Abstract
Activity of voltage-gated Cav1.3 L-type Ca2+ channels is required for proper hearing as well as sinoatrial node and brain function. This critically depends on their negative activation voltage range, which is further fine-tuned by alternative splicing. Shorter variants miss a C-terminal regulatory domain (CTM), which allows them to activate at even more negative potentials than C-terminally long-splice variants. It is at present unclear whether this is due to an increased voltage sensitivity of the Cav1.3 voltage-sensing domain, or an enhanced coupling of voltage-sensor conformational changes to the subsequent opening of the activation gate. We studied the voltage-dependence of voltage-sensor charge movement (QON-V) and of current activation (ICa-V) of the long (Cav1.3L) and a short Cav1.3 splice variant (Cav1.342A) expressed in tsA-201 cells using whole cell patch-clamp. Charge movement (QON) of Cav1.3L displayed a much steeper voltage-dependence and a more negative half-maximal activation voltage than Cav1.2 and Cav3.1. However, a significantly higher fraction of the total charge had to move for activation of Cav1.3 half-maximal conductance (Cav1.3: 68%; Cav1.2: 52%; Cav3.1: 22%). This indicated a weaker coupling of Cav1.3 voltage-sensor charge movement to pore opening. However, the coupling efficiency was strengthened in the absence of the CTM in Cav1.342A, thereby shifting ICa-V by 7.2 mV to potentials that were more negative without changing QON-V. We independently show that the presence of intracellular organic cations (such as n-methyl-D-glucamine) induces a pronounced negative shift of QON-V and a more negative activation of ICa-V of all three channels. These findings illustrate that the voltage sensors of Cav1.3 channels respond more sensitively to depolarization than those of Cav1.2 or Cav3.1. Weak coupling of voltage sensing to pore opening is enhanced in the absence of the CTM, allowing short Cav1.342A splice variants to activate at lower voltages without affecting QON-V.
Introduction
Ca2+ influx through voltage-gated Ca2+ channels (VGCCs) in the plasma membrane of electrically excitable cells causes membrane depolarization and triggers intracellular Ca2+-dependent signaling processes. Cav1.3 and Cav1.2, members of the so-called L-type Ca2+ channel family (LTCCs, Cav1), are widely expressed in many tissues, including muscle and neurons, sensory tissue, and endocrine cells (1–5). Work with genetically modified mice revealed different physiological roles for these two channel isoforms, even when expressed in the same cell (6–8). Differences in their voltage- and Ca2+-dependent gating properties underlie this functional diversity (6–8). Cav1.3 channels activate faster and at more negative voltages than Cav1.2 (9–11) and therefore sustain Ca2+ inward currents also at threshold voltages. This allows them to control autonomous pacemaking in the sinoatrial node (2,6) and adrenal chromaffin cells (7) and support upstroke potentials in neurons (12). Cav1.3 channels are also expressed in the substantia nigra pars compacta dopaminergic neurons (13) where they seem to contribute to dendritic Ca2+ signals linked to mitochondrial stress and the selective vulnerability of these neurons in Parkinson’s disease (14). Selective Cav1.3 channel block is currently pursued as a therapeutic option for neuroprotection in Parkinson’s disease. Although Cav1.3 channels activate at voltages that are more negative than all other Cav1 (Cav1.1, Cav1.2, and Cav1.4) and Cav2 high VGCCs (15), they cannot be classified as low VGCCs, such as T-type channels (16). Indirect comparisons of published data suggest that T-type channels activate and inactivate at voltages that are more negative than Cav1.3 (16), but a direct comparison of their gating properties, to our knowledge, does not exist.
Within the past few years, we have discovered that the activation voltage range (ICa-V) of Cav1.3 Ca2+ inward currents (ICa) can be shifted to potentials that are even more negative by alternative splicing within the C-terminus of its pore-forming α1-subunit (17,18). Alternative splicing generates Cav1.3 α1-subunits with shorter C-termini (e.g., Cav1.342A, Cav1.343S (18–20)) thereby removing a C-terminal modulatory domain (CTM) from long isoforms (Cav1.3L). This causes two apparently independent changes of channel gating:
-
1.
It moderates Ca2+-induced inactivation (CDI), an important autoinhibitory feedback mechanism of the channel. The molecular basis of this effect is well understood. As in other VGCCs, CDI is induced by Ca2+ binding to calmodulin (CaM) preassociated with the proximal C-terminus of α1-subunits (21). The CTM of Cav1.3 competitively interferes with CaM binding, and thereby reduces CDI (17,21).
-
2.
The presence of the CTM in long Cav1.3 splice variants reduces open probability at negative voltages and shifts the half-maximal activation voltage (V0.5(ICa)) by ∼10 mV to potentials that are more positive. This modulation has been reported by different laboratories, and occurs independently of CaM (11,18,19).
In contrast to modulation of CDI, it remains unclear how the CTM can affect voltage-dependent gating. Contemporary homology models of the pore-forming α1-subunit propose four voltage-sensing domains (transmembrane segments S1–S4 of each of the four homologous repeats), which undergo conformational changes upon de- and repolarization of the channel. The intramembrane movement of the four positively charged S4 helices represents the main moving part (22,23). The cytoplasmic linkers between S4 and S5 segments are tightly packed into the pore-forming segments (in particular S6 helices), and thereby couple the voltage-sensor movements (recorded as nonlinear charge movements QON and QOFF) to pore opening and closing (22). In the case of Cav1.3, the CTM must therefore interfere with this gating apparatus to induce the positive shift in ICa-V. Mechanistically, two possibilities exist: the CTM could affect the voltage sensitivity of the voltage-sensing mechanism itself such that higher voltages are required to move the charged S4 helix (inducing a shift in QON voltage-dependence (QON-V)). Alternatively, it could decrease the efficiency of coupling between voltage-sensor movements and subsequent pore opening, evident as a change in ICa-V but not QON-V parameters (24).
