Abstract
Purpose
The aim of the study was to determine the prognostic impact of lymph node (LN) involvement and sampling in patients with Wilms tumor (WT) and the minimum number of LNs needed for accurate staging.
Methods
We reviewed all patients with unilateral, nonmetastatic WT enrolled in the National Wilms Tumor Study 4 or 5. Data were abstracted on patient demographics, tumor histology, staging, number of LNs sampled, and disease-specific and overall patient outcomes.
Results
A total of 3409 patients had complete information on LN sampling. Five-year event-free survival (EFS) was lower in patients with nodal disease (P = .001); the effect of LN positivity was greater for patients with anaplastic (P = .047) than with favorable histology (P = .02). The likelihood of obtaining a positive LN was higher when sampling at least 7 LNs. However, after controlling for tumor histology and stage, the number of LNs sampled did not predict EFS variations (P = .75). Among patients with stage II disease, patients with LN sampling (P = .055) had improved EFS, largely reflecting poorer EFS in patients with anaplastic tumors (P = .03).
Conclusions
Lymph node sampling is particularly important for patients with stage II anaplastic WT. Although the likelihood of finding a positive LN was greater when more than 7 LNs were sampled, EFS was not impacted by the number of LNs sampled.
Keywords: Wilms tumor, Lymph node, Treatment outcome, Child
The goals of this study were to determine the prognostic impact of lymph node (LN) involvement treated with the regimens dictated in the National Wilms Tumor Study (NWTS) 4 and 5, to determine the influence of failure to sample LNs on patient outcome, and to determine whether the number of LNs present on pathologic analysis was associated with variations in event-free survival (EFS) in patients with unilateral, localized Wilms tumor (WT). This later information could then be used to determine whether there was a minimum number of LNs that should be sampled at initial surgery to ensure accurate staging. Because therapy for unilateral, nonmetastatic WT did not differ significantly between NWTS-4 and NWTS-5 (primarily, duration of therapy for favorable histology disease), we felt that the results from these 2 studies could be combined for analysis.
Wilms tumor is the most common renal tumor and the second most common intraabdominal tumor in children, with about 650 new cases annually in the United States [1]. Evaluation of different treatment strategies has been conducted through multi-institutional trials such as the NWTS Group beginning in 1969. Through this systematic evaluation, several prognostic factors have been identified for patients with WT. One significant prognostic factor is involvement of loco-regional LNs, first recognized by Jereb et al [2], as a predictor of relapse [3,4].
Histologic evaluation of LNs is essential because there is imperfect concordance between clinical assessment and histopathologic findings. Leape et al [5] and Othersen et al [6] reported that surgeons incorrectly identified histologically positive LNs as clinically negative 4% to 11% of the time. In addition, both reported that clinically positive LNs were histologically negative approximately 40% of the time. Therefore, histologic evaluation of LNs is a critical component of staging for WT even when the loco-regional nodes appear grossly uninvolved. Nodal assessment not only provides important prognostic information but is also a factor in determining the intensity of adjuvant therapy. Shamberger et al [4], on review of surgery-related factors and local recurrence in NWTS-4, reported that failure to sample LNs was associated with an increased risk of local recurrence. A subsequent study by Ehrlich et al [7] suggested that local relapse rates may be higher in stage II patients treated on NWTS-5 when LNs were not sampled. Taken together, these results suggest that patients in whom LNs are not sampled may be understaged and, therefore, receive inadequate therapy.
1. Methods
After obtaining institutional review board approval, we reviewed the records of all patients enrolled in the NWTS-4 and NWTS-5 studies who had undergone unilateral nephrectomy for nonmetastatic WT. Enrollment for NWTS-4 and NWTS-5 was ongoing between August 1986 and May 2002. Patients who received prenephrectomy chemotherapy or who underwent partial nephrectomy for bilateral (or unilateral) disease as well as those with evidence of metastatic disease were not included. Patients with clear cell sarcoma of the kidney, rhabdoid tumor of the kidney, or renal cell carcinoma on pathologic analysis were also excluded.
