Abstract
Juvenile neuronal ceroid lipofuscinosis is a childhood-onset neurodegenerative disease with prominent symptoms comprising a pediatric dementia syndrome: intellectual decline, mood and behavioral impairments, and loss of adaptive skills. We review the history of neurobehavioral features in juvenile neuronal ceroid lipofuscinosis and the work of the University of Rochester Batten Center to characterize the extent and progression of neurobehavioral symptoms over disease course, and discuss the relevance of neurobehavioral studies as an aid to understanding the clinical phenotype of juvenile Batten disease and potential targets for intervention.
Keywords: Behavior, cognition, neuropsychology, quality of life, Unified Batten Disease Rating Scale
Introduction
Juvenile neuronal ceroid lipofuscinosis, also known as juvenile Batten disease, is an autosomal recessively inherited neurodegenerative disease of childhood onset. It is caused by mutations in the ceroid-lipofuscinosis-3 gene located on the short arm of chromosome 16 (locus p.12.1). Approximately 80% to 85% of affected individuals are homozygous for a 1.02 kb deletion; most of the remaining children are heterozygous for this common deletion and another mutation.1 A comprehensive catalog of known disease-causing mutations for juvenile neuronal ceroid lipofuscinosis and the other neuronal ceroid lipofucinoses is maintained at the NCL Mutation and Patient Database: http://www.ucl.ac.uk/ncl/mutation.shtml. Juvenile neuronal ceroid lipofuscinosis is also a rare disease, with a worldwide incidence estimated to range from 0.2 to 7.0 per 100 000 births worldwide.2
Clinically, onset of juvenile neuronal ceroid lipofuscinosis occurs at approximately 5 to 6 years of age. On average, disease is evident in males a year earlier than in females.3 Although the temporal sequence of symptom onset varies, the most common symptom families recognize early on is vision loss, rapidly progressing to blindness within about one year, in both males and females. Symptom progression continues with loss of motor skills, loss of speech, a dementia syndrome comprising loss of cognition and adaptive skills, and emergence of behavioral and mood problems. Seizures are known to occur in juvenile neuronal ceroid lipofuscinosis but may not develop until several years after disease onset, and are less prominent than in the infantile and late-infantile forms of Batten disease, where they are likely to occur early in the disease course, and be frequent, severe, and difficult to control. The juvenile neuronal ceroid lipofuscinosis disease course unfolds slowly, with an average survival of 15 years from symptom onset to the end of life.3 There are unfortunately no established treatments that can stop, reverse, or prevent disease.
Neurobehavioral Studies of Juvenile Neuronal Ceroid Lipofuscinosis
History and Early Research Foundation
The first account of what would ultimately become known as the eponymous Batten disease was published in 1826 by physician Otto Christian Stengel (1795-1890). He described 4 siblings living in the copper mining town of Røros, Norway.4 All 4 children developed normally at first, but each experienced vision loss beginning at about 6 years of age. In conjunction with physical changes, including difficulty eating and weight loss, behavioral alterations were noted. Of the eldest child, a boy, Stengel wrote, “…his intellectual abilities slowed down, he became indifferent to things which had previously interested him…”4 Behavioral symptoms also developed; at 15 years of age, the child “…became more restless…his behavior was like someone suffering from mania.”4 Mild seizures were noted from about ages 9 to 15 years, but did not worsen until the later teen years. The 3 younger siblings each developed the same pattern of symptoms along a similar time course. In 1902, British ophthalmologist Frederick Batten presented 2 other cases, also siblings, with similar clinical features. The younger of 2 girls, at about age 6 years, “…became spiteful at school, had attacks of violent temper…her sight was failing, and ‘she looked out of the corners of her eyes to see an object’…the plantar reflex tended to give an extensor report.” 5The older sister, by age 10, had also experienced vision loss and seizures, and was living in an asylum because her “mental condition…deteriorated.”5 Though much of Batten's report focused on the ophthalmologic findings, this brief description of 2 children, like Stengel's, captured all core features of the disease: deteriorating vision with initial central field loss, motor impairments, and the prominent neurobehavioral symptoms of the disease — cognitive decline and behavioral and mood disturbances.
Formal characterization of neurobehavioral features of juvenile neuronal ceroid lipofuscinosis was first conducted more than 150 years after Stengel's first report, and was predominantly based on patient groups in Finland. These studies provided further details of associated behavioral and mood impairments, including anxious and depressed mood, aggressive behavior, and hallucinations.6-9 In a survey of 42 affected children, Santavuori and co-authors9 found high rates of physical restlessness (35%), aggression (40%), fears (45%), and sleep problems (30%). Ten percent of the sample also experienced psychotic symptoms (hallucinations and/or delusions), depression, and irritable mood. Bäckman and colleagues6 provided an extensive and standardized assessment of mood and behavior, using well-validated, norm-referenced parent and teacher ratings of child behavior. Cognitive decline was first quantitatively described in several case reports published in the 1990s; each presented intelligence test scores for children with clinical juvenile neuronal ceroid lipofuscinosis, including in some cases, repeat assessment that provided information on change in cognition over time.8,10,11
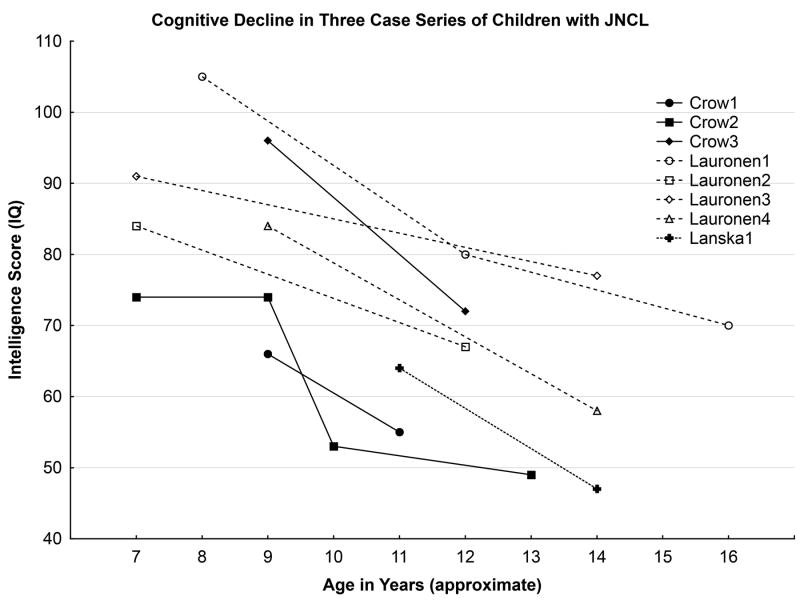
Figure 1 summarizes data available for the 8 cases, described in these 3 papers, who underwent repeat intellectual assessment. The longest follow-up period was approximately 7 years. Several challenges exist in interpreting these data, including the variable follow-up periods, differences in measures used that may limit direct comparability between assessments and among patients, and the use of retrospective data culled from clinical files, rather than prospectively obtained in uniform fashion. Nonetheless, even with this small group of cases, aggregated across 3 studies, 2 themes are evident: first, cognition declines over time, and second, the rate of decline appears variable. A subsequent study, also from Finland, reported prospective data on 12 children who completed intelligence testing at the time of diagnosis, and then were subsequently re-evaluated annually for up to 5 years;12 this investigation confirmed the inexorable decline in cognitive abilities over time, but as well, illustrated the variable progression among the children.
