Abstract
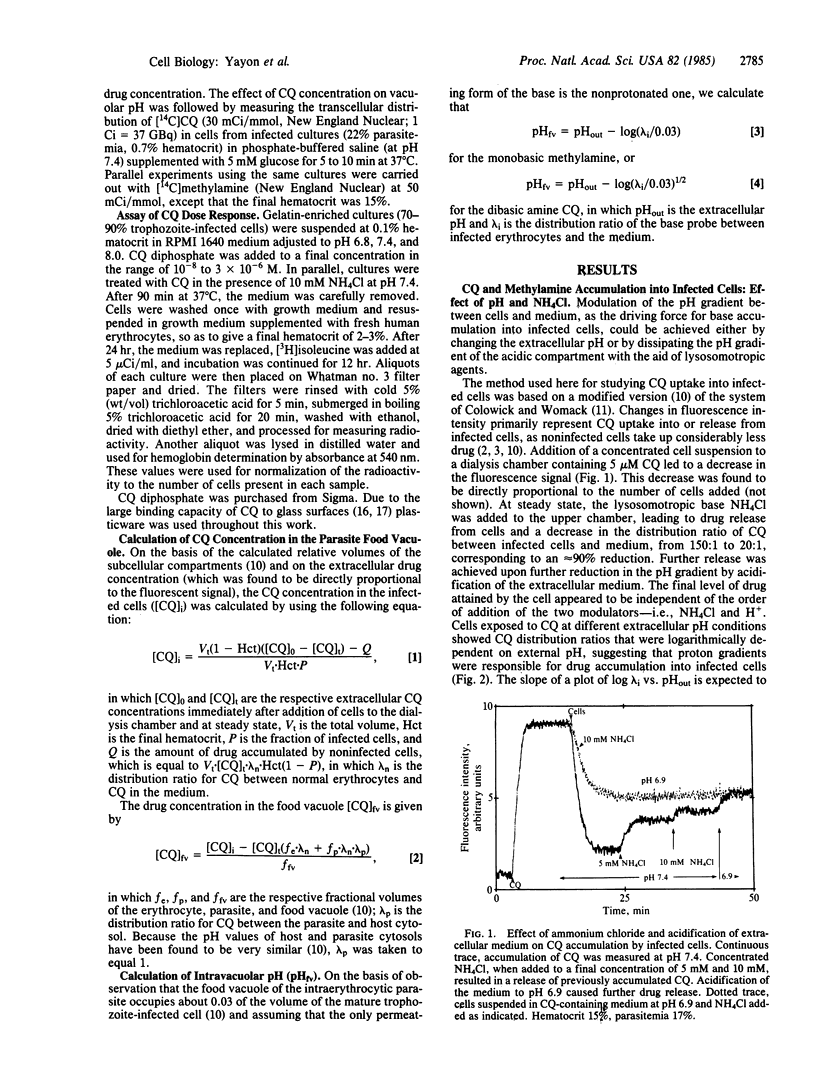
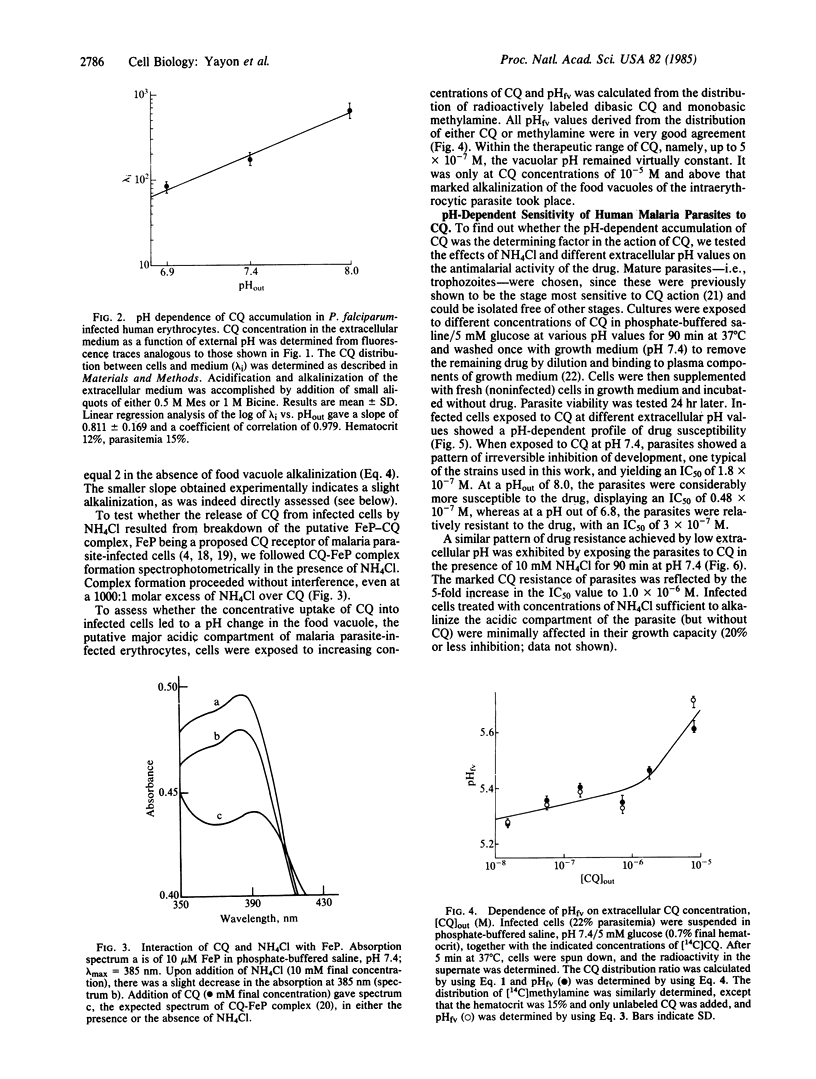
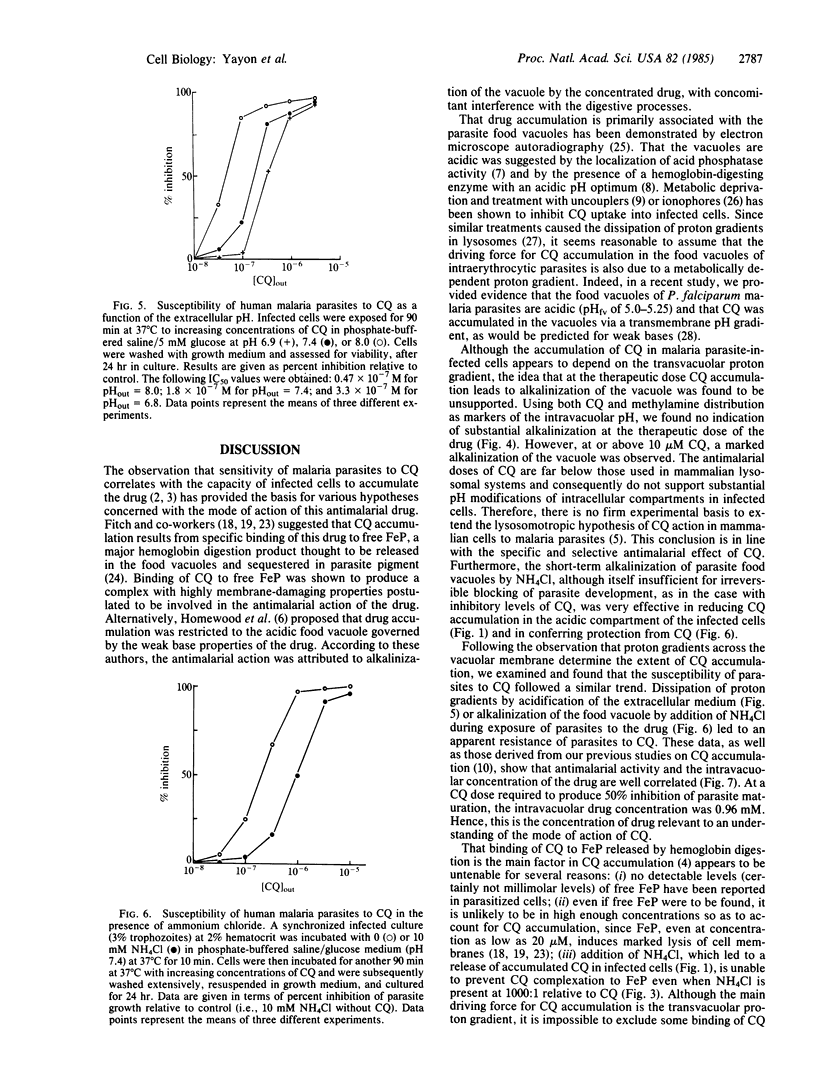
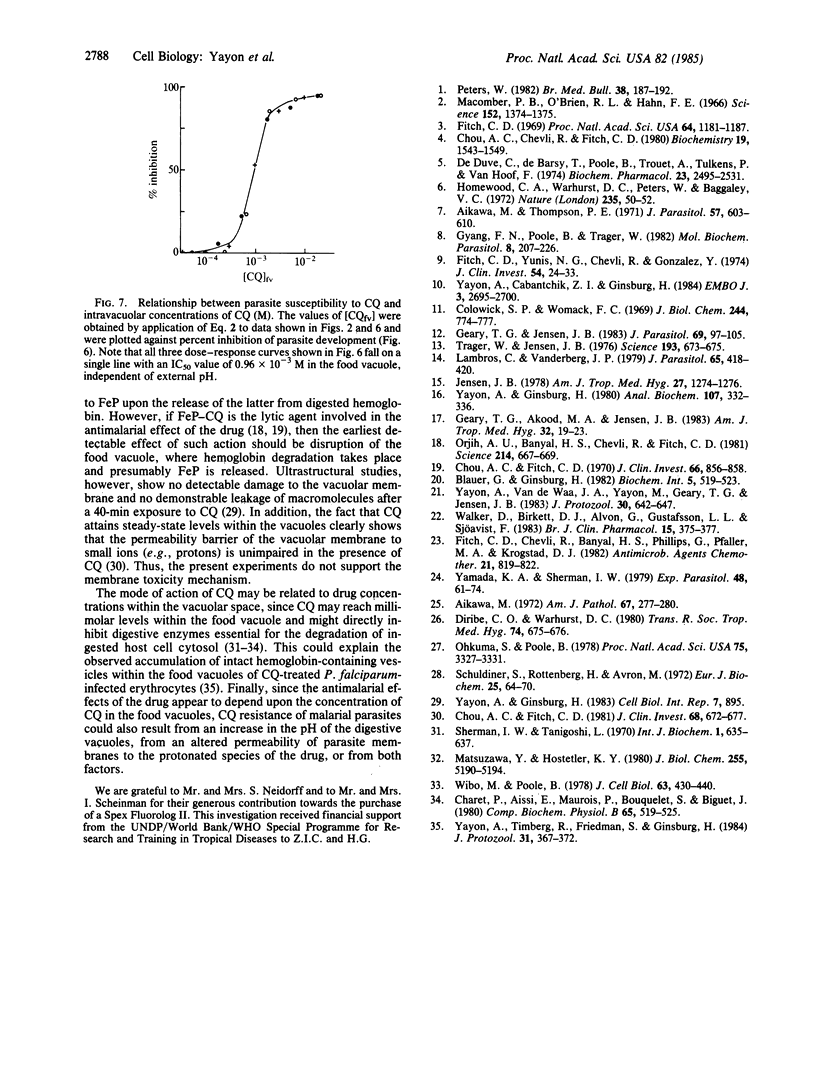
Chloroquine (CQ) accumulates in the acidic food vacuole of intraerythrocytic malaria parasites (Plasmodium falciparum) by virtue of its weak base properties. In the present work, the extent of CQ accumulation was determined by the transvacuolar pH gradient: modification of the latter--either by changing the external pH or by adding the acidotropic agent NH4Cl--led to a corresponding change in CQ distribution between cells and medium. Changes in pH gradient provoked a change in the susceptibility of parasites to CQ: at external pH values of 8.0, 7.4, and 6.8, the IC50 values for CQ were 0.48 X 10(-7) M, 1.8 X 10(-7) M, and 3.3 X 10(-7) M, respectively. Marked resistance to CQ (IC50 = 9.8 X 10(-7) M) was conferred upon cells by exposing them simultaneously to CQ and 10 mM NH4Cl, at pH 7.4. The final concentration of CQ attained within the acidic compartment of the parasite was correlated with inhibition of parasite growth. At therapeutic drug levels, CQ accumulation caused minor changes in the food vacuole pH, whereas at higher CQ concentrations substantial alkalinization was observed. The antimalarial activity of CQ is suggested to be exerted by the interference of the high concentrations of the accumulated drug with vital functions of the food vacuole.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. High-resolution autoradiography of malarial parasites treated with 3 H-chloroquine. Am J Pathol. 1972 May;67(2):277–284. [PMC free article] [PubMed] [Google Scholar]