Data collected included patient demographics, tumor histopathology, the number of LNs collected, the presence or absence of tumor in LN(s), and pathologic stage. All histopathologic data were abstracted from the central pathology report. In a small number of patients, slides of resected LNs were not sent for central review; in these cases, the institutional assessment of nodal involvement was used.
Event-free survival was defined as the time from study entry until the first occurrence of disease progression, relapse after response, or death as a first event from any cause. Follow-up for patients not experiencing an event was censored at the time of last follow-up. Kaplan-Meier curves were calculated to estimate 5-year EFS for various patient subsets. The EFS among patient subsets was compared using the log-rank test. The independent contribution of various factors to the prediction of EFS was assessed using the Cox proportional hazards model. Differences were considered statistically significant if P < .05. Analyses were performed using commercially available statistical software (SAS; Cary, NC).
2. Results
A total of 3913 patient records were reviewed. Thirty-eight cases were excluded from the analysis either because details about LN sampling or the status of the LNs sampled could not be determined or because data on disease stage were unavailable. For the remaining 3875 patients, tumor histology was favorable for 3591 patients (92.7%), focal anaplasia for 59 patients (1.5%), and diffuse anaplasia for 225 patients (5.8%). Four hundred sixty-six (12.5%) of these patients did not have nodal tissue analyzed. Of the remaining 3409 patients, 616 (18.1%) had at least 1 positive LN on histologic analysis. Resected LNs were not quantified in 194 patients (5.0%), often simply being described as “numerous” or “matted.” Of these patients, 29 (15.0%) had LN involvement. Data from these 194 patients were not included in the analysis evaluating the impact of the number of LNs sampled, although they were included in the analyses of the impact of LN involvement on outcome.
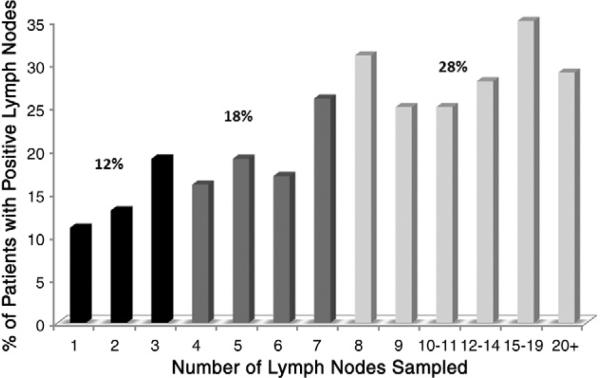
More LNs were removed in patients with nodal involvement. The median number of LNs present in the surgical specimens from patients with at least 1 positive LN was 5 (range, 1-42); in patients with negative LNs the median number present was 3 (range, 1-45) (P < .0001). Table 1 summarizes the percentage of patients with histologically confirmed LN involvement and the percentage of involved LNs stratified by the number of LNs assessed. The likelihood of finding a positive LN increased as more LNs were sampled (Fig. 1; P < .0001, χ2 test). The statistically significant relationship of LNs sampled and the percentage of patients with LN involvement suggests 3 groups of “percent positive”: 12% of patients with 1 to 2 LNs sampled, 18% of those with 3 to 6 LNs sampled, and 28% of patients with at least 7 LNs sampled had at least 1 positive LN. The number of LNs sampled was associated with disease stage: 19% of patients with stage I disease had 7 or more LNs sampled, compared with 24% with stage II disease and 33% of those with stage III disease (Table 2, P < .0001). Somewhat fewer LNs were sampled in the favorable histology tumors compared with those with anaplasia, although the difference was not statistically significant (Table 3, P = .07).
Table 1.