To address these questions, we established experimental conditions that allowed us to directly determine differences in gating properties that account for the more-negative ICa-V of Cav1.3 in comparison to Cav1.2 and of short Cav1.3 splice variants in comparison to long ones. We also systematically compared the gating properties of Cav1.3 with low-voltage-activated Cav3.1 T-type channels.
We found that Cav1.3 QON is significantly more voltage-sensitive than QON of Cav3.1 and Cav1.2. The coupling of voltage sensing to pore opening is less efficient in Cav1.3 but its negative QON-V still leads to ICa-V that is more negative than in Cav1.2. The CTM did not affect the voltage-dependence of QON of Cav1.3, indicating an inhibitory action on the transmission of voltage-sensor movements to pore opening. Surprisingly, intramembrane charge movement of Cav3.1 occurred at voltages more positive than that of Cav1.3, but the sensitive coupling of the voltage sensors to pore opening accounted for low voltage activation of its ICa-V. In the course of our work, we also discovered a strong effect of intracellular organic cations on the voltage-sensing machinery of all three VGCCs.
Methods
Cell culture and transient transfection
HEK 293 (human embryonic kidney) cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 2 mM L-glutamine (Cat. No. 25030-032; Gibco, Life Technologies, Carlsbad, CA), 10 units/mL penicillin (Cat. No. P-3032; Sigma Aldrich, St. Louis, MO), 10 μg/mL streptomycin (Cat. No. S-6501; Sigma), and with 10% v/v fetal calf serum (Cat. No. 10270-106; Gibco). Cells were grown under 5% CO2 and 37°C until they reached 80% confluency. They were split with 0.05% trypsin for cell dissociation, and passage did not exceed 20. Transient transfection was achieved as described in Bock et al. (19), using equimolar cDNA ratios encoding Cav1.3L, Cav1.2, or Cav3.1 (generously provided by Norbert Klugbauer) together with auxiliary subunits β3 and α2δ1. Cells were visualized by cotransfection of 1 μg GFP. Twenty-four hours after transfection, cells were plated on 35-mm polystyrene dishes, pretreated with 10 μg/mL poly-L-lysine. At 48–72 h after cell transfection, whole cell patch-clamp experiments were performed.
Whole-cell patch-clamp recordings
GFP-positive HEK 293 cells were recorded using the whole-cell patch-clamp configuration. Borosilicate glass electrodes, having a final resistance of 2–5 MΩ, were pulled with a micropipette puller (Sutter Instruments, Novato, CA) that was fire-polished (MF-830 microforge; Narishige, Tokyo, Japan). Data were digitized (Digitizer 1322A; Axon Instruments, Novato, CA) and recorded in the whole-cell patch-clamp configuration (Axopatch 200B; Axon Instruments).
Intracellular recording solutions used
NMDGint: NMDG (150 mM n-methyl-D-glucamine), 10 mM EGTA, 1 mM MgCl2, 10 mM HEPES, and 4 mM ATP-Mg, adjusted to pH 7.3 with MS (Methanesulfonate);
Csint: 135 mM CsCl, 10 mM Cs-EGTA, 1 mM MgCl2, 10 mM HEPES, and 4 mM ATP-Na2, adjusted to pH 7.3 with CsOH;
TRISint: 164 mM Tris, 10 mM EGTA, 1 mM MgCl2, 10 mM HEPES, and 4 mM ATP-Mg, adjusted to pH 7.3 with MS; and
TEAint: 160 mM TEA (triethanolamine), 10 mM EGTA, 1 mM MgCl2, 10 mM HEPES, and 4 mM ATP-Mg, adjusted to pH 7.3 with MS.
Extracellular solution used for QON recordings
Choline-Cl(Mg2+)ext: 150 mM choline-Cl, 16 mM MgCl2, 10 mM HEPES, 0.5 mM CdCl2, and 0.2 mM LaCl3, adjusted to pH 7.3 with CsOH.
Extracellular solutions used for ICa recordings
Choline-Clext: 150 mM choline-Cl, 15 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, adjusted to pH 7.3 with CsOH;
Csext: 150 mM CsCl, 15 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, adjusted to pH 7.3 with CsOH; and
NMDGext: 146 mM NMDG, 15 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, adjusted to pH 7.3 with HCl.
Cells were maintained at a holding potential of −80 mV, before a 25-ms- (ICa-V), or a 10-ms-long (QON-V) square-pulse depolarization (2-s interpulse interval). P/4 leak subtraction was applied. QON recordings were performed as described in Baig et al. (3).
To compare the QON-V of Cav1.3L and Cav1.342A, we quantified QON (QON, max) at Vrev after applying conditional prepulses in 5-mV steps to different potentials as previously described in McDonough et al. (24) and Baig et al. (3). During depolarizations to Vrev, we measured the QON not already moved during the prepulse. This allowed calculation of QON-V from the remaining QON at Vrev after the indicated prepulses (QON, post-pre). Using this protocol, we have previously also demonstrated that for Cav1.3L, QON-V is the same when Ca2+ in the extracellular solution is replaced by equimolar Mg+2 (+ 0.5 mM Cd2+ + 0.2 mM La3+, see solutions above). This revealed no differences in surface charge effects and ruled out that the voltage-dependence of QON is affected by the Mg2+-based extracellular solution used to block ionic current for QON-measurements (3).
