Abstract
Regulatory Factor X (RFX) transcription factors are important for development and are likely involved in the pathogenesis of serious human diseases including ciliopathies. While seven RFX genes have been identified in vertebrates and several RFX transcription factors have been reported to be regulators of ciliogenesis, the role of RFX7 in development including ciliogenesis is not known. Here we show that RFX7 in Xenopus laevis is expressed in the neural tube, eye, otic vesicles, and somites. Knockdown of RFX7 in Xenopus embryos resulted in a defect of ciliogenesis in the neural tube and failure of neural tube closure. RFX7 controlled the formation of cilia by regulating the expression of RFX4 gene, which has been reported to be required for ciliogenesis in the neural tube. Moreover, ectopic expression of Foxj1, which is a master regulator of motile cilia formation, suppressed the expression of RFX4 but not RFX7. Taken together, RFX7 plays an important role in the process of neural tube closure at the top of the molecular cascade which controls ciliogenesis in the neural tube.
Keywords: RFX, cilia, neural tube closure, Xenopus laevis
1. Introduction
Cilia are cellular organelles that are present on the surface of most vertebrate cells and play crucial roles in physiological and developmental processes (Eggenschwiler and Anderson, 2007; Ishikawa and Marshall, 2011; Pedersen et al., 2008). There are two major types of cilia. One is motile cilia that generate extracellular fluid flows such as mucus flow, cerebrospinal-fluid flow, and leftward flow in the node (Roy, 2009). The second type of cilia, called primary cilia, sense extracellular signals such as growth factors and hormones (Berbari et al., 2009; Gerdes et al., 2009; Goetz and Anderson, 2010; Singla and Reiter, 2006). Substantial studies have revealed that disorders in the formation or the function of cilia result in a wide range of human diseases such as primary ciliary dyskinesia, polycystic kidney disease, Joubert syndrome, Bardet–Biedl syndrome as well as Meckel-Gruber syndrome (Baker and Beales, 2009; Hildebrandt et al., 2011; Mougou-Zerelli et al., 2009; Sattar and Gleeson, 2011; Zariwala et al., 2007). Particularly, Joubert syndrome and Meckel-Gruber syndrome have been reported to be associated with neural tube defects (NTDs) (Delous et al., 2007; Lee et al., 2012). Mice studies have also shown that mutations in genes required for ciliogenesis, such as C2cd3, Fuz, Ift122, Intu as well as Inpp5e, result in NTDs (Harris and Juriloff, 2010). NTDs are a set of major congenital malformations that are caused by disrupting closure of the neural tube, where neural progenitors are known to have primary cilia (Bay and Caspary, 2012). NTDs divide into two forms, open and closed forms (Katsanis, 2006; Murdoch and Copp, 2010; Robinson et al., 2012). Open forms are seen in the cranial region with anencephaly and in the spinal region with spinal bifida. The entire craniospinal axis can be involved in cases of craniorachischisis. Closed forms can present with more subtle phenotypic changes that are skin covered, including encephalocele, meningocele, and spina bifida occulta. While genetic evidence indicates that the function of cilia in the neural tube is crucial for neural tube closure, the role of cilia during neural tube closure is not completely understood.
Cilia are dynamic structures that can form and resorb throughout development. For example, multiple cilia of the cerebral ventricles grow at precise developmental stages (Spassky et al., 2005). Epithelial cells in the cerebral ventricles first harbor primary cilia and switch to multiple ciliary growth under a specific developmental program. Another example is that mouse tracheal epithelial cells in primary cultures differentiate into multi-ciliated cells by exposure to an air-liquid interface (You et al., 2002), indicating that airway epithelial cells can be differentiated by various tracheal conditions during lung development. This differentiation is induced by the function of Multicilin, the Xenopus ortholog of human IDAS, which coordinately promotes cell-cycle exit, deuterosome-mediated centriole assembly and the gene expression required for motile ciliogenesis (Stubbs et al., 2012). Therefore, organisms must have developed regulatory mechanisms to induce the assembly of specific cilia subtypes in a temporal and spatial manner. Transcriptional regulation of ciliary gene expression is likely one of these mechanisms. In fact, genes encoding ciliary proteins in Patella vulgata are dynamically expressed during development of ciliated tissues and inhibition of transcription impaired cilia assembly (Damen et al., 1994). Recently, several transcription factors, which control the transcription of ciliary genes and are involved in ciliogenesis in vertebrates, have been identified; the RFX (Ashique et al., 2009; Bonnafe et al., 2004; Chung et al., 2012; Laurencon et al., 2007; Liu et al., 2007; Swoboda et al., 2000) and Foxj1 (forkhead box j1) (Brody et al., 2000; Jacquet et al., 2009; Stubbs et al., 2008; Yu et al., 2008) transcription factors. While Foxj1 is a master regulator of motile ciliogenic program in vertebrate (Stubbs et al., 2008; Yu et al., 2008), RFX transcription factors are involved in both primary and motile cilia formation. In vertebrates, seven RFX genes have been identified based on a highly conserved DNA binding domain that belongs to the winged-helix family of transcription factors (Aftab et al., 2008; Emery et al., 1996; Gajiwala et al., 2000). While only one RFX transcription factor, known as Daf19 in Caenorhabditis elegans, has been identified and reported to be a central regulator of ciliogenesis in C. elegans as well as Drosophila (Dubruille et al., 2002; Swoboda et al., 2000), it has been reported that not all RFX transcription factors are crucial for ciliogenesis in vertebrates. RFX1, RFX5 and RFX6 do not appear to be essential for ciliogenesis (Reith and Mach, 2001; Smith et al., 2010; Soyer et al., 2010; Steimle et al., 1995; Zhao et al., 2010), whereas RFX2 and RFX3 are more broadly required for the proper development of cilia (Bonnafe et al., 2004; Chung et al., 2012). Importantly, RFX4 has been reported to be a key regulator required for the development of cilia only in the neural tube that are critical in modulating Sonic Hedgehog (Shh) signaling (Ashique et al., 2009). Although RFX7 is ubiquitously and highly expressed in nearly all human tissues examined, with the highest expression in the brain (Aftab et al., 2008), very little is known about the role of RFX7 in ciliogenesis.