Guidelines for the reporting of clinical research data in the Journal of Pediatric Surgery
| Reported | Not applicable | Reporting detail |
|---|---|---|
| Methods | ||
| □ | ⊠ | The no. and practice type of all institutions where cases were performed |
| □ | ⊠ | The no. of surgeons who actually operated in the study (and the relative number of cases for each) |
| □ | ⊠ | The prior experience of participating surgeons in performing the reported intervention |
| ⊠ | □ | The precise timeline during which all patients were treated in the study (eg, Jan 1995 to March 1998) |
| ⊠ | □ | A clear description of how patients were selected into the study. This should include relevant inclusion and/or exclusion criteria. |
| ⊠ | □ | The no. of eligible patients at the study sites excluded during the timeline of the study |
| ⊠ | □ | A clear description of the study population from which the patients were selected. |
| ⊠ | □ | A clear description of the relevant diagnostic criteria used to identify cases |
| □ | ⊠ | A clear description of critical aspects of operative technique and perioperative care |
| □ | ⊠ | Statement as to whether any attempts were made to standardize operative technique or perioperative care (and how this was accomplished). |
| Results | ||
| ⊠ | □ | The range and mean of all relevant demographic and baseline variables |
| □ | ⊠ | The range and median (not mean) for length of follow-up reporting |
| ⊠ | □ | Relevant outcome variables are presented with appropriate measures of range and variability (eg, SD) |
| ⊠ | □ | Methods for measuring outcomes of interest are clearly described |
| ⊠ | □ | Statement regarding whether any data are missing (and how missing data are addressed in the analysis of outcome variables) |
| □ | ⊠ | No. and appropriate details regarding all complications |
| Additional details for studies reporting more than 1 treatment group (eg, controls) | ||
| □ | ⊠ | Mean and range for all relevant demographic and baseline variables for all treatment groups. |
| □ | ⊠ | The range and median (not mean) for length of follow-up reporting for each treatment group |
| □ | ⊠ | A precise timeline during which all patients were treated for each group |
| □ | ⊠ | Outcome variables being compared between groups are presented with appropriate measures of variability (eg, SD) |
| □ | ⊠ | Measures of type II error (P values) for comparison statistics are presented with actual values if P = .01 or larger (eg, P = NS and P < .05 are not acceptable) |
| □ | ⊠ | A description of how patients were selected into each treatment group |
| □ | ⊠ | A statement is made as to whether the same surgeons operated on patients from different treatment groups |
Articles concerning clinical research should follow a uniform set of reporting guidelines. The guidelines, listed above, were developed from sound clinical research principles and are designed to improve the reporting accuracy of clinical data pertaining to surgical conditions.
With more accurate and transparent reporting of study methodology and outcomes data, readers of the Journal will be better able to gauge the relevance of reported results to their own clinical practice. Although not all of the recommended reporting guidelines below are applicable to every clinical study, it is important that all details relevant to your study are clearly reported in the article. Please check the appropriate boxes below to verify compliance with these guidelines, and return this sheet with the article at the time of submission. Compliance with these guidelines in combination with subsequent content revisions will be considered by the editor in the final decision regarding publication of your article.
NS indicates not significant.
Fig. 1.
Number of patients with nodal involvement, stratified by number of LNs submitted for histopathologic analysis.
Table 2.
Number of LNs sampled per patient stratified by pathologic stage
| No. of LN sampled | Stage |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| 0 | 17% | 11% | 10% |
| 1-2 | 34% | 31% | 27% |
| 3-6 | 33% | 37% | 33% |
| 7+ | 16% | 21% | 30% |
Table 3.