Steady-state activation (G-V) relationships were derived from ICa-V curves. We did not analyze tail current-voltage relationships due to contamination of tail currents by off-gating current in Cav1.2 and Cav1.3 channels. ICa-V of individual experiments was fitted to a modified Boltzmann equation,
where Vrev is the extrapolated reversal potential, V is the test potential, I is the peak current amplitude, Gmax is the maximum slope conductance, V0.5 is the half-maximal activation voltage, and kact is the slope factor. For fitting ICa-V curves recorded in the presence of intracellular organic cations (no current reversal observed due to the lack of outward current), data points at test pulses to voltages >30–40 mV were excluded from fitting. QON-V and steady-state activation curves (G-V) were fitted to a Boltzmann equation,
where Gmax is the saturating value and kact is the slope factor. Junction potentials were individually calculated for every solution combination, using the software included in the PCLAMP 10.2 software suite (Molecular Devices, Sunnyvale, CA), and offline-subtracted. (Missing ion mobility values in PCLAMP 10.2 were collected from http://web.med.unsw.edu.au/phbsoft/mobility_listings.htm (accessed November 27, 2013)).
Junction potential corrections
Csint versus choline-Clext (−9.3 mV);
NMDGint versus choline-Clext (−8.5 mV);
TEAint versus choline-Clext (−4 mV);
Trisint versus choline-Clext (−5.6 mV);
Csint versus Csext (−2.4 mV);
Csint versus NMDGext (−13 mV);
NMDGint versus Csext (1.1 mV),
NMDGint versus choline-Cl(Mg2+)ext (−8.8 mV); and
Csint versus choline-Cl(Mg2+)ext (−9.5 mV).
Statistics
Data were analyzed with the softwares CLAMPFIT 10.2 (Axon Instruments) and SIGMA PLOT 12 (Systat Software, Chicago, IL). For statistical analysis GRAPHPAD PRISM 5.1 software (GraphPad Software, La Jolla, CA) was used, performing either one-way ANOVA with Bonferroni post-hoc test or Student’s t-test as given. Data are presented as mean ± SE. Significance level was set to α-error lower than p < 0.05 (∗), p < 0.01 (∗∗), and p < 0.001 (∗∗∗).
Results
Cav1.3 voltage sensors are highly sensitive to membrane potential changes but are weakly coupled to activation of ion conductance
To compare the voltage-dependence of charge movement (QON-V) with the ICa-V of different VGCCs we had to establish experimental conditions for reproducible recordings of QON over a large voltage range. This was achieved by using rat Cav1.3 α1-subunits that express at higher levels than human channels (but with indistinguishable current properties (25)) and by replacing Cs+ in our intracellular standard solution with NMDG to prevent contaminating outward currents. Inward currents were blocked by replacing Ca2+ with isomolar concentrations of Mg2+ and addition of 0.5 mM CdCl2 and 0.2 mM LaCl3. Using a prepulse protocol (Methods; see also below), we have previously shown that the voltage-dependence of QON is not affected by this solution exchange (3) and direct comparisons of ICa-V and QON-V are possible without corrections for surface charge shifts, as discussed in previous studies (24,26). Representative recordings of ICa (left panel) and QON (right panel) for Cav1.3L, Cav1.2, and Cav3.1 at different test potentials are illustrated in Fig. 1.
Figure 1.
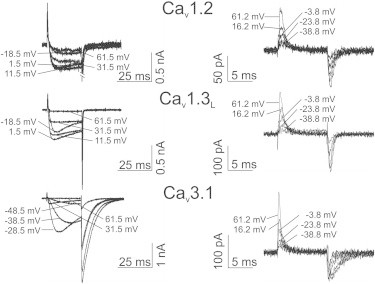
Representative current traces of ICa and QON. Representative current traces of ICa (left) and QON (right) are shown for Cav1.2, Cav1.3L, and Cav3.1 at different depolarizing voltages.
As in previous studies with Cs+-solution (3,18,25), the long splice variant of Cav1.3 (Cav1.3L) became activated at a voltage range that is more negative than Cav1.2 under identical experimental conditions with NMDG as the major intracellular cation (Fig. 2 A). Cav1.3L activated with a half-maximal activation voltage (V0.5(ICa)) of ∼10 mV more negative than Cav1.2 (Fig. 2 A; for statistics, see Table 1). To test whether this was due to a more refined voltage sensing, we also measured QON-V. Although the threshold voltage for induction of QON was similar for both channels, Cav1.3L displayed a steeper voltage-dependence as evident from the significantly lower slope factor and more-negative half-maximal activation voltage of QON (V0.5(QON)) (Fig. 2 B; and see Table 1 for statistics). A comparison of conductance (G-V) and QON–V is shown in Fig. 1 C.
Figure 2.
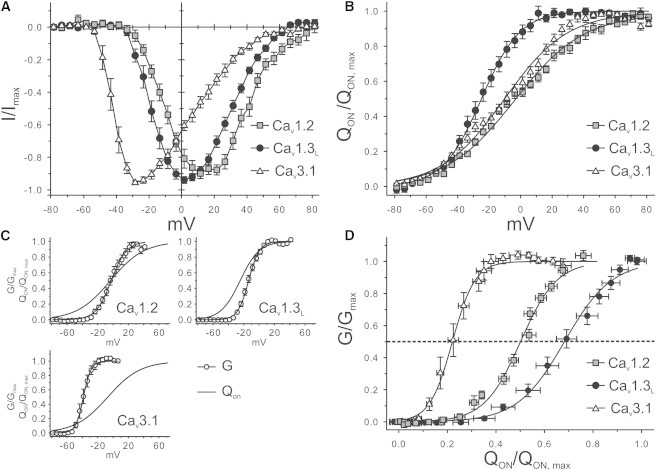
ICa and QON voltage-dependence of Cav1.2, Cav1.3L, and Cav3.1. The n-numbers and detailed statistics are given in Table 1. (A) Normalized ICa-V recorded with standard extracellular solution (choline-Clext) and intracellular NMDG (NMDGint). ICa-V was fitted to a modified Boltzmann function, as described in Methods. (B) Normalized QON-V of Cav1.2, Cav1.3L, and Cav3.1 recorded with choline-Cl(Mg2+)ext/NMDGint. (C) Comparison of the voltage-dependence of QON (solid line, Boltzmann fit from data in panel B) and conductance (G, calculated from data shown in panel A). (D) Normalized G/Gmax in comparison to normalized QON/QON, max measured at similar voltages (difference = 0.3 mV) to illustrate the fraction of observed gating charge required for activation of conductance. (Dashed line) Half-maximal conductance. Data points were obtained from the experiments given in Table 1. Pooled data were fitted to a sigmoid function to determine the fractional QON at half-maximal G. Values were significantly different among the three channels (determined by sum-of-squares F-test from GRAPHPAD PRISM, GraphPad Software).