Here we report that RFX7 is highly expressed in the central nervous system of X. laevis embryos, including the neural tube and eyes. Knockdown of RFX7 resulted in defects of cilia formation and neural tube closure. In addition, X. laevis RFX4 was also essential for ciliogenesis and neural tube closure, as previously shown in a mouse model (Ashique et al., 2009). Importantly, RFX7 controls the expression of RFX4 in the neural plate, and the failure of neural tube closure induced by knockdown of RFX7 was rescued by co-injection with RFX4 RNA. In addition, ectopic expression of Foxj1 suppressed the expression of RFX4 but not RFX7 in the neural plate. These data indicate that the role of RFX7 is upstream of RFX4 in the molecular cascade of cilia formation at the neural tube and is not affected by Foxj1, demonstrating that RFX7 plays a key role in neural tube ciliogenesis.
2. Results
2.1 RFX7 is essential for ciliogenesis in the neural tube
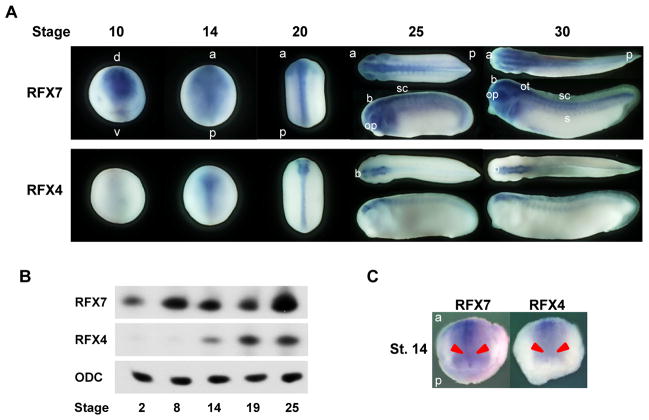
To test the possibility of a role for RFX7 in the formation of cilia, we first examined the spatial and temporal expression profile of the RFX7 gene in X. laevis embryos. The RFX7 gene was maternally expressed and continued to be expressed throughout embryogenesis (Fig. 1A, B). We observed that the RFX7 gene was expressed on the dorsal side of the ectoderm at stage 10 (gastrula) and in the neural plate at stage 14 (neurula) (Fig. 1A). In addition, RFX7 is barely detected in the gastrocoel roof plate (GRP), where motile cilia exist during the neurula stage (Schweickert et al., 2007), at stage 14 (Fig. 1C). Then, the expression of RFX7 was detected in the brain, spinal cord, eyes, otic vesicles, as well as the somites at the tailbud stage (Fig. 1B). RFX7 is highly expressed in the nervous system including the neural tube, suggesting RFX7 may be important for neural tube formation in Xenopus embryos.
Fig. 1.
RFX7 and RFX4 are expressed in the nervous system. (A) Spatial expression profiles of RFX7 and RFX4 genes. (B) Temporal expression profiles of RFX7 and RFX4 genes. (C) RFX7 and RFX4 are not expressed in the GRP at the stage 14. The GRP is indicated by red arrows. d: dorsal, v: ventral, a: anterior, p: posterior, b: brain, op: optic vesicles, ot: otic vesicles, sc: spinal cord, s: somites.
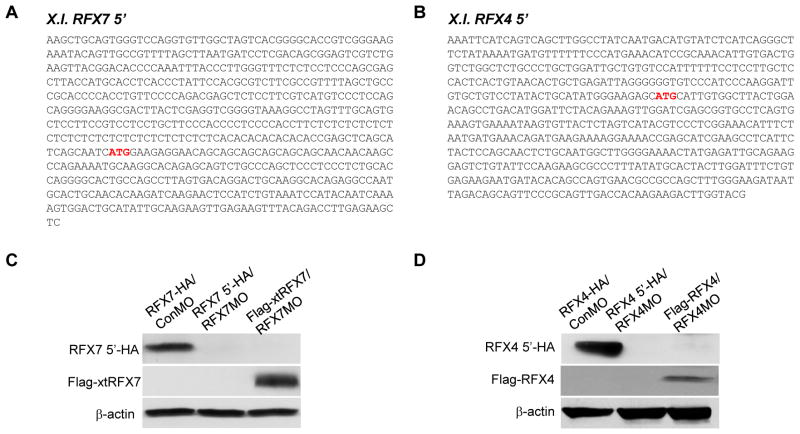
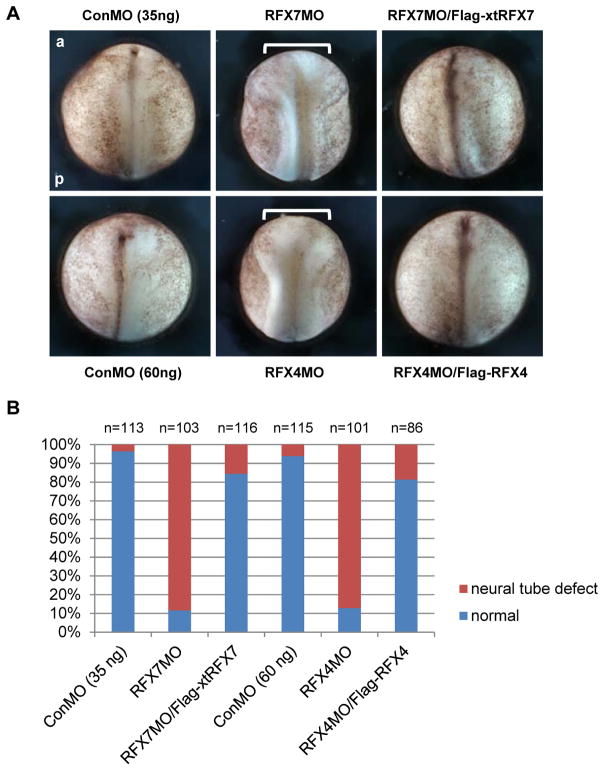
Since some vertebrate RFX transcription factors are necessary for ciliogenesis (Ashique et al., 2009; Bonnafe et al., 2004; Chung et al., 2012), we hypothesized that RFX7 is required for ciliogenesis in the neural tube. Various genes related to ciliogenesis are required for neural tube closure and some ciliopathies such as Joubert and Meckel-Gruber syndromes are associated with NTD (Murdoch and Copp, 2010; Vogel et al., 2012). Interestingly, several Bardet-Biedl syndrome (BBS) proteins that are localized to primary