Number of LNs sampled per patient stratified by histology
| No. of LN sampled | Histology |
|
|---|---|---|
| Anaplastic | Favorable | |
| 0 | 8% | 13% |
| 1-2 | 30% | 31% |
| 3-6 | 39% | 34% |
| 7+ | 24% | 22% |
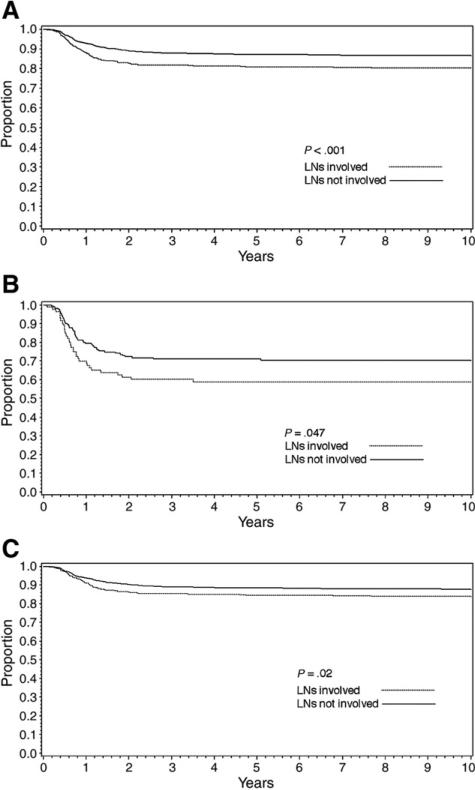
Overall, patients with LN involvement had poorer 5-year EFS than those with negative LNs (81% vs 87%, P < .001; Fig. 2A). The effect of LN positivity on EFS was somewhat greater for patients with anaplastic histology (59% vs 72%, P = .047, Fig. 2B) than for those with favorable histology (85% vs 88%, P = .02, Fig. 2C).
Fig. 2.
Effect of LN positivity on 5-year EFS: overall (A) and stratified by favorable (B) and anaplastic (C) histology.
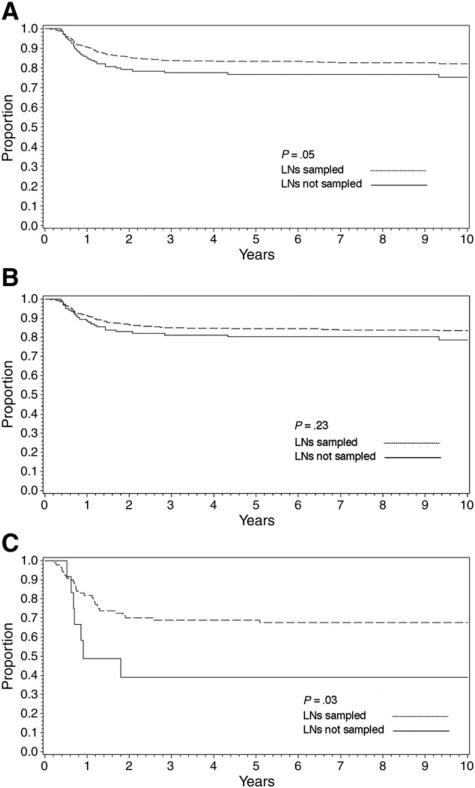
A total of 2575 patients had stage I or II disease (node negative). Overall, for these patients, the number of LNs sampled was not predictive of EFS (P = .24), with the 5-year EFS being 85% when no nodes were sampled, 88% when 1 to 2 nodes were sampled, 88% when 3 to 6 nodes were sampled, and 86% when 7+ nodes were sampled. When the patients with no LNs sampled were compared with those with any LN sampling, there was no significant difference in EFS overall (P = .23). Among patients with stage I disease, there was no difference in EFS by whether LNs were sampled, either overall (90% vs 92%, P = .43) or within histologic subsets (favorable histology, 91% vs 92%, P = .47; anaplastic histology, 73% vs 83%, P = .68). Among patients with stage II disease, there was a marginally statistically significant difference in EFS by whether LNs were sampled (77% vs 83%, P = .054, Fig. 3A), but this difference was mostly because of a difference in outcome for patients with anaplastic tumors (5-year EFS, no LNs sampled [n = 12], 39%; LNs sampled [n = 90], 69%; P = .03; Fig. 3B) and not for patients with favorable histology tumors (80% vs 84%, P = .23, Fig. 3C).
Fig. 3.
Effect of LN sampling on 5-year EFS in patients with stage I and II WTs: overall (A) and stratified by favorable (B) and anaplastic (C) histology.