Table 1.
Biophysical properties of VGCCs α1-subunits
| choline-Clext / NMDGint | V0.5(ICa) [mV] | Slope (ICa) [mV] | n |
|---|---|---|---|
| Cav1.2 | −3 ± 2 | 8.6 ± 0.6 | 10 |
| Cav1.3L | −13 ± 1 ∗∗∗ | 6.4 ± 0.3 ∗∗ | 14 |
| Cav1.342A | −20 ± 2 ∗∗∗, † | 5.6 ± 0.3 ∗∗∗ | 17 |
| Cav3.1 | −40 ± 2 ∗∗∗, †††, ‡‡‡ | 3.8 ± 0.3 ∗∗∗, †††, ‡‡ | 12 |
| choline-Clext/ Csint | |||
| Cav1.2 | 14 ± 2 §§§ | 11.2 ± 0.5 §§ | 28 |
| Cav1.3L | −4 ± 1 §§§ | 8.8 ± 0.2 §§§ | 19 |
| Cav3.1 | −35 ± 1 § | 5.3 ± 0.2 §§§ | 11 |
| choline-Cl(Mg2+)ext/ NMDGint | V0.5(QON) [mV] | Slope (QON) [mV] | n |
| Cav1.2 | −3 ± 2 | 22.6 ± 1.3 | 10 |
| Cav1.3L | −24 ± 2 ∗∗∗ | 11.6 ± 0.9 ∗∗∗ | 13 |
| Cav3.1 | −6 ± 2 ††† | 19.9 ± 0.9 ††† | 10 |
| choline-Cl(Mg2+)ext/ NMDGint | V0.5(QON) at Vrev[mV] | Slope (QON) at Vrev[mV] | |
| Cav1.3L | −29 ± 3 | 10.6 ± 1.9 | 12 |
| Cav1.342A | −27 ± 2 | 11.1 ± 0.7 | 10 |
Parameters (mean ± SE) were obtained by fitting data of ICa-V relationships or by fitting data of QON-V relationship, as described in methods. QON-V was either determined by measuring QON during pulses to different voltage steps or by measuring QON at Vrev after prepulses to different voltages (V0.5(QON) at Vrev). Statistical significances are indicated for comparison vs. Cav1.2 (∗, ∗∗, ∗∗∗), vs. Cav1.3L (†, ††, †††), and vs. Cav1.342A (‡, ‡‡, ‡‡‡) (one-way ANOVA with Bonferroni post-hoc test). §, §§, §§§ indicate statistical significance for intra-construct comparisons of parameters obtained with intracellular NMDG vs intracellular Cs (e.g. Cav1.2 choline-Clext/NMDGint vs. Cav1.2 choline-Clext/Csint) (Student’s t-test).
As a measure for the efficiency of voltage-sensor charge movement to pore opening, we plotted the fraction of total observed QON (QON/QON, max), required for the activation of G (G/Gmax) at each voltage (Fig. 2 D). Fitting the data to a sigmoid function revealed that significantly less total QON (52%, n = 10) was required to activate half-maximal conductance of Cav1.2 than of Cav1.3L (68%, n ≥ 13) (see legend to Fig. 2 D). These data indicate weaker coupling of voltage-sensor movements to pore opening in Cav1.3 as compared to Cav1.2. However, the more-sensitive QON charge movement still results in activation at voltages that are more negative than Cav1.2. Low-VGCCs (T-type; Cav3 family (27)) are known to activate at significantly lower voltages than Cav1.3L (28,29), also shown in our direct comparison with Cav1.3L (Fig. 2 A). This can be explained by the finding that only a small fraction of total Cav3.1 QON (22%, n ≥ 9; Fig. 2, B–D; and see Table 1) was required for half-maximal activation of conductance. This allows Cav3.1 ICa to activate at much lower voltages than Cav1.3 despite its more positive QON-V, which is similar to Cav1.2 (Fig. 2 B).
Our data show that the voltage-sensing machinery of Cav1.3 responds more sensitively to depolarizing stimuli than Cav1.2 and even Cav3.1. Despite its weaker coupling to pore opening, this allows Cav1.3L to carry ICa at voltages that are more negative than for Cav1.2.
Alternative splicing affects QON coupling to pore opening of Cav1.3 LTCCs
We next investigated whether the more-negative ICa-V previously observed for naturally occurring C-terminally short splice variants (such as Cav1.342A) as compared to the long Cav1.3L splice variants (18,19) is attributable to more refined voltage sensing or more efficient pore coupling. A negative shift was also observed when intracellular NMDG was used instead of Cs+ (Fig. 3, A and C) as in previous studies (18,19). The V0.5(ICa) for Cav1.342A was 7 ± 2 mV more negative (Fig. 3 A; for statistics, see Table 1) and inactivation of ICa was faster (due to more pronounced CDI as demonstrated in previous work (18)). Measuring QON for this short splice variant was more difficult than for Cav1.3L due to small (presumably Mg2+) inward currents contaminating the measurement of QON at voltages at ∼Vmax, but not at Vrev. We therefore determined QON-V at Vrev (Fig. 3 B) by measuring QON that remained after applying conditioning prepulses to different voltages (see Methods).