Figure 1.
Cognitive decline in intelligence test scores in 3 case series of children with juvenile neuronal ceroid lipofuscinosis.
Neurobehavioral Studies in Juvenile Neuronal Ceroid Lipofuscinosis – University of Rochester Batten Center
Background and Methods
Since 2002, the University of Rochester Batten Center has evaluated neurobehavioral features of juvenile neuronal ceroid lipofuscinosis using several items from the Unified Batten Disease Rating Scale in which the examiner (a neurologist) provides global clinical ratings (Clinical Global Impression scores) of the child's current cognitive, behavioral, and mood state.13,14 Independent assessment of neurobehavioral features was added in 2003. Table 1 lists the current neurobehavioral battery, which includes a direct, multi-domain neuropsychological evaluation and parent-based ratings of mood, behavior, adaptive function, and quality of life. Table 1 also provides a description of any testing modifications, and the rationale for doing so. Many children are enrolled in, and participate in, the neurobehavioral study during the annual family support meetings of the Batten Disease Support and Research Association and/or at clinical research visits to the University of Rochester Batten Center. Neurobehavioral assessments are completed as part of the UBDRS Natural History study and also as a stand-alone protocol for children who are only followed remotely, (eg, have not been seen in person for Unified Batten Disease Rating Scale exams). Children complete neuropsychological testing annually whenever possible. In addition, parent-based ratings are completed biannually.
Table 1. University of Rochester Batten Center's Neurobehavioral Assessment Protocol.
| A. DIRECT NEUROPSYCHOLOGICAL ASSESSMENT | |
|---|---|
| 1. WISC-IV23 Vocabulary | Verbal Intellectual Ability |
| a. First 4, picture-naming items are not presented, to accommodate vision loss in JNCL. Credit for these items only given if study subject earns full credit for first 2 verbal items, otherwise the total score for the test treats the first 4 items as ‘failed’; b. Study subject is asked to recite full alphabet to evaluate retention of a well-routinized skill whose attainment likely precedes symptom onset. It is scored separately from Vocabulary raw score as ‘pass/fail.’ | |
| 2. WISC-IV Digit Span* | Auditory attention, working memory |
| Verbal cues provided to visually impaired child, before each item is presented: “Here comes the next one” | |
| 3. WISC-IV Information | Fund of knowledge |
| No modifications | |
| 4. WISC-IV Similarities | Verbal abstract reasoning |
| No modifications | |
| 5. WRAML-245 Story Recall | Immediate, delayed, and recognition memory |
| No modifications | |
| 6. WRAML-2 Sentence Recall | Auditory attention, immediate recall |
| No modifications | |
| 7. Controlled Oral Word Association Test46 | Verbal fluency |
| Two tasks combined – phonemic (F-A-S) and semantic (animals; food) to extend ‘floor’ of task, as phonemic items are failed earlier. | |
| B. PARENT-PROXY RATINGS OF CHILD FUNCTION | |
|---|---|
| 1. PedsQL26 | Child Health-related Quality of Life |
| No modifications | |
| 2. Family Impact Module27 | Ratings of self (parental) HRQL and family QL. |
| No modifications | |
| 3. Child Behavior Checklist31 | Omnibus assessment of child behavior and mood |
| No modifications | |
| 4. Scales of Independent Behavior – Revised30 | Child adaptive function level |
| None, but use of SIB-R Short form for Visually Impaired, to reduce burden for completion | |
HRQL, health-related quality of life; QL, quality of life; WISC-IV, Wechsler Intelligence Scale for Children - Fourth Edition; WRAML-2, Wide Range Assessment of Memory and Learning - 2nd Edition.