3. Discussion
In this study, we have confirmed previous reports that LN involvement in children with WT has significant prognostic and therapeutic implications. However, the magnitude of the effect of LN positivity on EFS was more profound for patients with tumors of anaplastic histology rather than favorable histology. In the latter group, the difference in EFS was modest, although statistically significant, suggesting that current adjuvant therapy for patients with stage III disease owing to LN involvement is effective for favorable histology disease. In these patients, LN sampling provides important diagnostic information that enables proper staging and delivery of appropriate adjuvant therapy, ultimately rendering outcomes in this group similar to those with node-negative disease. This is not the case for tumors of anaplastic histology for which adjuvant therapy is much less effective and for which therapy for patients with stage II and III diseases did not differ on NWTS-4 and NWTS-5. Currently, patients with stage III disease receive a higher dose of ionizing radiation than for those with stage II disease.
Because intraoperative gross assessment of LNs is not reliable for determining tumor involvement, LN sampling for histologic evaluation is required.
The importance of LN sampling is highlighted by reviews of prior NWTS studies that have shown that failure to sample LNs at the time of nephrectomy was associated with an increased incidence of tumor recurrence [5,8,9]. Presumably, this reflects understaging of patients with occult LN metastases that were not detected and so effective; more intensive adjuvant stage-appropriate therapy was not administered. We found LN sampling to be important for stage II disease but not stage I tumors, likely reflecting the greater likelihood of occult metastases in the setting of renal sinus invasion or capsular penetration. Among patients with stage I disease, there was no difference in EFS by whether LNs were sampled, either overall or within histologic subsets. Interestingly, among patients with stage II disease, this difference was mostly because of a difference in outcome for patients with anaplastic tumors; LN sampling was not associated with a significant difference in EFS for patients with favorable histology tumors. Because some of these stage II patients with favorable histology disease likely had microscopic LN involvement missed because LNs were not sampled, these data suggest that most patients with occult LN involvement and favorable histology may be adequately treated with 2-drug chemotherapy.
It is difficult to explain why stage II patients with anaplastic histology and no LNs sampled should do worse than those documented to not have LN involvement, given that therapy was identical for patients with stage II and III diseases. We theorize that perhaps removal of LNs with microscopic anaplastic disease provided a therapeutic advantage given that adjuvant therapy for anaplastic WT is not very effective. However, because Dome et al [10] noted in their review of patients with anaplastic histology treated on NWTS-5 that, among patients with stage II anaplastic histology who had a recurrence, the initial site was never local, one must then hypothesize that the presence of occult LN metastases led to or was associated with an increase in distant recurrence that became manifest before the local recurrence.
Despite the importance of assessing loco-regional LN involvement, prior studies have suggested that extensive retroperitoneal LN dissection does not improve patient outcome as compared with LN sampling [5] and can be associated with significant procedure-related morbidity [11,12]. Less certain is how many LNs comprise an adequate sampling. Our analysis shows that the incidence of LN positivity increases with increasing numbers of LNs sampled; “yield,” in terms of identifying patients with positive LNs, appears to be maximal when at least 7 LNs are sampled. This analysis assumes that the number of LNs sampled is independent of the risk of having positive LNs. However, we cannot exclude the possibility that the surgeon removes more LNs when the intraoperative findings suggest more advanced disease (eg, a larger, more aggressive appearing primary tumor or clinical LN involvement), as initially suggested in a review by Leape et al [5]. If this were the case, tumor characteristics and/or LN involvement would be driving the number of LNs sampled rather than the converse. This possible bias is supported by the trend toward sampling more LNs in patients with stage II disease as compared with patients with stage I disease, in patients with tumors of anaplastic histology as compared with those of favorable histology, and in patients with LN involvement as compared with those without. Because neither the stage nor histology was known at the time of nephrectomy and LN sampling, these results suggest that operative findings may have biased the extent of LN sampling.