- Aikawa M., Thompson P. E. Localization of acid phosphatase activity in Plasmodium berghei and P. gallinaceum: an electron microscopic observation. J Parasitol. 1971 Jun;57(3):603–610. [PubMed] [Google Scholar]
- Chou A. C., Chevli R., Fitch C. D. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980 Apr 15;19(8):1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Fitch C. D. Hemolysis of mouse erythrocytes by ferriprotoporphyrin IX and chloroquine. Chemotherapeutic implications. J Clin Invest. 1980 Oct;66(4):856–858. doi: 10.1172/JCI109925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou A. C., Fitch C. D. Mechanism of hemolysis induced by ferriprotoporphyrin IX. J Clin Invest. 1981 Sep;68(3):672–677. doi: 10.1172/JCI110302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Fitch C. D., Chevli R., Banyal H. S., Phillips G., Pfaller M. A., Krogstad D. J. Lysis of Plasmodium falciparum by ferriprotoporphyrin IX and a chloroquine-ferriprotoporphyrin IX complex. Antimicrob Agents Chemother. 1982 May;21(5):819–822. doi: 10.1128/aac.21.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D. Chloroquine resistance in malaria: a deficiency of chloroquine binding. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1181–1187. doi: 10.1073/pnas.64.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Yunis N. G., Chevli R., Gonzalez Y. High-affinity accumulation of chloroquine by mouse erythrocytes infected with Plasmodium berghei. J Clin Invest. 