To date, 87 affected children and young adults have participated in neurobehavioral studies with us (47 males, 40 females). Thirteen children are unfortunately now deceased. Of the remaining, the average current age is 17.4 years (range, 7.5 – 28.2 years; standard deviation, 4.6 years). Not every child participates in all study activities each year, and the eldest children in the sample are typically unable to participate in direct neuropsychological evaluation. Therefore, the average age for participating children is provided, with results of respective analyses for any new results presented below. Most participants in the neurobehavioral studies have genetic confirmation of the juvenile neuronal ceroid lipofuscinosis diagnosis (homozygous for the common deletion, n = 49; compound heterozygous, n = 25; homozygous for a novel mutation, n = 1). Genotype is confirmed via review of patients' clinical records or through our lab.15 Participants without genetic confirmation meet the clinical definition for juvenile neuronal ceroid lipofuscinosis.16 All research activities have been conducted under study protocols approved by the University of Rochester Research Subjects Review Board, and all parents have completed an informed consent process and provided written permission for their child's participation in the study. Any new data presented herein were analyzed using Statistica (Version 10) by StatSoft (Tulsa, OK).17
Results
Behavioral Symptoms
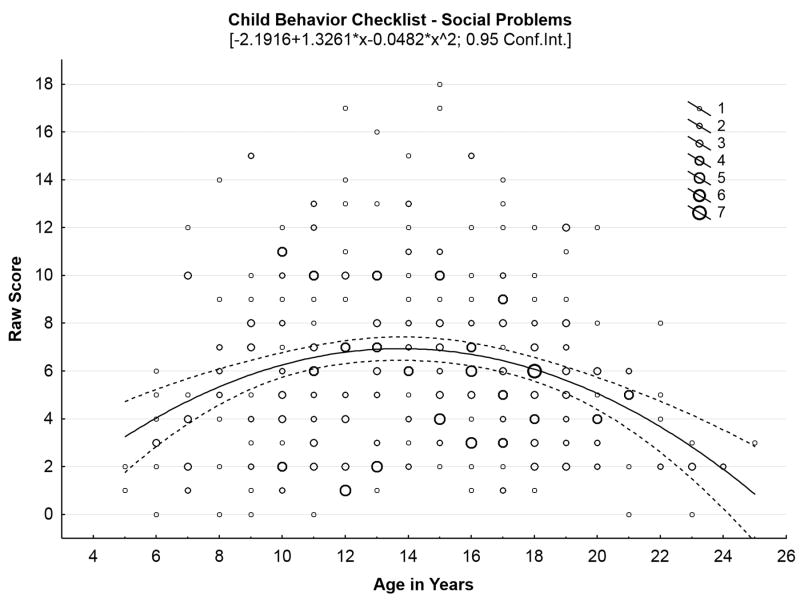
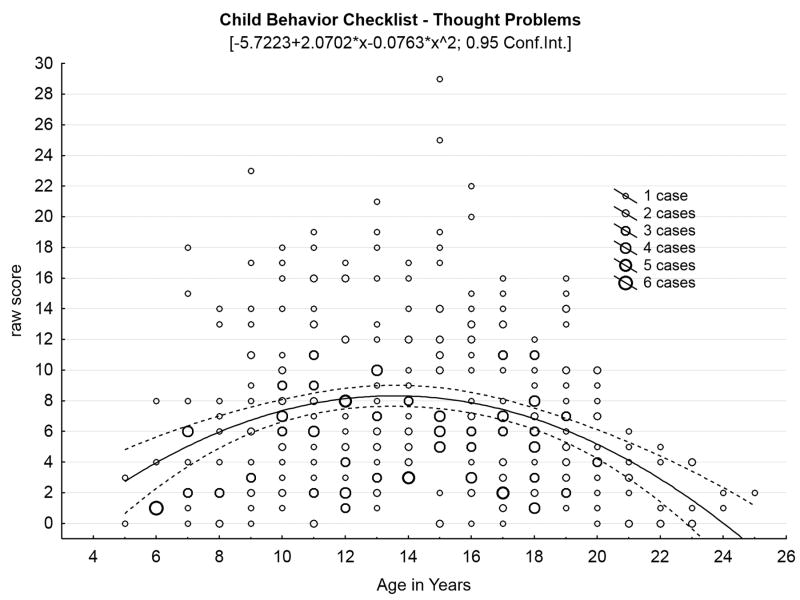
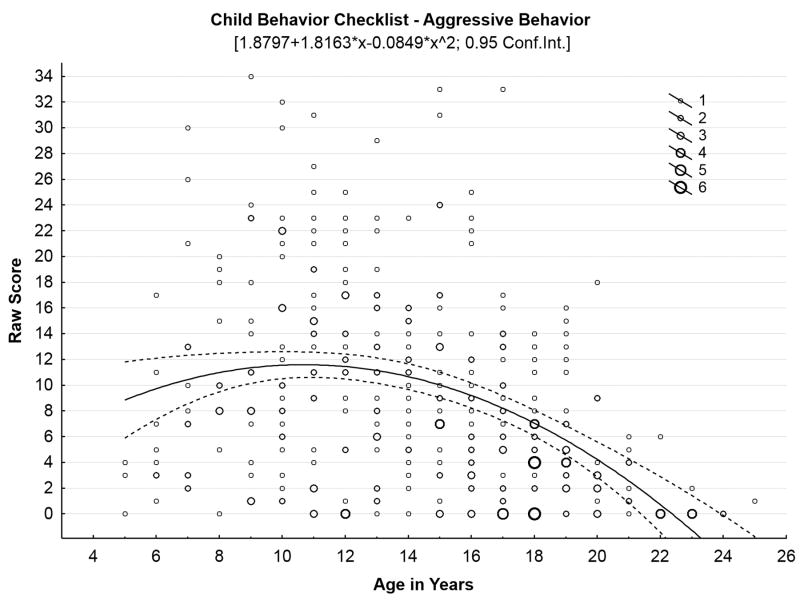
Our initial studies18-20 confirmed high rates of behavioral problems in children with juvenile neuronal ceroid lipofuscinosis. These symptoms, along with cognitive impairments, appear to precede onset of motor impairment,3 and do not appear to be influenced by genotype.21 Bäckman and colleagues6 reported sex differences in behavioral symptoms among a Finnish cohort of children with juvenile neuronal ceroid lipofuscinosis: females exhibited greater anxiety (per parent report) and greater social difficulties (per teacher report) in comparison with males, although these findings were not replicated in our North American sample.20 We also have hypothesized that behavioral and mood symptoms would follow an inverse U-shaped function in which problems initially worsen as disease advances, before dropping off in the later stages of the illness, as motor impairment limits the behavioral repertoire. Figure 2, Panels A-C illustrate this pattern for several behavioral domains, with scatterplots containing all observations at each time point for each child who has undergone parental ratings of behavior with the Child Behavior Checklist, representing 369 total evaluations of 70 children since 2003.
Figure 2.
Behavioral problems increase through middle teen years, then decrease in later years of disease. (A) Social problems by age in years. (B) Thought problems by age in years. (C) Aggressive problems by age in years.