cilia and basal bodies (Ansley et al., 2003) cooperate with non-canonical Wnt signaling in planar cell polarity (PCP) (Ross 2005) that is essential for convergent extension movements of cells (Elul et al., 1997; Keller et al., 1992; Wallingford et al., 2001). In fact, the BBS proteins are required for proper convergent extension movements (Gerdes et al., 2007). Since the failure of convergent extension movements of midline cells in the neural plate resulted in NTDs (Wallingford and Harland, 2002), primary cilia in the neural tube may be necessary for convergent extension movements to close the neural tube. To determine the role of RFX7 in neural tube ciliogenesis, we tested the effect of RFX7 knockdown in neural tube closure. Since the 5′ sequence in the X. laevis RFX7 gene, including the initiation codon, had not been identified, we performed 5′RACE to isolate a X. laevis RFX7 5′ clone including the 5′ UTR and the initiation codon (Fig. 2A). We designed antisense morpholino oligonucleotides (MO) against the X. laevis RFX7 gene to block RFX7 translation, and confirmed the effect of RFX7MO by immunoblotting (Fig. 2B). RFX7MO was injected into two dorsal blastomeres of 4-cell stage embryos to deliver RFX7MO to dorsal tissue. At stage 18 (late neurula), the neural folds in RFX7 morphants failed to be closed, while the neural folds in control embryos began to fuse (Fig. 3A, B). To confirm the specificity of this effect, rescue experiments of co-injecting X. tropicalis RFX7 RNA (Flag-xtRFX7), which is not recognized by RFX7MO (Fig. 2B), were performed. Co-injection with Flag-xtRFX7 RNA rescued neural tube closure defect induced by RFX7MO injection (Fig. 3A, B). This showed that RFX7 is required for the process of neural tube closure.
Fig. 2.
Isolation of X. laevis RFX7 5′ and X. laevis RFX4 5′ clones. (A) Sequences of X. laevis RFX7 5′ (GenBank accession number: KF543241) and X. laevis RFX4 5′ (GenBank accession number: KF543240). The predicted initiation codon is indicated by red characters. (B) RFX7MO and RFX4MO blocked protein translation of RFX7 5′ and RFX4 5′, respectively. MOs and RNAs were co-injected into 2 cell stage embryos and embryos were collected at stage 10. Protein extracts from these embryos were used for immunoblotting.
Fig. 3.
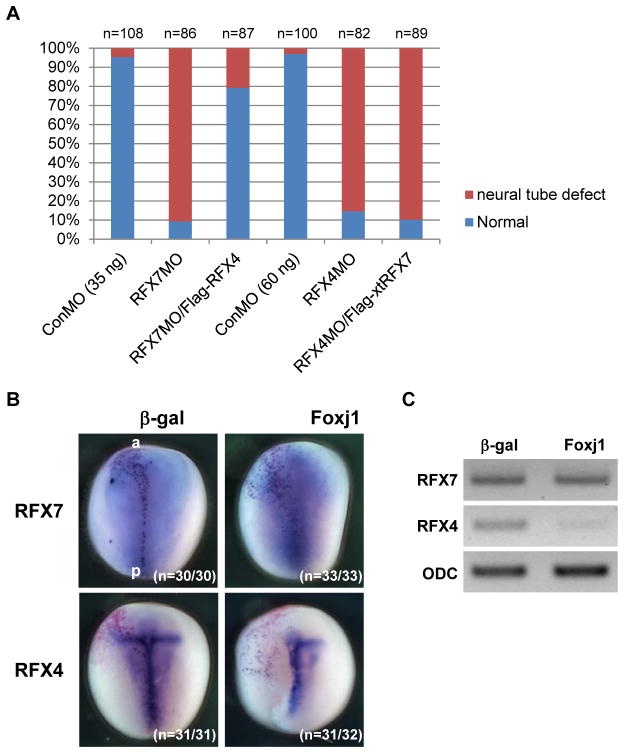
RFX7 and RFX4 are required for neural tube closure. (A) Dorsal view of bilaterally injected embryos at stage 18. Thirty-five nanograms of RFX7MO and 60 ng of RFX4MO were injected into two dorsal blastomeres of 4-cell stage embryos. One nanogram of Flag-xtRFX7 and Flag-RFX4 RNAs were used for rescue experiments. Brackets show the distance between the neural folds in RFX7 or RFX4 morphants. a: anterior, p: posterior. (B) The quantitative assessment of the injections in (A). At least three independent experiments were performed. “n” indicates the number of injected embryos.
