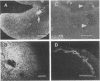
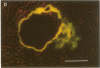
Abstract
A fundamental question concerning the development of the extracellular matrix is what factors control the arrangement of collagen fibrils within a tissue and at the same time allow for the great diversity of geometric forms exhibited by collagen. In this report, we test the possibility that physical forces within the embryo serve to organize collagen fibers into regular patterns. In particular, we test the prediction that patterns of stress having this morphogenetic function are generated by cell traction, the contractile force exerted by cells to propel themselves. To study the effects of these mechanical forces on the extracellular matrix, type I collagen was fluorescently labeled and injected into developing chicken wing buds. When the injected limbs were allowed to develop and then examined histologically, the exogenous collagen was found incorporated within normal connective tissues of the wing. The labeled collagen became arranged according to its site of injection, forming parts of tendons, perichondria, cartilages, perineuria, and blood vessels. Since the injected collagen formed a gel within minutes of its injection, the subsequent incorporation of this performed collagen within organized structures cannot be explained in terms of molecular self-assembly or other mechanisms occurring during collagen deposition. These results demonstrate that, within developing tissues, patterns of forces exist that are capable of physically rearranging collagen and determining its long-range order.
Full text
PDF

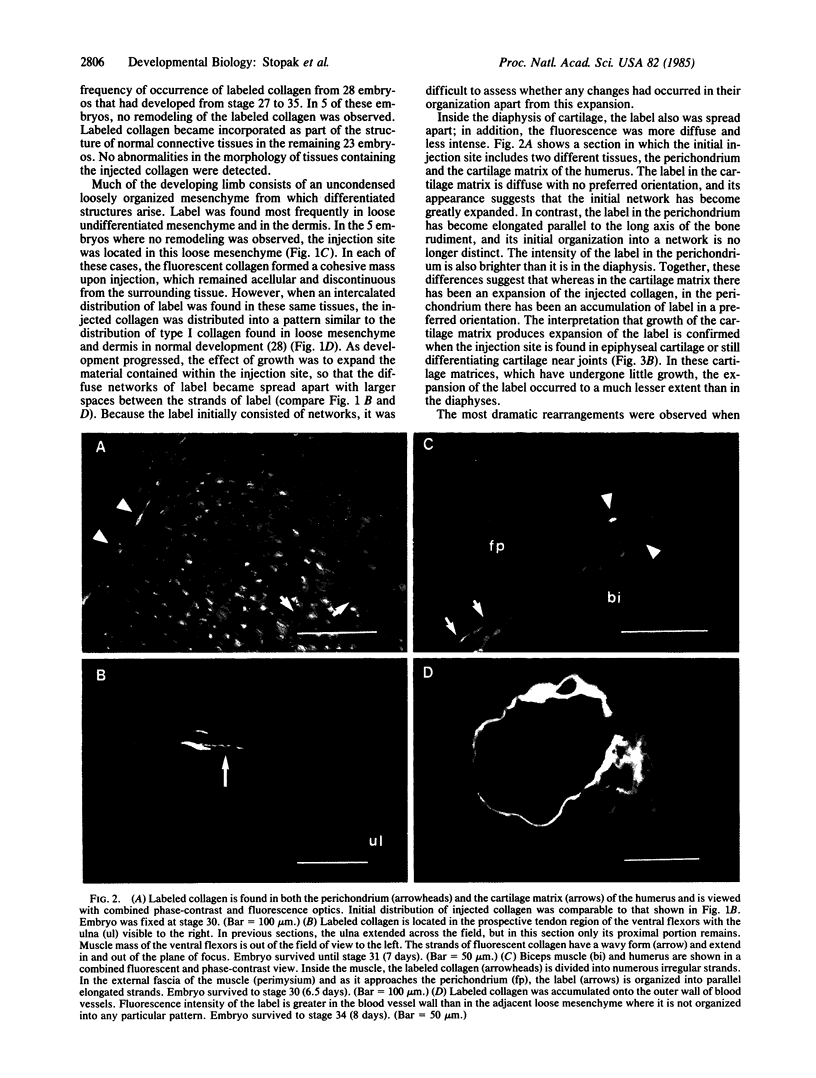


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Kubai L., Knighton D., Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974 Dec;41(2):391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- Bell E., Ehrlich H. P., Buttle D. J., Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981 Mar 6;211(4486):1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows C. G., Melcher A. H., Aubin J. E. Contraction and organization of collagen gels by cells cultured from periodontal ligament, gingiva and bone suggest functional differences between cell types. J Cell Sci. 1981 Aug;50:299–314. doi: 10.1242/jcs.50.1.299. [DOI] [PubMed] [Google Scholar]
- Dessau W., von der Mark H., von der Mark K., Fischer S. Changes in the patterns of collagens and fibronectin during limb-bud chondrogenesis. J Embryol Exp Morphol. 1980 Jun;57:51–60. [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. P., Selwood L., Day A., Wolpert L. The mechanism of cellular orientation during early cartilage formation in the chick limb and regenerating amphibian limb. Exp Cell Res. 1974 Feb;83(2):287–296. doi: 10.1016/0014-4827(74)90341-3. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Lamke C. R. Reorganization of hydrated collagen lattices by human skin fibroblasts. J Cell Sci. 1984 Mar;66:51–63. doi: 10.1242/jcs.66.1.51. [DOI] [PubMed] [Google Scholar]
- Gross J. Collagen biology: structure, degradation, and disease. Harvey Lect. 1974;68:351–432. [PubMed] [Google Scholar]
- Gross J., Highberger J. H., Schmitt F. O. COLLAGEN STRUCTURES CONSIDERED AS STATES OF AGGREGATION OF A KINETIC UNIT. THE TROPOCOLLAGEN PARTICLE. Proc Natl Acad Sci U S A. 1954 Aug;40(8):679–688. doi: 10.1073/pnas.40.8.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. K., Stopak D., Warner P. Generation of spatially periodic patterns by a mechanical instability: a mechanical alternative to the Turing model. J Embryol Exp Morphol. 1984 Apr;80:1–20. [PubMed] [Google Scholar]