Cognitive symptoms
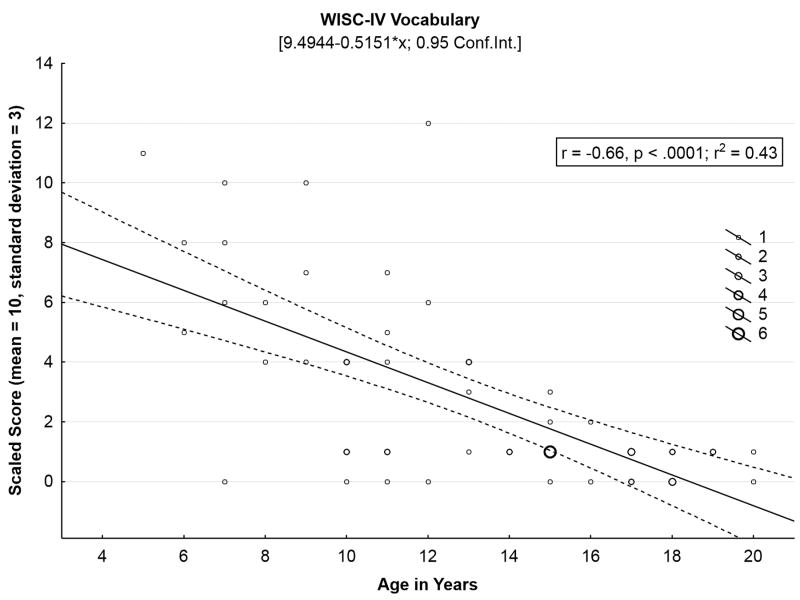
A total of 152 neuropsychological evaluations of 70 children have been conducted since 2003, either through our specific neurobehavioral study protocol or as an add-on to the Unified Batten Disease Rating Scale natural history study (Table 2). The cognitive test battery incorporates an estimation of global verbal intellectual ability, and tests of new learning and memory, language fluency, and attention and working memory. As shown in Table 1, several age-appropriate tasks used in the early years of our project22 were piloted with older participants (≥ 17 years old) but were not retained because they were too difficult for young adults with juvenile neuronal ceroid lipofuscinosis to complete. Neuropsychological function in all areas evaluated is significantly lower than expected in comparison with same-age peers. Scaled scores are shown here; in healthy children these scores will remain stable (mean, 10; standard deviation, 3) as they are benchmarked against age-based expected performance. In juvenile neuronal ceroid lipofuscinosis, however, scaled scores tend to drop off with increasing age, reflecting a failure to keep pace with expected development. The Vocabulary subtest from the Wechsler Intelligence Scale for Children-IV23 illustrates this pattern of cognitive decline (Figure 3). Fifty-seven children (26 females, 31 males; mean age, 13.5 years; standard deviation, 4.0 years; range, 5.7 to 20.9 years) have completed the Wechsler Vocabulary task at least once. Taking the most recent evaluation for each child, it is possible to see sharp declines in Wechsler Vocabulary (Pearson r = -.64) as a function of chronological age.
Table 2. Neuropsychological Evaluations by Number of Assessments Completed.
| Number of Assessments | Number of Participants |
|---|---|
| 1 | 35 |
| 2 | 15 |
| 3 | 9 |
| 4 | 3 |
| 5 | 4 |
| 6 | 1 |
| 7 | 2 |
| 8 | 1 |
Figure 3.
Wechsler Intelligence Scale for Children - Fourth Edition Vocabulary test score, a proxy for verbal intellectual ability, declines sharply with age, in juvenile neuronal ceroid lipofuscinosis.
Psychosocial Impacts in Juvenile Neuronal Ceroid Lipofuscinosis
One set of studies has reported that parents of juvenile neuronal ceroid lipofuscinosis-affected children experienced higher levels of depression in comparison with parents of children with chronic but non-life-limiting illnesses,24,25 but also that higher levels of ‘optimism’ were associated with lower levels of family conflict, anxiety, and depression. We hypothesized that neurobehavioral symptoms in affected individuals would be negatively associated with the well-being of parents and the family as a whole. The Pediatric Quality of Life (PedsQL) Family Impact Module is a health-related quality of life assessment to evaluate parent- and family-level quality of life in relation to child health.26,27 The PedsQL Family Impact Module Total Score collectively describes both parental ratings of parents' own health-related quality of life and family-wide well-being, in relation to a child's recent (past month) health state: “…as a result of your child's health, how much of a problem have you had with…”27
In an initial sample of 44 affected individuals (27 males, 17 females; mean age, 14.0 years; standard deviation, 5.16 years) we found a significant, moderate correlation between the PedsQL Family Impact Module Total Score and total number and severity of juvenile neuronal ceroid lipofuscinosis child behavioral problems (Pearson r = -53, P <.001).28 In a follow-up assessment with the most recent evaluation completed for all children who have participated (n = 51: 26 males, 25 females; mean age,16.3 years; standard deviation, 5.1 years; range, 7.4 – 32.0 years), we also see that child behavior problems are significantly associated with parent self-ratings of reduced emotional, social, and cognitive well-being on the total Parent Health-related Quality of Life score of the Peds QL Family Impact Module (Table 3). Interestingly, these relationships were present for child ‘internalizing’ symptoms (i.e., anxious, withdrawn, depressed mood, social problems) but not ‘externalizing’ symptoms (aggression, non-compliance, and defiance).
Table 3. Relationships Among Parent Health-related Quality of Life, and Child Behavior Problems.
| Child Behavior Checklist | ||
|---|---|---|
| Internalizing symptoms | Externalizing symptoms | |
| Family Impact Module | ||
| Parent Emotional HRQL | -.41** | -.04 (ns) |
| Parent Social HRQL | -.39** | -.02 (ns) |
| Parent Cognitive HRQL | -.52** | -.21 (ns) |
| Parent Physical HRQL | -.22 (ns) | -.07 (ns) |
Higher CBCL scores indicate greater problems; higher FIM and PedsQL scores indicate better HRQL; higher SIB-R scores indicate better adaptive function skills.