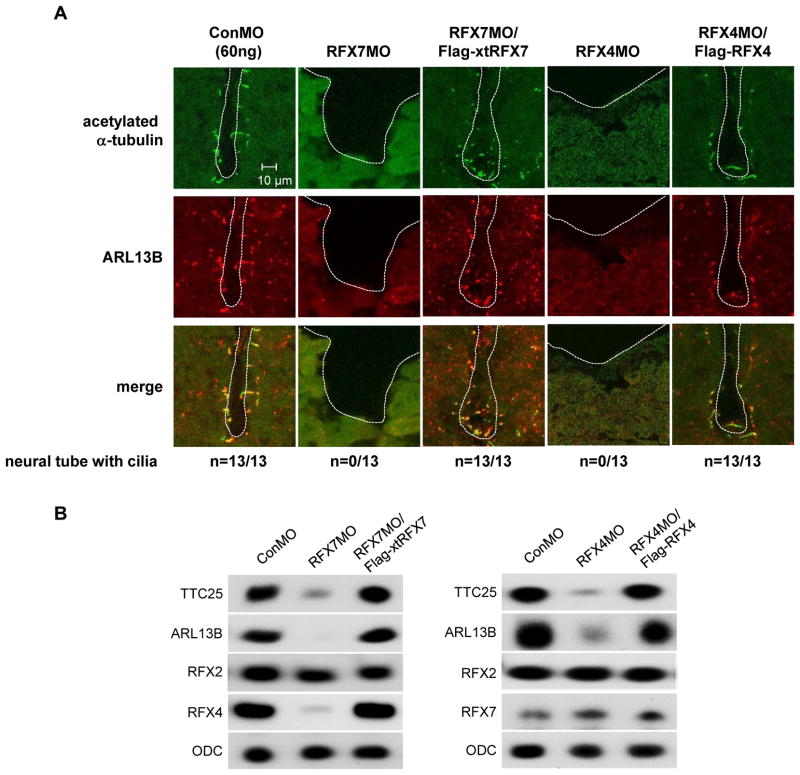
To determine if NTD in RFX7 morphants is correlated with defects in ciliogenesis of the neural tube, we visualized cilia axonemes in the neural tube by immunostaining with anti-acetylated-α-tubulin antibody (Chung et al., 2012; Stubbs et al., 2008; Suzuki et al., 2010). Immunostaining with an antibody against ADP-ribosylation factor like protein 13B (ARL13B), a protein that localizes not only to cilia but also to tubular-vesicular structures (Barral et al., 2012; Caspary et al., 2007), was also performed. At stage 23 (early tailbud), acetylated-α-tubulin and ARL13B positive cilia were detected in the neural tube of control embryos but not RFX7 morphants, and this effect of RFX7MO was rescued by co-injection with Flag-xtRFX7 RNA (Fig. 4A). We also tested the expression of TTC25, which is localized in cilia axonemes and is required for neural tube closure (Hayes et al., 2007). The expression of TTC25 was reduced in RFX7 morphants and its expression was re-stored by co-injection of Flag-xtRFX7 RNA (Fig. 4B). These demonstrated that RFX7 controls ciliogenesis in the neural tube.
Fig. 4.
RFX7 and RFX4 are necessary for ciliogenesis in the neural tube. (A) Transverse section view of the neural tube at stage 23. Cilia are visualized by staining with acetylated α-tubulin (green) and ARL13B (red) antibodies. ARL13B is also expressed in cells without cilia at the neural tube. In RFX7 and RFX4 morphants, cilia were not detected. All sections from whole embryos were examined to detect cilia. White dashed lines outline the lumen of the neural tube. The number of embryos examined is indicated. (B) MOs and RNA injected embryos were collected at stage 23 and RNAs isolated from injected embryos were used to examine the gene expression. ODC was used as an internal control.
Taken together, these results indicate that RFX7 is involved in the process of neural tube closure by regulating neural tube ciliogenesis.
2.2 RFX7 is upstream of RFX4 during neural tube closure
Studies done using mouse models have shown that RFX4 is required for ciliogenesis only in the neural tube (Ashique et al., 2009). In Xenopus embryos, RFX4 is mainly expressed in the nervous system (Fig. 1A, B) (Chung et al., 2012). Interestingly, RFX4 was not detected in the GRP at stage 14 (Fig. 1C). These data suggest that the role of mouse RFX4 in neural tube ciliogenesis is conserved in Xenopus and the function of RFX4 is associated with RFX7. We first examined the role of RFX4 in neural tube ciliogenesis of Xenopus embryos by knockdown of RFX4. A X. laevis RFX4 5′ clone including the 5′ UTR and the initiation codon was isolated by 5′ RACE and RFX4MO was designed based on this sequence (Fig. 2A). After the effect of RFX4MO was confirmed by immunoblotting (Fig. 2B), RFX4MO was injected into two dorsal blastomeres of 4-cell stage embryos. At stage 18, the neural folds in RFX4 morphants failed to close and this defect was rescued by co-injection of X. laevis RFX4 RNA (Flag-RFX4), whose initiation site was replaced by the Flag-tag (Fig. 3A, B). Ciliogenesis in the neural tube of RFX4 morphants was also impaired and this defect was rescued by co-injection with Flag-RFX4 RNA (Fig. 4A, B), confirming that RFX4 is also essential for neural tube ciliogenesis in Xenopus embryos. To determine the correlation between RFX7 and RFX4 during neural tube closure, we tested whether RFX7 morphants could be rescued by co-injection with Flag-RFX4 RNA or vice versa. RFX7MO with Flag-RFX4 RNA or RFX4MO with Flag-xtRFX7 RNA were injected into the dorsal side of embryos at the 4-cell stage. Interestingly, RFX4 rescued the defect in neural tube closure of RFX7 morphants but RFX7 could not rescue the defect of neural tube closure in RFX4 morphants (Fig. 5A). Furthermore, the expression of RFX4 gene was reduced in RFX7 morphants and this reduction was restored by co-injection with Flag-xtRFX7 RNA (Fig. 4B). Interestingly, the expression of RFX2, which is required for both motile and primary ciliogenesis of Xenopus embryos (Chung et al., 2012), was not changed in both RFX7 and RFX4 morphants (Fig. 4B). These data demonstrate that RFX7 plays a crucial role in neural tube closure upstream of RFX4. Next, we asked whether Foxj1, a master regulator of motile ciliogenesis (Cruz et al., 2010; Stubbs et al., 2008; Yu et al., 2008), regulates the expression of RFX7 and RFX4. Surprisingly, the expression of RFX4 but not RFX7 was reduced by ectopic expression of Foxj1 (Fig. 5B, C). These results demonstrate that RFX4 expression is controlled positively by RFX7 or negatively by Foxj1 during neural tube closure.