Figure 3.
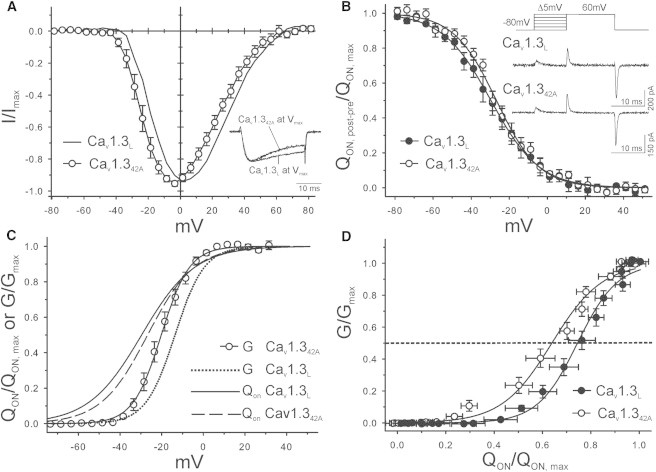
ICa and QON voltage-dependence of Cav1.3L in comparison to Cav1.342A. The n-numbers and detailed statistics are given in Table 1. (A) ICa-V of Cav1.3L and Cav1.342A recorded with standard recording solutions (choline-Clext/NMDGint). Fits were generated as described in Fig. 2A. (B) QON-V measured for Cav1.3L (n = 12) and Cav1.342A (n = 10) using the prepulse protocol (inset) as described in Methods with Mg2+-containing solution. (Inset) Example traces for both constructs obtained by depolarization to the reversal potential after a prepulse to −28.8 mV, which causes partial movement of QON. The value QON after the prepulse (QON, post-pre) was normalized to QON, max. For Cav1.3L the same gating parameters were obtained using this and the protocol in Fig. 2B. (C) Comparison of the QON-V and G-V relationships of Cav1.3L and Cav1.342A. (Solid and dashed lines) Boltzmann fits of QON-V data obtained from experiments illustrated in panel B (dotted line is the same as in Fig. 2C (Cav1.3L) for comparison). (D) Data representation as in Fig. 2D. Data points were obtained from the experiments given in Table 1. Pooled data were fitted to a sigmoid function to determine the fractional QON at half-maximal G. Values were significantly different between the two splice variants (determined by sum-of-squares F-test using GRAPHPAD PRISM, GraphPad Software).
Such prepulses moved part of QON in a voltage-dependent manner and allowed calculation of QON-V from the remaining QON at Vrev after the indicated prepulses (QON, post-pre). This protocol has originally been described by McDonough et al. (24) and was validated by us previously (3) and in this study (see legend to Fig. 3 B). Despite the more-negative V0.5(ICa) for Cav1.342A, no significant difference in the QON-V was observed between long and short Cav1.3 constructs (Fig. 3 B; for statistics, see Table 1). G-V curves for both splice variants are shown in Fig. 3 C in relation to their QON-V (for analysis, see Fig. 2 D). Half-maximal G of Cav1.342A required significantly less QON than the long isoform (68 vs. 79%, n ≥ 10) (Fig. 3 D). Taken together, these findings demonstrate that the presence of the intramolecular protein interaction forming the CTM in Cav1.3 LTCCs modulates the ICa-V activation of Cav1.3 by reducing the coupling efficiency between voltage sensing and pore opening.
Cation composition strongly affects voltage-dependent VGCC gating
Comparison of our ICa-V relationships measured using intracellular NMDG (Figs. 2 and 3) with our previously published data employing intracellular Cs+ revealed an ∼10 mV shift of V0.5(ICa) toward voltages that were more negative for Cav1.3L (Fig. 4 A). In these experiments, an identical extracellular solution with choline as the major extracellular cation, and 15 mM Ca2+ as the charge carrier, was employed (3,9,18,19,25) (Fig. 4 A). We corrected all data for junction potentials precisely calculated for all our solutions as described in the Methods, ruling out differences in junction potentials as an explanation for this difference. To further characterize this unexpected finding, we also recorded Cav1.2 and Cav3.1 ICa-V relationships with NMDG (NMDGint) or Cs+ containing intracellular solution (Csint). This revealed a negative shift also for these channel types (Table 1) and thus ruled out a Cav1.3-specific effect of NMDG. Next, we tested whether the effect was mimicked by other large organic cations. Tris and TEA caused a similar negative shift of ICa-V like NMDG as shown for Cav1.3L (Fig. 4 D). We also quantified effects on QON for Cav1.3L. Intracellular NMDG also shifted QON-V by approximately the same extent as ICa-V (Fig. 4 B, inset; Cav1.3L: n = 12; Cav1.342A: n = 9; p = 0.02, Student’s t-test), suggesting that the NMDG effect is due to voltage-sensing that is more refined rather than by pore-coupling (Fig. 4 C).
Figure 4.