CBCL, Child Behavior Checklist; FIM, Family Impact Module; HRQL, health-related quality of life; JNCL, juvenile neuronal ceroid lipofuscinosis;
The clinical course of juvenile neuronal ceroid lipofuscinosis involves progressive loss of independent adaptive skills such as mobility, feeding, and communication.20 In association with decrements in adaptive skills, caregiver burden is presumed to increase, but this has not yet been empirically studied. A needs-assessment survey conducted in Great Britain to assess service gaps to juvenile neuronal ceroid lipofuscinosis children and families identified various areas of caregiver need, including support for management of neurobehavioral problems, and respite care.29 We have examined the relationship between adaptive function, using the Scales of Independent Behavior-Revised30 and family burden, using the Family Impact Module.27 Twenty-five parents have completed assessment of child adaptive function and parent/family burden at the same time, providing concurrent information on child's adaptive function and parental and family well-being (17 males, 8 females; mean age, 16.3 years; standard deviation, 6.1 years; range, 16.3 – 7.4 years). Using the most recently completed assessment, there was not a significant relationship between adaptive function in an affected child and the total PedsQL Family Impact Module score, which summarizes both parental health-related quality of life and family function (Pearson r = .15, P = .47). However, PedsQL Family Impact Module-based parental ratings of problems in ‘family relationships’ (ie, conflicts, stress, or tension among family members, lack of communication, difficulty working together as a family to solve problems or make decisions) was moderately negatively associated with adaptive function, as assessed with the Scales of Independent Behavior - Revised (Pearson r = -.48, P <.05).
Neurobehavioral and Other Natural History Data
The multidisciplinary nature of the University of Rochester Batten Center natural history studies of juvenile neuronal ceroid lipofuscinosis facilitates ‘cross-talk’ among different sets of data gathered on the same cohort of affected individuals. A composite neuropsychological score based on all cognitive tests in the protocol provided external validation of the Unified Batten Disease Rating Scale Clinical Global Impression rating of cognition (1 = no cognitive impairment; 5 = severely impaired), in a relatively small sample of 18 affected children.19 We have replicated this finding using the most recent concurrent evaluations for both neuropsychological assessment and a Unified Batten Disease Rating Scale exam in 59 children and young adults (31 males, 28 females: mean age, 13.5 years; standard deviation, 13.0 years; range, 5.7-20.9 years). Higher scores on the Clinical Global Impression (i.e., clinician rating of poor cognition) are associated with lower scores on the neuropsychological tests, also indicating poor cognition: Spearman ρ = -0.64.
Table 4 shows the individual Spearman correlations between the Clinical Global Impression ratings and performance on each cognitive domain (by individual neuropsychological test); all are significant. Similarly, the Child Behavior Checklist,31 a widely accepted measure of child behavior, has demonstrated external validity of the Unified Batten Disease Rating Scale clinician-based PedsQL Family Impact Module ratings of mood and behavior. Parent ratings of Child Behavior Checklist Internalizing symptom clusters (anxious/depressed, and withdrawn/depressed mood) were significantly associated with clinician ratings of sad mood, apathy, and anxiety. Likewise, parent ratings of aggressive behavior correlated robustly with clinician ratings of aggression to self or others.21 We have also used neurobehavioral data to evaluate the hypothesis that children who are heterozygous for the common deletion plus a novel mutation may experience an attenuated disease course. Linking the Child Behavior Checklist with genotyping results available through the Unified Batten Disease Rating Scale study, we did not see a significant difference in behavior problems by genotype group.21
Table 4. Spearman Rank-order Correlation Coefficients: Associations between UBDRS Clinician Global Impression (CGI) of Cognitive Function and Neuropsychological Test Performance (n = 59).
| Neuropsychological Test Raw Scores | UBDRS CGI |
|---|---|
| WISC-IV Vocabulary (n = 54) | -.53** |
| WISC-IV Digit Span (n = 56) | -.49** |
| WRAML-2 Story Memory: immediate recall (n = 58) | -.65** |
| WRAML-2 Story Memory: delayed recall (n = 58) | -.69** |
| WRAML-2 Story Memory: recognition (n = 58) | -.60** |
| WRAML-2 Sentence recall (n = 56) | -.52** |
| Controlled Oral Word Association (F-A-S) (n = 55) | -.36* |
ρ < .05;
ρ < .001
CGI, Clinician Global Impression; UBDRS, Unified Batten Disease Rating Scale; WISC-IV, Wechsler Intelligence Scale for Children – 4th Edition; WRAML-2, Wide Range Assessment of Memory and Learning - 2nd Edition.
Looking Forward – Challenges and Opportunities
Longitudinal neurobehavioral assessment of children with juvenile neuronal ceroid lipofuscinosis faces several challenges. First, well-established measures of cognition and behavior are ideally developed using representative samples of healthy, normally-developing children.32 This enables appropriate benchmarking of individual performance in relation to age-expected skills, but also may restrict the sensitivity of measures for evaluating children at the lowest levels of ability, as is the case for children in the later years of their disease course. In contrast, measures developed to assess children with static cognitive or developmental delay (eg, intellectual disability; autism spectrum disorders) may be appropriate for evaluating the significant limitations experienced by older children with juvenile Batten disease, but may not provide adequate information about capability during the earliest stages of disease, when cognition and/or behavior may still be developmentally normal. Shapiro and Balthazor provide a roadmap for the evaluation of children with dementing conditions, including use of repeatable, reliable measures that “are capable of measuring widely disparate abilities.”33
Second, the particular combination of symptoms in juvenile neuronal ceroid lipofuscinosis — dementia and loss of vision and speech intelligibility — also places limits on assessment. Many intelligence tests evaluate both verbal and visual reasoning skills, and even some ‘verbal’ tasks such as picture naming require visual identification. In children with juvenile neuronal ceroid lipofuscinosis, vision loss excludes tests of nonverbal/visual reasoning, but loss of speech intelligibility eventually also limits the ability of the child's ability to provide verbal responses to language-based tasks. In addition, mood and behavioral impairments can limit compliance with the evaluation process. The wide geographic dispersal of individuals affected by this rare disease, and the eventual physical limitations of affected children, preclude consistent and in-person re-evaluation at fixed intervals.
