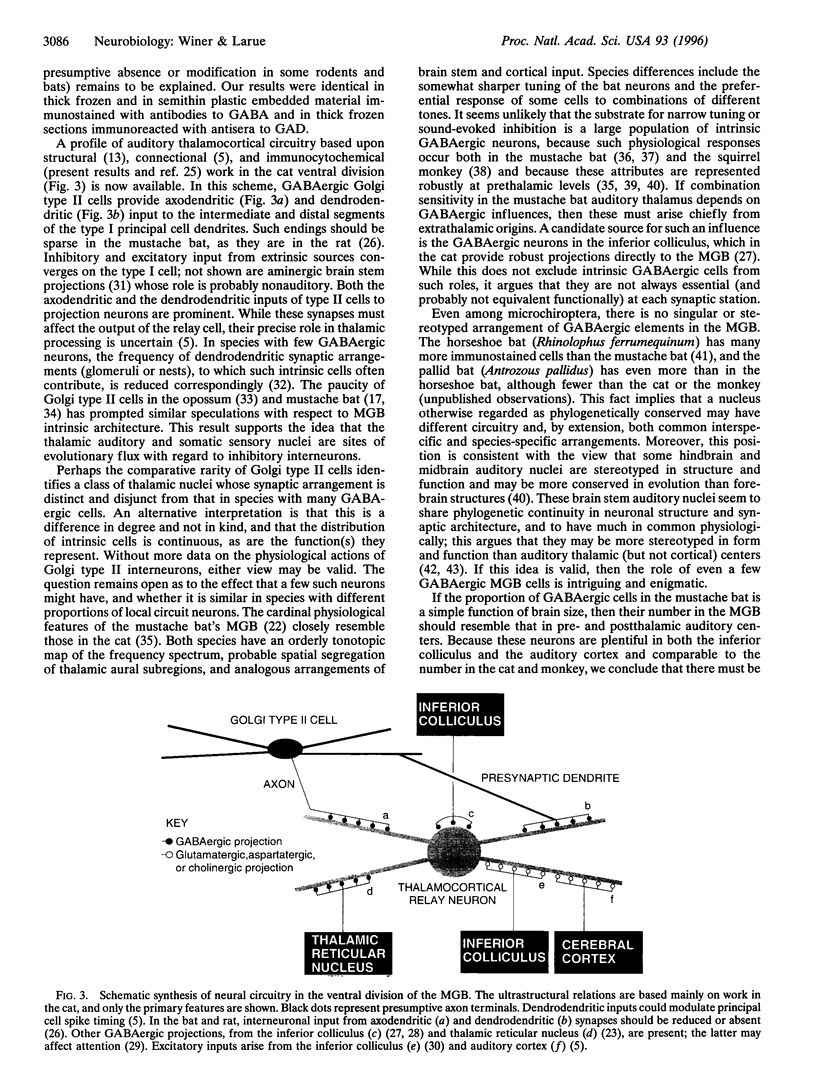
- Pritz M. B., Stritzel M. E. Percentage of intrinsic and relay cells in a thalamic nucleus projecting to general cortex in reptiles, Caiman crocodilus. Brain Res. 1987 Apr 14;409(1):146–150. doi: 10.1016/0006-8993(87)90751-7. [DOI] [PubMed] [Google Scholar]
- Schofield B. R., Cant N. B. Organization of the superior olivary complex in the guinea pig. I. Cytoarchitecture, cytochrome oxidase histochemistry, and dendritic morphology. J Comp Neurol. 1991 Dec 22;314(4):645–670. doi: 10.1002/cne.903140403. [DOI] [PubMed] [Google Scholar]
- Spreafico R., Frassoni C., Arcelli P., De Biasi S. GABAergic interneurons in the somatosensory thalamus of the guinea-pig: a light and ultrastructural immunocytochemical investigation. Neuroscience. 1994 Apr;59(4):961–973. doi: 10.1016/0306-4522(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Vater M., Kössl M., Horn A. K. GAD- and GABA-immunoreactivity in the ascending auditory pathway of horseshoe and mustached bats. J Comp Neurol. 1992 Nov 8;325(2):183–206. doi: 10.1002/cne.903250205. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Larue D. T. Anatomy of glutamic acid decarboxylase immunoreactive neurons and axons in the rat medial geniculate body. J Comp Neurol. 1988 Dec 1;278(1):47–68. doi: 10.1002/cne.902780104. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Larue D. T., Pollak G. D. GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. J Comp Neurol. 1995 May 8;355(3):317–353. doi: 10.1002/cne.903550302. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Wenstrup J. J., Larue D. T. Patterns of GABAergic immunoreactivity define subdivisions of the mustached bat's medial geniculate body. J Comp Neurol. 1992 May 1;319(1):172–190. doi: 10.1002/cne.903190114. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Wenstrup J. J. The neurons of the medial geniculate body in the mustached bat (Pteronotus parnellii). J Comp Neurol. 1994 Aug 8;346(2):183–206. doi: 10.1002/cne.903460203. [DOI] [PubMed] [Google Scholar]