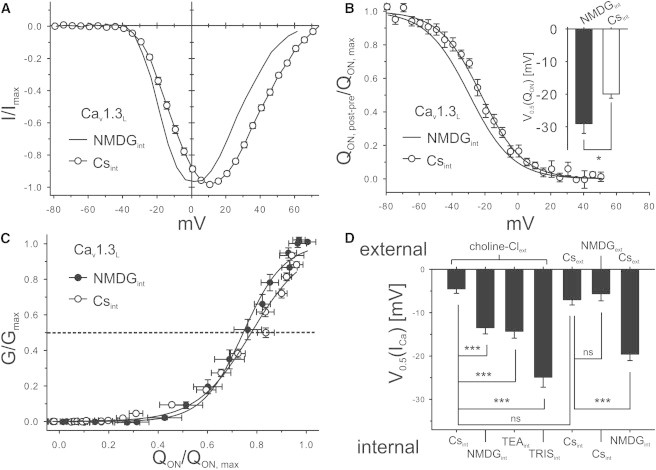
Effect of intracellular cations on Cav1.3L voltage-dependence. (A) ICa-V of Cav1.3L recorded with either Csint or NMDGint. (For comparison, ICa-V of Cav1.3L recorded with NMDGint is shown as a line, as taken from Fig. 2A.) Fits were generated as described in Fig. 2A. For statistics, see panel D. (B) QON-V of Cav1.3L, recorded at Vrev as in Fig. 3B with either Cs+ (Csint) or NMDG (NMDGint) as the major intracellular cation. For comparison, the QON-V of Cav1.3L in NMDGint is illustrated (line taken from Fig. 3B). (Inset) Statistical comparison of the V0.5 values (Csint: −20 ± 1 mV, n = 9; NMDGint: −29 ± 3 mV, n = 12; p = 0.022, Student’s t-test). (C) Normalized G/Gmax to normalized QON/QON,max (replotted from Fig. 3D as 1-QON, post-pre/QON, max). (D) V0.5(ICa) values for Cav1.3L, recorded with different internal and external cation-based solutions, as indicated (for solution composition, see Methods) (n ≥ 9). For calculation of statistical significance, one-way ANOVA with Bonferroni post-hoc test was performed (p < 0.05, ∗; p < 0.005, ∗∗; p < 0.001, ∗∗∗).
To test whether changes in the voltage-dependence of gating are also observed by corresponding changes of the extracellular solution, we exchanged choline-Cl (in our standard extracellular solution) by either CsCl (Csext) or NMDG-Cl (NMDGext), closely resembling the changes in the internal solutions. The V0.5(ICa) values obtained with the various combinations of intra- and extracellular solutions are illustrated in Fig. 4 D. When intracellular Cs+ was present, the replacement of extracellular choline by NMDG or Cs+ did not cause a change in V0.5(ICa). Intracellular NMDG also caused a negative shift with extracellular Cs+, as observed with extracellular choline. Taken together, our data revealed that intracellular but not extracellular organic cations can enhance the coupling efficiency of Ca2+ channel voltage sensors to membrane depolarization.
Discussion
Our study was motivated by two previous observations regarding Cav1.3 function:
-
1.
Cav1.3L channels were previously reported to activate at lower voltages than Cav1.2. This special feature has been discovered in heterologous expression studies (9,10) but was subsequently confirmed for native Cav1.3 currents in sinoatrial node cells (6), cochlear inner hair cells (2), and adrenal chromaffin cells (7). It allows Cav1.3 channels to sustain subthreshold inward currents and thus serve as a pacemaker channel in the sinoatrial node and chromaffin cells and shape firing patterns of neurons (12).
-
2.
C-terminal splicing removes a CTM (11,18,19), which can further shift the channel's ICa-V to more-negative voltages in short splice variants.
Because these splice variants are expressed in a tissue-dependent manner, it is likely that they contribute to the fine-tuning of Cav1.3 channel activity in different tissues. Here we present data showing that the voltage sensors of Cav1.3 respond more readily to depolarizing stimuli than those of Cav1.2 and Cav3.1. This ensures that despite the weaker coupling of voltage sensing to pore opening, Cav1.3 currents can activate at lower membrane potentials than Cav1.2. Moreover, alternative splicing enhances the efficiency of coupling between charge movement and pore opening, explaining the even lower activation voltage range of naturally occurring short splice variants lacking the CTM.
Based on contemporary structural models of the voltage-gated cation channel family, mainly derived from x-ray structures of voltage-gated K+- (30) and bacterial Na+-channels (31), a negative shift in the voltage-dependence of channel conductance (G-V) may, in principle, result via two possible mechanisms:
-
1.
Values of QON-V that are more negative. Even if the efficiency of voltage-sensor coupling to the pore remains unchanged, this should shift G-V to voltages that are more negative. An example is the deletion of a ‘gating brake’ in T-type channel α1-subunits. This causes a negative shift and steeper voltage-dependence (slope) of the QON-V relationship (29) paralleled by a corresponding negative shift in G-V (32).
-
2.
QON-V is unaltered but the efficiency of coupling to pore opening is enhanced. This has been reported for the LTCC activators FPL64176 and BayK8644, which induce strong changes in the kinetics and gating of Cav1.2 currents (24,33), including a shift of the ICa-V relationship to potentials that are more hyperpolarized (33). However, they do not affect the voltage-dependence of QON (24,33).
Here we clearly demonstrate that the more-negative activation range of Cav1.3 as compared to Cav1.2 is not due to a more efficient coupling. Instead, coupling is even weaker, as evident from a higher fractional QON required for V0.5(ICa). However, QON of Cav1.3 displayed a steeper voltage-dependence and thereby still permits a more-negative ICa-V. We show that this is in contrast to the mechanism imposed by alternative splicing. We found that in the absence of the CTM, the QON-V does not change but more channel activation (i.e., fractional conductance) is observed at a given percentage of maximal QON. Similar to the findings obtained with FPL64176 (24), this can be interpreted as more efficient coupling between voltage-sensor movements and pore opening.