1974 Jul;54(1):24–33. doi: 10.1172/JCI107747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary T. G., Akood M. A., Jensen J. B. Characteristics of chloroquine binding to glass and plastic. Am J Trop Med Hyg. 1983 Jan;32(1):19–23. doi: 10.4269/ajtmh.1983.32.19. [DOI] [PubMed] [Google Scholar]
- Geary T. G., Jensen J. B. Lack of cross-resistance to 4-aminoquinolines in chloroquine-resistant Plasmodium falciparum in vitro. J Parasitol. 1983 Feb;69(1):97–105. [PubMed] [Google Scholar]
- Homewood C. A., Warhurst D. C., Peters W., Baggaley V. C. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972 Jan 7;235(5332):50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Macomber P. B., O'Brien R. L., Hahn F. E. Chloroquine: physiological basis of drug resistance in Plasmodium berghei. Science. 1966 Jun 3;152(3727):1374–1375. doi: 10.1126/science.152.3727.1374. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4'-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J Biol Chem. 1980 Jun 10;255(11):5190–5194. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjih A. U., Banyal H. S., Chevli R., Fitch C. D. Hemin lyses malaria parasites. Science. 1981 Nov 6;214(4521):667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- Peters W. Antimalarial drug resistance: an increasing problem. Br Med Bull. 1982 May;38(2):187–192. doi: 10.1093/oxfordjournals.bmb.a071757. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Walker O., Birkett D. J., Alván G., Gustafsson L. L., Sjöqvist F. Characterization of chloroquine plasma protein binding in man. Br J Clin Pharmacol. 1983 Mar;15(3):375–377. doi: 10.1111/j.1365-2125.1983.tb01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. A., Sherman I. W. Plasmodium lophurae: composition and properties of hemozoin, the malarial pigment. Exp Parasitol. 1979 Aug;48(1):61–74. doi: 10.1016/0014-4894(79)90055-9. [DOI] [PubMed] [Google Scholar]
- Yayon A., Cabantchik Z. I., Ginsburg H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984 Nov;3(11):2695–2700. doi: 10.1002/j.1460-2075.1984.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A., Ginsburg H. A method for the measurement of chloroquine uptake in erythrocytes. Anal Biochem. 1980 Sep 15;107(2):332–336. doi: 10.1016/0003-2697(80)90392-9. [DOI] [PubMed] [Google Scholar]
- Yayon A., Ginsburg H. Chloroquine inhibits the degradation of endocytic vesicles in human malaria parasites. Cell Biol Int Rep. 1983 Nov;7(11):895–895. doi: 10.1016/0309-1651(83)90207-2. [DOI] [PubMed] [Google Scholar]
- Yayon A., Timberg R., Friedman S., Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J Protozool. 1984 Aug;31(3):367–372. doi: 10.1111/j.1550-7408.1984.tb02981.x. [DOI] [PubMed] [Google Scholar]
- Yayon A., Vande Waa J. A., Yayon M., Geary T. G., Jensen J. B. Stage-dependent effects of chloroquine on Plasmodium falciparum in vitro. J Protozool. 1983 Nov;30(4):642–647. doi: 10.1111/j.1550-7408.1983.tb05336.x. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