Fig. 5.
The expression of RFX4 is regulated by RFX7 and Foxj1. (A) RFX4 rescued NTD in RFX7 morphants. The bar-graphs show the quantitative assessment of the injections. At least three independent experiments were performed. “n” indicates the number of injected embryos. (B) Five hundred picograms of Foxj1 RNA was injected into a dorsal blastomere of 4-cell stage embryos and the gene expression was examined at stage 14 by whole mount in situ hybridization. β-gal staining (red) indicates the injected side. The number of embryos examined is indicated. (C) Foxj1 RNA was injected into two dorsal blastomeres of 4-cell stage embryos and the gene expression was examined at stage 14 by RT-PCR.
3. Discussion
The analysis presented here reveals a new role of RFX7 and the molecular hierarchy of RFX transcription factors in ciliogenesis at the neural tube. RFX7 regulates the expression of RFX4 to form cilia in the neural tube as well as complete neural tube closure. Additionally, ectopic expression of Foxj1, which is a master regulator of motile ciliogenesis, suppressed the expression of RFX4 but not RFX7. This study indicates the molecular crosstalk among regulators for primary and motile ciliogenesis is an important factor to determine the fate of cilia subtypes.
While the molecular machinery underlying cilia structure and function has been well studied, the transcriptional control of genes required for cilia assembly still remains poorly understood (Gherman et al., 2006; Ishikawa and Marshall, 2011; Thomas et al., 2010). RFX transcription factors have been shown to play key roles in ciliogenesis in C. elegans, Drosophila, and vertebrates (Thomas et al., 2010). In mice, RFX3 is necessary for ciliogenesis in the node that controls left-right asymmetric patterning (Bonnafe et al., 2004), the growth of primary cilia in the endocrine pancreas (Ait-Lounis et al., 2007), and motile ciliogenesis in the brain (Baas et al., 2006; El Zein et al., 2009). In zebrafish, RFX3 has also been reported to be involved in the motile ciliogenic program (Liu et al., 2007). In Xenopus, RFX2 is essential for the formation of motile cilia in the GRP and the multi-ciliated cells in the skin as well as cilia in the neural tube (Chung et al., 2012). These studies demonstrate that RFX2 and RFX3 regulate the formation of both motile and primary cilia. Our work shows that RFX7 is involved in ciliogenesis at the neural tube by controlling the expression of RFX4, which have been reported to govern the growth of cilia at mice neural tube (Ashique et al., 2009). In the neural tube, there are two subtypes of cilia; cilia in the floor plate (FP) and primary cilia found elsewhere in the neural tube (Cruz et al., 2010). FP cilia are longer than non-FP primary cilia and exhibit a 9+0 arrangement with nine peripheral doublet microtubules in mice. Although the evidence of the motility of these cilia has not been shown yet, the molecular and ultrastructure data indicate that FP cilia are similar to motile cilia found in the node. According to our data, knockdown of either RFX7 or RFX4 results in defects of cilia at both FP and non-FP, indicating that RFX7 and RFX4 are required for the formation of both cilia subtypes in the neural tube (Fig. 4). While RFX7 is uniformly expressed in the neural plate, the expression of RFX4 is highly expressed in the midline of the neural plate, from which FP is originated (Fig. 1). In addition, RFX4 is not expressed in the GRP, a Xenopus analog of mice node (Schweickert et al., 2007) (Fig. 1C). These suggest that the high expression level of RFX4 may affect some specific feature of cilia on the FP cells, which is not clearly known. While RFX7 and RFX4 are required for the formation of both primary cilia on the non-FP cells and longer cilia on the FP cells that maybe or may not be motile, their function seems to be limited in the neural tube. This reason still remains unclear. Comparison of the characteristics of cilia between neural tube and other tissues as well as identification of ciliary genes regulated by only RFX7 and/or RFX4 may provide a clue to address this interesting question.
Foxj1 is required for motile ciliogenesis in vertebrates (Brody et al., 2000; Jacquet et al., 2009; Stubbs et al., 2008; Yu et al., 2008). In mice, Foxj1 is expressed at the FP cells of the neural tube and can alter the structure of cilia on the FP cells which are longer than primary cilia found elsewhere in the neural tube (Cruz et al., 2010). Interestingly, mice and chick studies showed that Foxj1 is sufficient but not necessary for the formation of long cilia on the FP cells (Cruz et al., 2010). Since Foxj1 is also expressed in the midline of the Xenopus neural plate at stage 15 when the neural plate is closing to form the neural tube (Stubbs et al., 2008), the role of Foxj1 in the Xenopus neural tube is expected to be conserved. Our study demonstrates that ectopic expression of Foxj1 suppressed the RFX4 expression in the midline of the neural plate (Fig. 5), indicating that Foxj1 can control the expression of RFX4. This regulatory mechanism and importance of the correlation between Foxj1 and RFX4 in neural tube ciliogenesis remain unclear. However, the spatial and temporal crosstalk among transcription factors expressed in the neural tube including Foxj1 and RFXs may induce the specific combination of ciliary gene expression and determine the fate of cilia subtypes in the limited region of the neural tube. Investigating this crosstalk will be an important issue in development of neural tube ciliogenesis.