We have attempted to strike a balance among these challenges by using standardized, psychometrically-sound measures, but with pre-specified modifications that accommodate the unique phenotype and neurodegenerative course in juvenile Batten disease, and the evaluation of both age-adjusted scores and individual, unadjusted raw scores; declines over time in the latter signify a loss of previously attained skills.33 The use of standard measures enables us to quantify the magnitude of impairment in relation to age-expected performance. Also, in order to include children who cannot be directly evaluated due to travel constraints, or who cannot directly provide information about themselves in later stages of disease, we have designed a protocol that relies heavily upon proxy report information (from parents), obtained via phone interview and mailed questionnaires. The use of remote, live video assessment (telemedicine) has already been validated for the Unified Batten Disease Rating Scale34 and is currently being used to minimize travel burden for juvenile Batten disease families participating in a clinical trial.35 Future studies may also address the utility of telemedicine approaches for the neurobehavioral assessment; this is an area of growing interest for evaluation of cognitively impaired populations where in-person evaluations may be difficult to complete.36-39
The past 2 decades of research on juvenile neuronal ceroid lipofuscinosis have shifted from the bench to bedside, including focus by a number of investigators on the neurobehavioral phenotype and natural history of the disease. Additionally, there has been growing interest in the exploration of potential therapeutic candidates for treatment or prevention of disease. An ongoing Phase II clinical trial is evaluating the safety and tolerability of mycophenolate mofetil35 (ClinicalTrials.gov Identifier: NCT01399047); the efficacy of this therapy is not yet known. While families wait for disease-modifying treatment, we must also consider interventions that could mitigate the everyday impact of dementia-related symptoms. Pharmacologic treatments have been evaluated; 14 Finnish children with juvenile Batten disease were placed on specific antidepressant or antipsychotic medications; approximately 70% were deemed to have a “good or satisfactory” response40. However, conclusions are limited in this study which was not randomized — all children received medication — and clinical outcome was determined qualitatively by parents and treating clinicians in an unblinded fashion.41 Behavioral interventions for juvenile neuronal ceroid lipofuscinosis dementia management have not yet been formally evaluated, but are considered effective and are recommended as a first-line approach for dementia management in older adults.42 Our current understanding of the cognitive and behavioral features of juvenile neuronal ceroid lipofuscinosis can inform the development of tailored psychosocial interventions for pediatric dementia, and also can guide evaluation of response to pharmacologic symptom management. Declines in memory, attention, and general reasoning abilities may limit children's abilities to benefit from the typical corrective learning experiences inherent to many behavior management programs.43 Also, although both internalizing and externalizing symptoms are clinically elevated in juvenile neuronal ceroid lipofuscinosis, only the former appear to be significantly related to parents' self-rated quality of life, suggesting a focus on this relationship for further study and potential intervention. Lastly, our understanding of the neurobehavioral natural history of juvenile Batten disease may be incorporated into the study of disease-modifying therapies, into determining eligibility, as part of safety assessments, as a marker of brain function, and/or into the evaluation of long-term treatment effects.33,44.
Neurobehavioral symptoms have long been described as a prominent feature of juvenile Batten disease, and were recognized as having negative impact even in Frederick Batten's early account of a young girl who was institutionalized due to intellectual decline, rather than physical symptoms of disease. From these earliest reports, characterization of the neurobehavioral features of juvenile neuronal ceroid lipofuscinosis has been relevant to our understanding of this disease, and will continue to be so.
Acknowledgments
We gratefully acknowledge Dr. Erika F Augustine, MD, for her review and thoughtful comments on the manuscript. Melanie Fridl Ross, MSJ, ELS provided editing assistance. We especially thank the many affected individuals and their families for their participation in our research.
Funding: The authors and/or their research staff currently receive financial support for the research described herein from:
National Institute of Neurological Diseases and Stroke: 5K23 NS058756-01 “Neurobehavioral Outcomes of Degenerative Neurologic Diseases” (Adams)
National Institute of Neurological Diseases and Stroke: 5R01NS060022-04 “Clinical and Neuropsychological Investigations in Batten Disease” (Mink)
National Institute of Neurological Diseases and Stroke/University of Minnesota: 5U54NS065768-02 “Longitudinal Studies in Batten Disease” (Mink)
Batten Disease Support and Research Association: “Cellcept for Treatment of Juvenile Neuronal Ceroid Lipofuscinosis (JUMP)”. ClinicalTrials.gov Identifier: NCT01399047.
FDA Office of Orphan Products Development: “Cellcept for Treatment of Juvenile Neuronal Ceroid Lipofuscinosis (JUMP)” ClinicalTrials.gov Identifier: NCT01399047.
Past research support:
Batten Disease Support & Research Association
Luke & Rachel Batten Foundation
Footnotes
Presented at the Neurobiology of Disease in Children Symposium: Batten Disease, in conjunction with the 41st Annual Meeting of the Child Neurology Society, Huntington Beach, California, October 31, 2012.
The URBC study group consists of: Neurologists: Erika F Augustine, MD, Joanna Blackburn, MD, Leon Dure, MD, Jennifer Kwon, MD, MPH, Frederick Marshall, MD, Jonathan Mink, MD, PhD, Denia Ramirez, MD, MPH, PhD; Neuropsychologists: Heather Adams, PhD, Julie Eisengart, PhD; Molecular Genetics: Paul Rothberg, PhD; Biochemist: David Pearce, PhD; Biostatisticians: Michael McDermott, PhD; Christopher Beck, PhD; Clinical Coordinator: Amy Vierhile, MS; Research Coordinators: Elisabeth de Blieck, MPA, Nicole Newhouse, BA, RN, Alyssa Thatcher, BA; Medical, Graduate, and Undergraduate Students: Ankita Agarwal, BA, Jennifer Cialone, MD, Rachel Jordan, MD, Erika Levy-Wexler, MD, Tiffani McDonough, MD, Samantha Potter, Jennifer Riehl, MD, Shayne Ragbeer, BA, MA, Katherine Rose, BA, Sabrina Seehafer, PhD, Erin Stachowski, BA, Melissa Wang, MD, Kim Worcester, BA.
Author Contributions: H Adams prepared the manuscript. All members of the URBC study group have contributed to the research activities described herein.
Declaration of Conflicting Interests: H Adams and JW Mink serve on the Medical Advisory Board for the Batten Disease Support & Research Association.