It is unclear how the CTM can moderate this coupling. Molecular studies using mutant and chimeric channel constructs will be difficult to perform because this modulatory domain is part of a larger structure consisting not only of the two noncovalently interacting putative α-helices (PCRD, DCRD) of the modulatory domain itself but also of channel-bound CaM (18,34). Based on our observations, it is most likely that the CTM targets the interaction of pore-forming helices (primarily S6) with the S4-S5 linkers that are considered the main structural determinants of QON-V to pore opening (22,23,35,36). Another possibility is interference of the distal C-terminus with the channels II-III linker. Such an interaction, which is also modulated by A-kinase anchoring proteins, has recently been described in Cav1.2 α1-subunits (37).
What structural differences mediate the steeper voltage-dependence of Cav1.3 and the higher coupling efficiency of Cav3.1? The amino-acid sequence within the S4 segments including the positive charges of Cav1.3 and Cav1.2 are highly conserved (see alignment in Fig. S1 in the Supporting Material) and are unlikely to explain the difference in their QON-V relationships. Structural features outside S4 must therefore play a crucial role. Mutations in S6 segments forming the activation gate or the S5-S6 linker, which couples voltage-sensors to the gate, can induce QON-V shifts to voltages that are more negative. This has been reported by us (for Cav1.3 (3)) and others (for Cav3.2 and Cav2.3 (35,38)). Interdomain cytoplasmic linkers (shown for N-terminal regions of the I-II loop in the case of Cav3 channels) can also affect the voltage-dependence of QON-V by serving as a ‘gating brake’ (29). The high sequence similarity of Cav1.3 α1 subunits with Cav1.2 also outside S4 regions provides an excellent opportunity to identify the structural determinants accounting for its uniquely steep voltage-dependence using chimeric approaches.
The high coupling efficiency of Cav3.1 channels is also not readily explained by charge differences in the S4 segments (see Fig. S1). Assuming that all four voltage sensors have to move completely for pore opening, the fact that only ∼25% of QON charge are moved when conductance is already fully activated (Fig. 2) could indicate that the activation of only one of the four voltage sensors is sufficient for activating the channel gate.
During the course of our studies, we also discovered that intracellular organic cations sensitize voltage-responses of all three VGCCs investigated. A shift to more negative ICa-V was initially observed when intracellular Cs+ was replaced by NMDG, but was also found for Tris and TEA. In contrast to splicing, NMDG affected QON-V with no major change in pore coupling, suggesting that it primarily affects voltage sensing itself. This also demonstrates that ion permeation is not required for this voltage shift. This modulation is unlikely to have been caused by a high affinity interaction with NMDG; it was not observed when only 15 mM of Cs+ were replaced by NMDG (n = 4, not shown).
To investigate the possibility of passive charge screening effects, we measured changes in Cav1.3L gating with all possible combinations of equimolar concentrations of NMDG and Cs+ in the intra- and extracellular solutions. Considerably less is known about passive-charge-screening effects of organic cations as compared to mono- or divalent inorganic cations (39–43). Therefore, although unlikely, the possibility of passive charge screening effects cannot be completely excluded. Alternatively, intracellular organic cations may somehow more specifically interfere with the gating apparatus. An example has previously been described for Kv1.2 channels (44) by showing that internal cations are able to occupy the inner cavity of the open channel. Thereby they prevent closing of the inner pore gate and stabilize the open state of the voltage sensors (44). This was not associated with a shift in QON-V, but caused a slowing of off-gating currents, especially with such larger cations as NMDG and TEA. However, we were not able to detect a major off-gating current-stabilizing effect of NMDG on Cav1.3L (n ≥ 9, not shown) or a slowing of ICa deactivation kinetics.
Independent from the molecular mechanism, our findings clearly emphasize that recording buffer compositions have to be considered when comparing biophysical parameters across different studies examining the voltage-dependent gating properties of VGCCs.
Acknowledgments
The authors thank Norbert Klugbauer for the Cav3.1 cDNA construct; Ed Perez-Reyes for the β3-subunit cDNA; Petronel Tuluc for valuable discussions and support; and Gospava Stojanovic, Jennifer Müller, and Germana Gratl for competent technical assistance.
This work was supported by the Austrian Science Fund (F44020, W11010 to J.S.) and the University of Innsbruck.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons-Attribution Noncommercial License (http://creativecommons.org/licenses/by-nc/2.0/), which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Andreas Lieb, Email: andreas.lieb@student.uibk.ac.at.
Jörg Striessnig, Email: joerg.striessnig@uibk.ac.at.