4. Experimental procedures
4.1 Embryo Manipulations
Eggs were artificially fertilized by using testis homogenate and cultivated in 0.1× Marc’s Modified Ringer’s solution (MMR) (Peng, 1991). Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967).
4.2 DNA Constructs
X. laevis RFX4 5′ and X. laevis RFX7 5′were cloned using the GeneRacer Kit (Life Technologies). To generate RFX4 5′-HA and RFX7 5′-HA, X. laevis RFX4 5′ and X. laevis RFX7 5′were sub-cloned into the 5′ side of HA-tag sequence in pCS2+2HA vector, respectively. X. laevis RFX4 full length (fused with RFX4 5′ and EST clone [accession number BP692211]), X. tropicalis RFX7 full length (accession number NM_001097380) and X. laevis Foxj1a (accession number BC077846) were sub-cloned into pCS2+3Flag vector. Antisense morpholino oligonucleotides (MO) were obtained from Gene Tools. The MO sequences were as followed:
RFX7MO; 5′-GCTGTTCAGTAAGCCACAATGCAT-3′
RFX4MO; 5′-GCTGCTGTTCCTCTTCCATGATTGC-3′
ConMO; 5′-CCTCTTACCTCAGTTACAATTTATA-3′
4.3 Microinjection of Synthetic RNA and Morpholino Oligonucleotides
Capped synthetic RNAs were generated by in vitro transcription with SP6 polymerase, using the mMessage mMachine kit (Ambion, Inc.). For microinjections, embryos were injected with 5 – 10 nl of the specified amount of RNA in 3% Ficoll in 0.1 x MMR and cultured in 0.1 x MMR until the desired stage. nucβ-gal RNA for whole mount in situ hybridization was injected as a tracer.
4.4 Beta-Galactosidase Staining and Whole Mount In Situ Hybridization
Embryos were fixed with MEMFA (0.1 M MOPS, 2 mM EGTA [pH8.0], 1 mM MgSO4 and 3.7 % formaldehyde) containing 0.02% Triton-X for 30 minutes at room temperature. Galactosidase activity was visualized with the RedGal substrate (Research Organics) in staining buffer (5 mM K3[Fe(CN)6], 5 mM K4[Fe(CN)6], 2 mM MgCl2 in PBS). After staining, embryos were re-fixed with MEMFA for 30 minutes. Whole mount in situ hybridization was performed as described previously (Harland, 1991; Kiyota et al., 2008; Takada et al., 2005) by using Digoxigenin (Roche Applied Science)-labeled antisense RNA probes and BM purple (Roche Applied Science) for the chromogenic reaction.
4.4 Immunoblotting
Embryos were homogenized in RIPA lysis buffer (50 mM Tris-HCl: pH 7.4, 150 mM NaCl, 5 nM MgCl2, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, Protease Inhibitor Cocktail), and embryonic protein extracts were used for immunoblotting with α-HA (Santa Cruz Biotechnology, Inc) and α-Flag (Sigma) antibodies.
4.5 Immunohistochemistry
Published procedures were used for staining (Suzuki et al., 2007) and thick sectioning (Becker and Gard, 2006) with minor modifications. Embryos were fixed in MEMFA for 2 – 3 hours at room temperature. Fixed embryos were dehydrated completely in methanol at −20 °C for at least several hours and rehydrated consecutively with PBS. After rinsing in PBT (0.1% Triton X-100 in PBS), embryos were incubated with 10% goat serum in PBT at room temperature for at least 1 hour. Primary antibodies used were mouse anti-acetylated-α-tubulin (1:500, Sigma) and rabbit anti-ARL13B (1:250, ProteinTech). Primary antibodies were detected with Cy2 donkey anti-mouse IgG and Cy5 donkey anti-rabbit IgG (1:500, Jackson ImmunoResearch), respectively. Antibodies were diluted in 10% goat serum in PBT. Images were taken by confocal microscopy.
4.6 Semi-quantitative RT-PCR Analysis
Total RNA was isolated with TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. Semi-quantitative RT-PCR was performed as described previously (Kato et al., 1999; Manojlovic and Stefanovic, 2012). Primer sequences are followed.
ODC: Forward 5′-ACATGGCATTCTCCCTGAAG-3′, Reverse 5′-TGGCCCAAGGCTAAAGTTG-3′
TTC25: Forward 5′-TCCTGAAAGGAGCCAGAAGA-3, Reverse 5′-GCGTGTCCAGGTACAGGATT-3′
ARL13B: Forward 5′-AGTGCTCTGCTGGCGATAAT-3′, Reverse 5′-ACTGCTCTGCTGGCGATAAT-3′
RFX4: Forward 5′-TCCAAGCTGGGCACTTTACT-3′, Reverse 5′-GAACCCAACACAGGAAGCAT-3′
RFX7: Forward 5′-GCCAACTCCAACTCCAACAT-3′, Reverse 5′-TAGGTGTGAACGCAAATGGA-3′.