Ethical Approval: All research activities have been conducted under study protocols approved by the University of Rochester Research Subjects Review Board, and all parents have completed an informed consent process and provided written permission for their child's participation in the study.
References
- 1.Phillips SN, Benedict JW, Weimer JM, Pearce DA. CLN3, the protein associated with batten disease: structure, function and localization. J Neurosci Res. 2005 Mar 1;79(5):573–583. doi: 10.1002/jnr.20367. [DOI] [PubMed] [Google Scholar]
- 2.Williams R. NCL incidence and prevalence data. In: Mole S, RE W, Goebel H, editors. The neuronal ceroid lipofuscinoses: Batten Disease. 2. Oxford: Oxford University Press; 2011. [Google Scholar]
- 3.Cialone J, Adams H, Augustine E, et al. Females experience a more severe disease course in batten disease. J Inherit Metabol Dis. 2011;35(3):549–555. doi: 10.1007/s10545-011-9421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stengel O. Report about a strange illness in four siblings in the vicinity of Røros. Hager P, Andersen T, translators. Eyr. 1826;1(1):347–352. (by Stengel, physician at the Røros copper mine) [in Norwegian] [Google Scholar]
- 5.Batten F. Paper presented at: Transactions of the Opthalmological Society of the United Kingdom. XXIII. London: Cerebral degeneration with symmetrical changes in the maculae in two members of a family; pp. 1902–1903. [Google Scholar]
- 6.Bäckman ML, Santavuori PR, Åberg LE, Aronen ET. Psychiatric symptoms of children and adolescents with juvenile neuronal ceroid lipofuscinosis. J Intellect Disabil Res. 2005;49(1):25–32. doi: 10.1111/j.1365-2788.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Boustany RM, Filipek P. Seizures, depression and dementia in teenagers with Batten disease. J Inherit Metab Dis. 1993;16(2):252–255. doi: 10.1007/BF00710257. [DOI] [PubMed] [Google Scholar]
- 8.Lauronen L, Munroe PB, Järvelä I, et al. Delayed classic and protracted phenotypes of compound heterozygous juvenile neuronal ceroid lipofuscinosis. Neurology. 1999 Jan 15;52(2):360–365. doi: 10.1212/wnl.52.2.360. [DOI] [PubMed] [Google Scholar]
- 9.Santavuori P, Linnankivi T, Jaeken J, Vanhanen S-L, Telakivi T, Heiskala H. Psychological symptoms and sleep disturbances in neuronal ceroid-lipofuscinoses (NCL) J Inherit Metab Dis. 1993;16:245–248. doi: 10.1007/BF00710255. [DOI] [PubMed] [Google Scholar]
- 10.Crow YJ, Tolmie JL, Howatson AG, Patrick WJA, Stephenson JBP. Batten Disease in the west of Scotland 1974-1995 including five cases of the juvenile form with granular osmiophilic deposits. Neuropediatrics. 1997;28:140–144. doi: 10.1055/s-2007-973690. [DOI] [PubMed] [Google Scholar]
- 11.Lanska DJ, Lanska MJ. Kluver-Bucy syndrome in juvenile neuronal ceroid lipofuscinosis. J Child Neurol. 1994 Jan;9(1):67–69. doi: 10.1177/088307389400900117. [DOI] [PubMed] [Google Scholar]
- 12.Lamminranta S, Åberg LE, Autti T, et al. Neuropsychological test battery in the follow-up of patients with juvenile neuronal ceroid lipofuscinosis. J Intellect Disabil Res. 2001;45(1):8–17. doi: 10.1046/j.1365-2788.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall F, de Blieck E, Mink J, et al. Unified Batten disease rating scale: description and reliability. Ann Neurol. 2004;56(Suppl 8):S102. [Google Scholar]
- 14.Marshall FJ, de Blieck EA, Mink JW, et al. A clinical rating scale for Batten disease: reliable and relevant for clinical trials. Neurology. 2005 Jul 26;65(2):275–279. doi: 10.1212/01.wnl.0000169019.41332.8a. [DOI] [PubMed] [Google Scholar]
- 15.Rothberg PG, Ramirez-Montealegre D, Frazier SD, Pearce DA. Homogeneous Polymerase Chain Reaction Nucleobase Quenching Assay to Detect the 1-kbp Deletion in CLN3 That Causes Batten Disease. J Mol Diagn. 2004 Aug 1;6(3):260–263. doi: 10.1016/S1525-1578(10)60519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams R, Mole S. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology. 2012;79(2):183–191. doi: 10.1212/WNL.0b013e31825f0547. [DOI] [PubMed] [Google Scholar]
- 17.StatSoft Inc. STATISTICA (data analysis software system) [computer program] Version 10.2011 [Google Scholar]
- 18.Adams H, de Blieck E, Dure L, et al. Obsessive/Compulsive Symptoms in Juvenile Neuronal Ceroid Lipofuscinosis (Batten Disease) Ann Neurol. 2004;56(suppl 8):S127. [Google Scholar]
- 19.Adams H, de Blieck E, Dure L, Marshall F, Mink J, Pearce DA. Neuropsychological and behavioral symptoms in Juvenile Neuronal Ceroid Lipofuscinosis (NCL-3; Batten's Disease) Neurology. 2004;62(Suppl 5):A68–69. [Google Scholar]
- 20.Adams H, de Blieck EA, Mink JW, et al. Standardized assessment of behavior and adaptive living skills in juvenile neuronal ceroid lipofuscinosis. Dev Med Child Neurol. 2006;48(4):259–264. doi: 10.1017/S0012162206000570. [DOI] [PubMed] [Google Scholar]
- 21.Adams H, Beck C, Levy E, et al. Genotype does not predict severity of behavioral phenotype in Juvenile Neuronal Ceroid Lipofuscinosis (Batten Disease) Developmental Medicine & Child Neurology. 2010;52(7):637–643. doi: 10.1111/j.1469-8749.2010.03628.x. PMID:20187884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams H, Kwon J, Marshall FJ, de Blieck EA, Pearce DA, Mink JW. Neuropsychological symptoms of Juvenile-onset Batten Disease: Experiences from Two Studies. Journal of Child Neurology. 2007 doi: 10.1177/0883073807302603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psychological Corporation. Wechsler Intelligence Scale for Children - Fourth Edition Manual. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- 24.Labbe EE. Emotional states and perceived family functioning of caregivers of chronically ill children. Psychol Rep. 1996;79:1233–1234. doi: 10.2466/pr0.1996.79.3f.1233. [DOI] [PubMed] [Google Scholar]