Supporting Material
References
- 1.Namkung Y., Skrypnyk N., Shin H.S. Requirement for the L-type Ca2+ channel α1D subunit in postnatal pancreatic β-cell generation. J. Clin. Invest. 2001;108:1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platzer J., Engel J., Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 3.Baig S.M., Koschak A., Bolz H.J. Loss of CaV1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat. Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 4.Striessnig J., Koschak A. Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene knockout models. Channels (Austin) 2008;2:233–251. doi: 10.4161/chan.2.4.5847. [DOI] [PubMed] [Google Scholar]
- 5.Marcantoni A., Carabelli V., Carbone E. Calcium channels in chromaffin cells: focus on L and T types. Acta Physiol. (Oxf.) 2008;192:233–246. doi: 10.1111/j.1748-1716.2007.01815.x. [DOI] [PubMed] [Google Scholar]
- 6.Mangoni M.E., Couette B., Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc. Natl. Acad. Sci. USA. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcantoni A., Vandael D.H., Carbone E. Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J. Neurosci. 2010;30:491–504. doi: 10.1523/JNEUROSCI.4961-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Striessnig J., Bolz H.J., Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koschak A., Reimer D., Striessnig J. Alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 10.Xu W., Lipscombe D. Neuronal CaV1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan B.Z., Jiang F., Soong T.W. Functional characterization of alternative splicing in the C terminus of L-type CaV1.3 channels. J. Biol. Chem. 2011;286:42725–42735. doi: 10.1074/jbc.M111.265207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson P.A., Tkatch T., Surmeier D.J. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J. Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan C.S., Guzman J.N., Surmeier D.J. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 14.Surmeier D.J., Guzman J.N., Sanchez-Padilla J. Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease. Cell Calcium. 2010;47:175–182. doi: 10.1016/j.ceca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catterall W.A., Perez-Reyes E., Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 17.Singh A., Hamedinger D., Striessnig J. C-terminal modulator controls Ca2+-dependent gating of Cav1.4 L-type Ca2+ channels. Nat. Neurosci. 2006;9:1108–1116. doi: 10.1038/nn1751. [DOI] [PubMed] [Google Scholar]
- 18.Singh A., Gebhart M., Koschak A. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J. Biol. Chem. 2008;283:20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bock G., Gebhart M., Koschak A. Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. J. Biol. Chem. 2011;286:42736–42748. doi: 10.1074/jbc.M111.269951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Striessnig J., Pinggera A., Kaur G., Bock G., Tuluc P. L-type calcium channels in heart and brain. WIREs Membr Transp Signal. 2014;2014 doi: 10.1002/wmts.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Yang P.S., Yue D.T. Enzyme-inhibitor-like tuning of Ca2+ channel connectivity with calmodulin. Nature. 2010;463:968–972. doi: 10.1038/nature08766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen M.O., Jogini V., Shaw D.E. Mechanism of voltage gating in potassium channels. Science. 2012;336:229–233. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- 23.Catterall W.A. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonough S.I., Mori Y., Bean B.P. FPL 64176 modification of CaV1.2 L-type calcium channels: dissociation of effects on ionic current and gating current. Biophys. J. 2005;88:211–223. doi: 10.1529/biophysj.104.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieb A., Scharinger A., Striessnig J. Structural determinants of CaV1.3 L-type calcium channel gating. Channels (Austin) 2012;6:197–205. doi: 10.4161/chan.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones L.P., Wei S.K., Yue D.T. Mechanism of auxiliary subunit modulation of neuronal α1E calcium channels. J. Gen. Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Reyes E., Cribbs L.L., Lee J.H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Reyes E., Lory P. Molecular biology of T-type calcium channels. CNS Neurol. Disord. Drug Targets. 2006;5:605–609. doi: 10.2174/187152706779025508. [DOI] [PubMed] [Google Scholar]
- 29.Karmažínová M., Baumgart J.P., Lacinová L. The voltage dependence of gating currents of the neuronal Cav3.3 channel is determined by the gating brake in the I-II loop. Pflugers Arch. 2011;461:461–468. doi: 10.1007/s00424-011-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long S.B., Tao X., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 31.Payandeh J., Scheuer T., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Reyes E. Characterization of the gating brake in the I-II loop of CaV3 T-type calcium channels. Channels (Austin) 2010;4:453–458. doi: 10.4161/chan.4.6.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan J.S., Yuan Y., Palade P. Kinetic effects of FPL 64176 on L-type Ca2+ channels in cardiac myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2000;361:465–476. doi: 10.1007/s002100000219. [DOI] [PubMed] [Google Scholar]
- 34.Johny M.B., Yang P.S., Yue D.T. Dynamic switching of calmodulin interactions underlies Ca2+ regulation of Cav1.3 channels. Nat. Com. 2013;4:1717. doi: 10.1038/ncomms2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wall-Lacelle S., Hossain M.I., Parent L. Double mutant cycle analysis identified a critical leucine residue in the IIS4S5 linker for the activation of the CaV2.3 calcium channel. J. Biol. Chem. 2011;286:27197–27205. doi: 10.1074/jbc.M111.237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haddad G.A., Blunck R. Mode shift of the voltage sensors in Shaker K+ channels is caused by energetic coupling to the pore domain. J. Gen. Physiol. 2011;137:455–472. doi: 10.1085/jgp.201010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altier C., Dubel S.J., Bourinet E. AKAP79 modulation of L-type channels involves disruption of intramolecular interactions in the CaV1.2 subunit. Channels (Austin) 2012;6:157–165. doi: 10.4161/chan.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demers-Giroux P.O., Bourdin B., Parent L. Cooperative activation of the T-type CaV3.2 channel: interaction between domains II and III. J. Biol. Chem. 2013;288:29281–29293. doi: 10.1074/jbc.M113.500975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdulkader F., Arcisio-Miranda M., Procopio J. Surface potential determination in planar lipid bilayers: a simplification of the conductance-ratio method. J. Biochem. Biophys. Methods. 2007;70:515–518. doi: 10.1016/j.jbbm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin S.G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J. Gen. Physiol. 1971;58:667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green W.N., Andersen O.S. Surface charges and ion channel function. Annu. Rev. Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- 42.Wilson D.L., Morimoto K., Brown A.M. Interaction between calcium ions and surface charge as it relates to calcium currents. J. Membr. Biol. 1983;72:117–130. doi: 10.1007/BF01870319. [DOI] [PubMed] [Google Scholar]
- 43.Becchetti A., Arcangeli A., Wanke E. Intra- and extracellular surface charges near Ca2+ channels in neurons and neuroblastoma cells. Biophys. J. 1992;63:954–965. doi: 10.1016/S0006-3495(92)81665-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodchild S.J., Xu H., Fedida D. Basis for allosteric open-state stabilization of voltage-gated potassium channels by intracellular cations. J. Gen. Physiol. 2012;140:495–511. doi: 10.1085/jgp.201210823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