Acknowledgments
We thank the National Institute for Basic Biology (NIBB) for a plasmid and R. Didier for supporting our experiments. We also thank Drs. N. Ueno, M. Suzuki and J. B. Wallingford for critical suggestions to experimental procedures and Dr. K. Sakabe for valuable comments to this manuscript. This work was supported by NIH grant (GM087641 to YK, to DK059466 to BS, AA019845 to ZM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aftab S, Semenec L, Chu JS, Chen N. Identification and characterization of novel human tissue-specific RFX transcription factors. BMC Evol Biol. 2008;8:226. doi: 10.1186/1471-2148-8-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Lounis A, Baas D, Barras E, Benadiba C, Charollais A, Nlend Nlend R, Liegeois D, Meda P, Durand B, Reith W. Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes. 2007;56:950–959. doi: 10.2337/db06-1187. [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Choe Y, Karlen M, May SR, Phamluong K, Solloway MJ, Ericson J, Peterson AS. The Rfx4 transcription factor modulates Shh signaling by regional control of ciliogenesis. Sci Signal. 2009;2:ra70. doi: 10.1126/scisignal.2000602. [DOI] [PubMed] [Google Scholar]
- Baas D, Meiniel A, Benadiba C, Bonnafe E, Meiniel O, Reith W, Durand B. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. The European journal of neuroscience. 2006;24:1020–1030. doi: 10.1111/j.1460-9568.2006.05002.x. [DOI] [PubMed] [Google Scholar]
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. American journal of medical genetics. Part C, Seminars in medical genetics. 2009;151C:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- Barral DC, Garg S, Casalou C, Watts GF, Sandoval JL, Ramalho JS, Hsu VW, Brenner MB. Arl13b regulates endocytic recycling traffic. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21354–21359. doi: 10.1073/pnas.1218272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay SN, Caspary T. What are those cilia doing in the neural tube? Cilia. 2012;1:19. doi: 10.1186/2046-2530-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker BE, Gard DL. Visualization of the cytoskeleton in Xenopus oocytes and eggs by confocal immunofluorescence microscopy. Methods Mol Biol. 2006;322:69–86. doi: 10.1007/978-1-59745-000-3_6. [DOI] [PubMed] [Google Scholar]
- Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Molecular and cellular biology. 2004;24:4417–4427. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Developmental cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chung MI, Peyrot SM, LeBoeuf S, Park TJ, McGary KL, Marcotte EM, Wallingford JB. RFX2 is broadly required for ciliogenesis during vertebrate development. Developmental biology. 2012;363:155–165. doi: 10.1016/j.ydbio.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C, Ribes V, Kutejova E, Cayuso J, Lawson V, Norris D, Stevens J, Davey M, Blight K, Bangs F, Mynett A, Hirst E, Chung R, Balaskas N, Brody SL, Marti E, Briscoe J. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development (Cambridge, England) 2010;137:4271–4282. doi: 10.1242/dev.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen WG, van Grunsven LA, van Loon AE. Transcriptional regulation of tubulin gene expression in differentiating trochoblasts during early development of Patella vulgata. Development (Cambridge, England) 1994;120:2835–2845. doi: 10.1242/dev.120.10.2835. [DOI] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Bertheleme JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Ruther U, Schneider-Maunoury S, Attie-Bitach T, Saunier S. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nature genetics. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Dubruille R, Laurencon A, Vandaele C, Shishido E, Coulon-Bublex M, Swoboda P, Couble P, Kernan M, Durand B. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development (Cambridge, England) 2002;129:5487–5498. doi: 10.1242/dev.00148. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annual review of cell and developmental biology. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zein L, Ait-Lounis A, Morle L, Thomas J, Chhin B, Spassky N, Reith W, Durand B. RFX3 governs growth and beating efficiency of motile cilia in mouse and controls the expression of genes involved in human ciliopathies. J Cell Sci. 2009;122:3180–3189. doi: 10.1242/jcs.048348. [DOI] [PubMed] [Google Scholar]
- Elul T, Koehl MA, Keller R. Cellular mechanism underlying neural convergent extension in Xenopus laevis embryos. Developmental biology. 1997;191:243–258. doi: 10.1006/dbio.1997.8711. [DOI] [PubMed] [Google Scholar]
- Emery P, Durand B, Mach B, Reith W. RFX proteins, a novel family of DNA binding proteins conserved in the eukaryotic kingdom. Nucleic acids research. 1996;24:803–807. doi: 10.1093/nar/24.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajiwala KS, Chen H, Cornille F, Roques BP, Reith W, Mach B, Burley SK. Structure of the winged-helix protein hRFX1 reveals a new mode of DNA binding. Nature. 2000;403:916–921. doi: 10.1038/35002634. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nature genetics. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nature genetics. 2006;38:961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature reviews. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods in cell biology. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol. 2010;88:653–669. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- Hayes JM, Kim SK, Abitua PB, Park TJ, Herrington ER, Kitayama A, Grow MW, Ueno N, Wallingford JB. Identification of novel ciliogenesis factors using a new in vivo model for mucociliary epithelial development. Developmental biology. 2007;312:115–130. doi: 10.1016/j.ydbio.2007.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jacquet BV, Salinas-Mondragon R, Liang H, Therit B, Buie JD, Dykstra M, Campbell K, Ostrowski LE, Brody SL, Ghashghaei HT. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development (Cambridge, England) 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Shi Y, He X. Neuralization of the Xenopus embryo by inhibition of p300/CREB-binding protein function. J Neurosci. 1999;19:9364–9373. doi: 10.1523/JNEUROSCI.19-21-09364.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis N. Ciliary proteins and exencephaly. Nature genetics. 2006;38:135–136. doi: 10.1038/ng0206-135. [DOI] [PubMed] [Google Scholar]