- 25.Labbe EE, Lopez I, Murphy L, O'Brien C. Optimism and psychosocial functioning in caring for children with Battens and other neurological diseases. Psychol Rep. 2002 Jun;90(3 Pt 2):1129–1135. doi: 10.2466/pr0.2002.90.3c.1129. [DOI] [PubMed] [Google Scholar]
- 26.Varni J, Seid M, Rode C. The PedsQL™: Measurement model for the Pediatric Quality of Life Inventory™. Medical Care. 1999;37(2):126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL™ Family Impact Module: Preliminary reliability and validity. Health and Quality of Life Outcomes. 2004;2(55):1–6. doi: 10.1186/1477-7525-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams H, deBlieck E, Kwon J, et al. Paper presented at: 11th International Congress on Neuronal Ceroid Lipofuscinosis (Batten Disease) Rochester, NY: Jul, 2007. Quality of life in Batten Disease: Affected children, parents, and siblings. [Google Scholar]
- 29.Scambler S. Care in Partnership: A study into the social care needs of young people with Juvenile Batten's Disease and their families. [Accessed May 7, 2013]; See Ability: Seeing beyond disability. Published online: http://web.archive.org/web/20040218194003/http://www.seeability.org/randd/caresec3.htm;
- 30.Bruininks RH, Woodcock RW, Weatherman RE, Hill BK. Scales of Independent Behavior - Revised: Comprehensive Manual. Itasca: Riverside; 1996. [Google Scholar]
- 31.Achenbach TM. Child Behavior Checklist/6-18. T.M. Achenbach; 2002. [Google Scholar]
- 32.Baron IS. Neuropsychological evaluation of the child. Oxford: Oxford University Press; 2004. [Google Scholar]
- 33.Shapiro E, Balthazor M. Metabolic and neurodegenerative disorders. In: Yeates K, Ris M, Taylor H, editors. Pediatric neuropsychology: Research, theory, and practice. New York: NY: The Guilford Press; 2000. pp. 171–205. [Google Scholar]
- 34.Cialone J, Augustine EF, Newhouse N, Vierhile A, Marshall FJ, JW M. Quantitative telemedicine ratings in Batten disease: implications for rare disease research. Neurology. 2011;77(20):1808–1811. doi: 10.1212/WNL.0b013e3182377e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batten Disease Support and Research Association, FDA Office of Orphan Products Development, University of Rochester. Cellcept for Treatment of Juvenile Neuronal Ceroid Lipofuscinosis (JUMP) [Accessed May 6, 2013];ClinicalTrials.gov Identifier: NCT01399047. ClinicalTrials.gov.
- 36.Barton C, Morris R, Rothlind J, Yaffe K. Video-telemedicine in a memory disorders clinic: evaluation and management of rural elders with cognitive impairment. Telemedicine Journal & E-Health. 2011;17(10):789–793. doi: 10.1089/tmj.2011.0083. [DOI] [PubMed] [Google Scholar]
- 37.Breitling LP, Wolf M, Muller H, Raum E, Kliegel M, Brenner H. Large-scale application of a telephone-based test of cognitive functioning in older adults. Dementia & Geriatric Cognitive Disorders. 2010;30(4):309–316. doi: 10.1159/000319896. [DOI] [PubMed] [Google Scholar]
- 38.Mundt JC, Geralts DS, Moore HK. Dial “T” for testing: Technological flexibility in neuropsychological assessment. Telemedicine Journal & E-Health. 2006;12(3):317–323. doi: 10.1089/tmj.2006.12.317. [DOI] [PubMed] [Google Scholar]
- 39.Stain HJ, Payne K, Thienel R, Michie P, Carr V, Kelly B. The feasibility of videoconferencing for neuropsychological assessments of rural youth experiencing early psychosis. Journal of Telemedicine & Telecare. 2011;17(6):328–331. doi: 10.1258/jtt.2011.101015. [DOI] [PubMed] [Google Scholar]
- 40.Bäckman ML, Åberg LE, Aronen ET, Santavuori PR. New antidepressive and antipsychotic drugs in juvenile neuronal ceroid lipofuscinoses -- a pilot study. Eur J Paediatr Neurol. 2001;5(Suppl A):163–166. doi: 10.1053/ejpn.2000.0455. [DOI] [PubMed] [Google Scholar]
- 41.Backman ML, Aberg LE, Aronen ET, Santavuori PR. New antidepressive and antipsychotic drugs in juvenile neuronal ceroid lipofuscinoses -- a pilot study. Eur J Paediatr Neurol. 2001;5(Suppl A):163–166. doi: 10.1053/ejpn.2000.0455. [DOI] [PubMed] [Google Scholar]
- 42.Logson R, McCurry S S, T L. Evidence-based psychological treatments for disruptive behavior in individuals with dementia. Psychology and Aging. 2007;22(1):28–36. doi: 10.1037/0882-7974.22.1.28. [DOI] [PubMed] [Google Scholar]
- 43.Kazdin A. Parent management training: Evidence, outcomes, issues. J Am Acad Child Adolesc Psychiatr. 1997;36(10):1349–1356. doi: 10.1097/00004583-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004 Aug 1;104(3):881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 45.Sheslow D, Adams W. WRAML2. Wide Range Assessment of Memory and Learning. Second Edition. Wilmington, DE: Wide Range, Inc; 2003. [Google Scholar]
- 46.Lezak M. Neuropsychological assessment. Third. New York: Oxford University Press; 1995. [Google Scholar]
- 47.Reynolds CR, Bigler ED. Test of Memory and Learning. Examiner's Manual. Austin: Pro-Ed; 1994. [Google Scholar]
- 48.Psychological Corporation. WAIS-III, WMS-III Technical Manual. San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- 49.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals - Third Edition. Technical Manual. San Antonio, TX: The Psychological Corporation; 1995. [Google Scholar]