Our finding that the percentage of patients with positive LNs increased as the number of LNs sampled increased, with a plateau in the LN positivity rate at about 28% at 7 or more LNs sampled, is different from the 3-node threshold recently reported by Godzinski et al [13]. This small study, conducted by the International Society of Paediatric Oncology (SIOP) Nephroblastoma Trial and Study Committee, demonstrated that the likelihood of LN positivity did not increase when more than 3 nodes were sampled. Although the precise number of LNs needed to ensure accurate characterization of nodal status differed between our study and the SIOP group (and may reflect the relative number of patients in each study), both studies concluded that extensive LN sampling appeared to confer no clinical benefit. Moreover, a significant difference in the conduct of this trial compared with NWTS protocols was that all SIOP patients received chemotherapy before nephrectomy and LN sampling.
Interestingly, despite the higher incidence of positive LNs with the greater number sampled, after accounting for the effects of stage and histology, the number of LNs sampled was not predictive of EFS when considering all patients or only those without nodal disease. This is in contrast to other solid tumors such as bladder cancer [14-16], colon cancer [17,18], and others [19,20], where disease outcome has been correlated with the number of LNs sampled. This difference might be explained by the fact that those solid tumors are generally less sensitive to adjuvant chemotherapy than WT.
Although it is difficult to determine to what degree our observations reflect the influences of surgeon bias and location of LNs sampled, our data suggest that accurate staging of patients with unilateral WT does not require sampling of a large number of LNs. The reason for this is unclear. One possibility is that lymphatic spread from a WT progresses in an anatomically orderly manner in which a “sentinel” lymph is involved first. This LN may be recovered frequently because it is either adherent to the resected tumor specimen itself or is within the hilar LNs in the immediate vicinity of the renal vessels and thus more often removed during the surgical procedure. The removal of additional LNs, then, might not improve detection of occult metastases or influence outcome; in this case, the increased incidence of positivity with greater sampling noted previously may simply reflect the biases described above.
Critical, then, is that the nodes that are sampled are hilar in location, as opposed, for example, to being mesenteric LNs, which are not likely to be a primary site of lymphatic drainage for a renal tumor. A clearer understanding of the location of positive LNs would help confirm the validity of this hypothesis. In this study, we did not specifically investigate the location of positive LNs as a variable because in many cases, LNs in pathology reports were coded for alternate locations (eg, “mesenteric”), and location of the positive LNs was not consistently recorded in all pathology reports. However, when LN sampling was performed, most patients had hilar LNs submitted for analysis within the nephrectomy specimen. In addition, no patient had positive distant LNs in the setting of negative hilar LNs. This finding is consistent with the early observation in NWTS-2 that EFS and overall survival were higher when positive LNs were confined to the renal hilum as compared with when they extended more distantly along the periaortic region, again suggesting an anatomically orderly progression of LN spread [21].
At present, the number of LNs sampled during nephrectomy for WT is left to the discretion of the operating surgeon. In the interest of minimizing surgical morbidity, surgeons may opt to limit LN sampling to those nodes that are most safely removed, thereby striking a balance between the need to ensure that LN sampling accurately reflects the nodal disease status and the desire to minimize morbidity in an already complex surgical procedure. Our review of the NWTS-4 and NWTS-5 databases would suggest that LNs collected from the hilar region are probably adequate to ensure accurate staging and that extensive LN dissection is not necessary. We have intentionally refrained from a quantitative recommendation because surgeons rarely identify individual LNs and, so, at the time of surgery, do not generally have a precise count of how many have been removed. It is likely that further detailed investigation into the pattern of LN spread and location of involved LNs will help to further refine this recommendation. Nevertheless, our study confirms the importance of sampling at least some LNs. Although this is true primarily for patients with anaplastic WT, all patients should undergo LN sampling because the surgeon does not know the tumor histology at the time of nephrectomy.