- Harris A. K., Stopak D., Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981 Mar 19;290(5803):249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- Harris A. K., Wild P., Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980 Apr 11;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Leader W. M., Stopak D., Harris A. K. Increased contractile strength and tightened adhesions to the substratum result from reverse transformation of CHO cells by dibutyryl cyclic adenosine monophosphate. J Cell Sci. 1983 Nov;64:1–11. doi: 10.1242/jcs.64.1.1. [DOI] [PubMed] [Google Scholar]
- Markwald R. R., Fitzharris T. P., Bolender D. L., Bernanke D. H. Sturctural analysis of cell:matrix association during the morphogenesis of atrioventricular cushion tissue. Dev Biol. 1979 Apr;69(2):634–654. doi: 10.1016/0012-1606(79)90317-8. [DOI] [PubMed] [Google Scholar]
- Mattey D. L., Garrod D. R. Organization of extracellular matrix by chick embryonic corneal epithelial cells in culture and the role of fibronectin in adhesion. J Cell Sci. 1984 Apr;67:171–188. doi: 10.1242/jcs.67.1.171. [DOI] [PubMed] [Google Scholar]
- Mauger A., Demarchez M., Herbage D., Grimaud J. A., Druguet M., Hartmann D., Sengel P. Immunofluorescent localization of collagen types I and III, and of fibronectin during feather morphogenesis in the chick embryo. Dev Biol. 1982 Nov;94(1):93–105. doi: 10.1016/0012-1606(82)90072-0. [DOI] [PubMed] [Google Scholar]
- Nakatsuji N., Gould A. C., Johnson K. E. Movement and guidance of migrating mesodermal cells in Ambystoma maculatum gastrulae. J Cell Sci. 1982 Aug;56:207–222. doi: 10.1242/jcs.56.1.207. [DOI] [PubMed] [Google Scholar]
- Ohsawa S., Yasui N., Ono K. Contraction of collagen gel by the dedifferentiated chondrocytes. Cell Biol Int Rep. 1982 Aug;6(8):767–774. doi: 10.1016/0309-1651(82)90169-2. [DOI] [PubMed] [Google Scholar]
- Oster G. F., Murray J. D., Harris A. K. Mechanical aspects of mesenchymal morphogenesis. J Embryol Exp Morphol. 1983 Dec;78:83–125. [PubMed] [Google Scholar]
- Shah J. S., Palacios E., Palacios L. Development of crimp morphology and cellular changes in chick tendons. Dev Biol. 1982 Dec;94(2):499–504. doi: 10.1016/0012-1606(82)90366-9. [DOI] [PubMed] [Google Scholar]
- Shellswell G. B., Bailey A. J., Duance V. C., Restall D. J. Has collagen a role in muscle pattern formation in the developing chick wing? 1. An immunofluorescence study. J Embryol Exp Morphol. 1980 Dec;60:245–254. [PubMed] [Google Scholar]
- Steinberg B. M., Smith K., Colozzo M., Pollack R. Establishment and transformation diminish the ability of fibroblasts to contract a native collagen gel. J Cell Biol. 1980 Oct;87(1):304–308. doi: 10.1083/jcb.87.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopak D., Harris A. K. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982 Apr;90(2):383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev Biol. 1979 Aug;71(2):228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- Tuan R. S., Lynch M. H. Effect of experimentally induced calcium deficiency on the developmental expression of collagen types in chick embryonic skeleton. Dev Biol. 1983 Dec;100(2):374–386. doi: 10.1016/0012-1606(83)90232-4. [DOI] [PubMed] [Google Scholar]