- Keller R, Shih J, Sater A. The cellular basis of the convergence and extension of the Xenopus neural plate. Dev Dyn. 1992;193:199–217. doi: 10.1002/aja.1001930302. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Kato A, Altmann CR, Kato Y. The POU homeobox protein Oct-1 regulates radial glia formation downstream of Notch signaling. Developmental biology. 2008;315:579–592. doi: 10.1016/j.ydbio.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Laurencon A, Dubruille R, Efimenko E, Grenier G, Bissett R, Cortier E, Rolland V, Swoboda P, Durand B. Identification of novel regulatory factor X (RFX) target genes by comparative genomics in Drosophila species. Genome Biol. 2007;8:R195. doi: 10.1186/gb-2007-8-9-r195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, Schlossman AM, Merriman B, Attie-Bitach T, Logan CV, Glass IA, Cluckey A, Louie CM, Lee JH, Raynes HR, Rapin I, Castroviejo IP, Setou M, Barbot C, Boltshauser E, Nelson SF, Hildebrandt F, Johnson CA, Doherty DA, Valente EM, Gleeson JG. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nature genetics. 2012;44:193–199. doi: 10.1038/ng.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development (Cambridge, England) 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- Manojlovic Z, Stefanovic B. A novel role of RNA helicase A in regulation of translation of type I collagen mRNAs. RNA. 2012;18:321–334. doi: 10.1261/rna.030288.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougou-Zerelli S, Thomas S, Szenker E, Audollent S, Elkhartoufi N, Babarit C, Romano S, Salomon R, Amiel J, Esculpavit C, Gonzales M, Escudier E, Leheup B, Loget P, Odent S, Roume J, Gerard M, Delezoide AL, Khung S, Patrier S, Cordier MP, Bouvier R, Martinovic J, Gubler MC, Boddaert N, Munnich A, Encha-Razavi F, Valente EM, Saad A, Saunier S, Vekemans M, Attie-Bitach T. CC2D2A mutations in Meckel and Joubert syndromes indicate a genotype-phenotype correlation. Human mutation. 2009;30:1574–1582. doi: 10.1002/humu.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Copp AJ. The relationship between sonic Hedgehog signaling, cilia, and neural tube defects. Birth Defects Res A Clin Mol Teratol. 2010;88:633–652. doi: 10.1002/bdra.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) North-Holland Publishing Co; Amsterdam, The Netherlands: 1967. [Google Scholar]
- Pedersen LB, Veland IR, Schroder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- Peng HB. Xenopus laevis: Practical uses in cell and molecular biology. Solutions and protocols. Methods in cell biology. 1991;36:657–662. [PubMed] [Google Scholar]
- Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, Greene ND, Copp AJ, Stanier P. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Human mutation. 2012;33:440–447. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. The motile cilium in development and disease: emerging new insights. Bioessays. 2009;31:694–699. doi: 10.1002/bies.200900031. [DOI] [PubMed] [Google Scholar]
- Sattar S, Gleeson JG. The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Developmental medicine and child neurology. 2011;53:793–798. doi: 10.1111/j.1469-8749.2011.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Smith SB, Qu HQ, Taleb N, Kishimoto NY, Scheel DW, Lu Y, Patch AM, Grabs R, Wang J, Lynn FC, Miyatsuka T, Mitchell J, Seerke R, Desir J, Vanden Eijnden S, Abramowicz M, Kacet N, Weill J, Renard ME, Gentile M, Hansen I, Dewar K, Hattersley AT, Wang R, Wilson ME, Johnson JD, Polychronakos C, German MS. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775–780. doi: 10.1038/nature08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyer J, Flasse L, Raffelsberger W, Beucher A, Orvain C, Peers B, Ravassard P, Vermot J, Voz ML, Mellitzer G, Gradwohl G. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development (Cambridge, England) 2010;137:203–212. doi: 10.1242/dev.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimle V, Durand B, Barras E, Zufferey M, Hadam MR, Mach B, Reith W. A novel DNA-binding regulatory factor is mutated in primary MHC class II deficiency (bare lymphocyte syndrome) Genes & development. 1995;9:1021–1032. doi: 10.1101/gad.9.9.1021. [DOI] [PubMed] [Google Scholar]
- Stubbs JL, Oishi I, Izpisua Belmonte JC, Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nature genetics. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nature cell biology. 2012;14:140–147. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Hara Y, Takagi C, Yamamoto TS, Ueno N. MID1 and MID2 are required for Xenopus neural tube closure through the regulation of microtubule organization. Development (Cambridge, England) 2010;137:2329–2339. doi: 10.1242/dev.048769. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Satoh A, Ide H, Tamura K. Transgenic Xenopus with prx1 limb enhancer reveals crucial contribution of MEK/ERK and PI3K/AKT pathways in blastema formation during limb regeneration. Developmental biology. 2007;304:675–686. doi: 10.1016/j.ydbio.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Molecular cell. 2000;5:411–421. doi: 10.1016/s1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- Takada H, Hattori D, Kitayama A, Ueno N, Taira M. Identification of target genes for the Xenopus Hes-related protein XHR1, a prepattern factor specifying the midbrain-hindbrain boundary. Developmental biology. 2005;283:253–267. doi: 10.1016/j.ydbio.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Thomas J, Morle L, Soulavie F, Laurencon A, Sagnol S, Durand B. Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol Cell. 2010;102:499–513. doi: 10.1042/BC20100035. [DOI] [PubMed] [Google Scholar]
- Vogel TW, Carter CS, Abode-Iyamah K, Zhang Q, Robinson S. The role of primary cilia in the pathophysiology of neural tube defects. Neurosurgical focus. 2012;33:E2. doi: 10.3171/2012.6.FOCUS12222. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development (Cambridge, England) 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. The International journal of developmental biology. 2001;45:225–227. [PubMed] [Google Scholar]
- You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. American journal of physiology. Lung cellular and molecular physiology. 2002;283:L1315–1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nature genetics. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–450. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- Zhao M, Wu X, Zhang Q, Luo S, Liang G, Su Y, Tan Y, Lu Q. RFX1 regulates CD70 and CD11a expression in lupus T cells by recruiting the histone methyltransferase SUV39H1. Arthritis research & therapy. 2010;12:R227. doi: 10.1186/ar3214. [DOI] [PMC free article] [PubMed] [Google Scholar]