References
- 1.Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21:172–81. doi: 10.1002/mpo.2950210305. [DOI] [PubMed] [Google Scholar]
- 2.Jereb B, Tournade MF, Lemerle J, et al. Lymph node invasion and prognosis in nephroblastoma. Cancer. 1980;45:1632–6. doi: 10.1002/1097-0142(19800401)45:7<1632::aid-cncr2820450719>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Breslow N, Sharples K, Beckwith JB, et al. Prognostic factors in nonmetastatic, favorable histology Wilms tumor. Results of the Third National Wilms Tumor Study. Cancer. 1991;68:2345–53. doi: 10.1002/1097-0142(19911201)68:11<2345::aid-cncr2820681103>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg. 1999;229:292–7. doi: 10.1097/00000658-199902000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leape LL, Breslow NE, Bishop HC. The surgical treatment of Wilms tumor: results of the National Wilms Tumor Study. Ann Surg. 1978;187:351–6. doi: 10.1097/00000658-197804000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Othersen HB, Jr, DeLorimer A, Hrabovsky E, et al. Surgical evaluation of lymph node metastases in Wilms tumor. J Pediatr Surg. 1990;25:330–1. doi: 10.1016/0022-3468(90)90079-o. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich PF, Ritchey ML, Hamilton TE, et al. Quality assessment for Wilms tumor: a report from the National Wilms Tumor Study-5. J Pediatr Surg. 2005;40:208–12. doi: 10.1016/j.jpedsurg.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 8.Zhuge Y, Cheung MC, Yang R, et al. Improved survival with lymph node sampling in Wilms tumor. J Surg Res. 2011;167:e199–203. doi: 10.1016/j.jss.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Raval MV, Bilimoria KY, Bentrem DJ, et al. Nodal evaluation in Wilms tumors: analysis of the national cancer data base. Ann Surg. 2010;251:559–65. doi: 10.1097/SLA.0b013e3181cc95d7. [DOI] [PubMed] [Google Scholar]
- 10.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms tumor: results from the fifth National Wilms Tumor Study. J Clin Oncol. 2006;24:2352–8. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 11.Williams SB, McDermott DW, Winston D, et al. Morbidity of open retroperitoneal lymph node dissection for testicular cancer: contemporary perioperative data. BJU Int. 2010;105:918–21. doi: 10.1111/j.1464-410X.2009.08888.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiser AC, Lindgren BW, Ritchey ML, et al. Chylous ascites following surgical treatment for Wilms tumor. J Urol. 2003;170:1667–9. doi: 10.1097/01.ju.0000085655.48806.87. [DOI] [PubMed] [Google Scholar]
- 13.Godzinski J, de Kraker J. Is the number of lymph nodes sampled at Wilms tumor nephrectomy predictive for detection of the regional extension of the disease? International Society of Paediatric Oncology, SIOP XXXVI Congress Meeting. Pediatr Blood Cancer. 2004;43:329. Abstracts. [Google Scholar]
- 14.Koppie TM, Vickers AJ, Vora K, et al. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer. 2006;107:2368–74. doi: 10.1002/cncr.22250. [DOI] [PubMed] [Google Scholar]
- 15.Herr HW, Bochner BH, Dalbagni G, et al. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295–8. [PubMed] [Google Scholar]
- 16.Bochner BH, Herr HW, Reuter VE. Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol. 2001;166:2295–6. [PubMed] [Google Scholar]
- 17.Tepper JE, O'Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–63. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 18.Sobin LH, Greene FL. TNM classification: clarification of number of regional lymph nodes for pNo. Cancer. 2001;92:452. doi: 10.1002/1097-0142(20010715)92:2<452::aid-cncr1342>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998;71:340–3. doi: 10.1006/gyno.1998.5254. [DOI] [PubMed] [Google Scholar]
- 20.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–24. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 21.Breslow N, Churchill G, Beckwith JB, et al. Prognosis for Wilms tumor patients with nonmetastatic disease at diagnosis—results of the second National Wilms Tumor Study. J Clin Oncol. 1985;3:521–31. doi: 10.1200/JCO.1985.3.4.521. [DOI] [PubMed] [Google Scholar]