Abstract
The human retrovirus human T cell lymphotropic virus type-I (HTLV-1) is the etiologic agent of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Axonal degeneration in HAM/TSP patients occurs without neuron infection, with the secreted viral Tax protein proposed to be involved. We previously found that Tax secreted into the culture medium of MT-2 cells (HTLV-1-infected cell line) produced neurite retraction in neuroblastoma cells differentiated to neuronal type. To assess the relevance of Tax posttranslational modifications on this effect, we addressed the question of whether Tax secreted by MT-2 cells and peripheral blood mononuclear cells (PBMCs) of HTLV-1-infected subjects is modified. The interaction of Tax with calreticulin (CRT) that modulates intracellular Tax localization and secretion has been described. We studied Tax localization and modifications in MT-2 cells and its interaction with CRT. Intracellular Tax in MT-2 cells was assessed by flow cytometry, corresponding mainly to a 71-kDa protein followed by western blot. This protein reported as a chimera with gp21 viral protein—confirmed by mass spectrometry—showed no ubiquitination or SUMOylation. The Tax–CRT interaction was determined by confocal microscopy and coimmunoprecipitation. Extracellular Tax from HAM/TSP PBMCs is ubiquitinated according to western blot, and its interaction with CRT was shown by coimmunoprecipitation. A positive correlation between Tax and CRT secretion was observed in HAM/TSP PBMCs and asymptomatic carriers. For both proteins inhibitors and activators of secretion showed secretion through the endoplasmic reticulum–Golgi complex. Tax, present in PBMC culture medium, produced neurite retraction in differentiated neuroblastoma cells. These results suggest that Tax, whether ubiquitinated or not, is active for neurite retraction.
Introduction
Human T cell lymphotropic virus type-I (HTLV-1), the first human retrovirus discovered in the early 1980s, is the etiologic agent of two human pathologies: adult T cell leukemia (ATL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP).1,2 HAM/TSP is a progressive neurological disease characterized by a central axonopathy, probably due to an axoplasmic transport disorder that produces a selective loss of long axons of corticospinal tracts.3,4 Regarding infection, T-CD4+ cells are the in vivo reservoir of the virus, mainly CD4+FoxP3+cells or Treg.5–7 Human cerebral endothelial cells have been shown to be susceptible to retroviral infection, producing a dysfunction of the blood–brain barrier by alteration in the expression of tight-junction proteins.8 This could be an important mechanism for the infiltration of infected lymphocytes into the central nervous system (CNS) and also facilitates astrocyte infection.
Despite increasing knowledge about HTLV-1, the molecular mechanisms in HAM/TSP and the progression of the disease are still unknown since HTLV-1 does not infect in vivo neurons.9 HAM/TSP has been associated with the expression and secretion of HTLV-1 Tax pleiotropic protein that exerts a role in viral and cellular transcription, cellular proliferation, and transformation.4,10–16 Among the viral proteins, Tax is chronically detected in the cerebrospinal fluid of HAM/TSP patients.17 Incubation of human SH-SY5Y neuroblastoma cells with culture medium of MT-2 cells (an HTLV-1-infected cell line that secretes viral Tax protein) produces neurite retraction and an increase in Tau phosphorylation at T181.18
Tax, a 40-kDa protein, undergoes posttranslational modifications such as phosphorylation, ubiquitination, SUMOylation, and acetylation.15,16,19–24 Phosphorylation is critical for Tax transactivation via both the ATF/CREB and NF-κB pathways.19,25 Ubiquitinated Tax is associated with cytoplasmic location, while SUMOylation is a nuclear retention signal of Tax resulting in NF-κB transcriptional activation.20–24 Acetylation, predominantly in the nucleus, also facilitates NF-κB activation and positively correlates with Tax phosphorylation, being improved by previous SUMOylation.15,25
Recently, a critical role of K63-linked polyubiquitination of Tax has been shown at lysines K4 to K8 for Tax-induced NF-κB activation.26,27 This modification is essential for Tax binding to NEMO/IKK-γ and IKK activation, while SUMOylation is dispensable. Tax nuclear import/export would occur through carrier- and energy-independent transport mechanisms; Tax may also have a carrier function.12,28,29 Nevertheless, no Tax posttranslational modification studies have been performed in constitutively HTLV-1-infected lymphocytes (MT-2 cells) and in secreted products from HTLV-1 lymphocytes of infected individuals.
Alefantis et al.30 reported that the calcium-binding protein calreticulin (CRT) interacts with Tax-GFP in BHK transfected cells, and the fluorescence signal was located near or at the nuclear membrane. They also observed in infected cells, including MT-2 cells, a higher expression of CRT compared with noninfected cell lines. Expression of Tax in Hep2 cells leads to a redistribution of CRT from the nucleus to the cytoplasm as shown by confocal microscopy with colocalization of Tax, CRT, NEMO/IKK-γ, and RelA in cytoplasmic dotted structures.31
Tax contains a number of putative secretory signals including YTNI and DHE, and interacts with cellular secretory pathway proteins in both a transfected cell line (BHK-21) and in an HTLV-1-infected cell line (C8166 cells, a human CD4+ T lymphoblastoid cell line).13 The secretion of Tax would occur by classical secretory pathways involving a vesicular transport mechanism through the endoplasmic reticulum (ER) and Golgi complex.11,13,28
The detection of Tax in cerebrospinal fluid of HAM/TSP patients and the neurite retraction produced by secreted products from MT-2 cells suggest that Tax could be a relevant protein in the pathogeny. In the present study, we followed Tax modifications with ubiquitin, SUMO-1, and SUMO-2/3 in MT-2 cells and in peripheral blood mononuclear cell (PBMC) culture medium from HTLV-1-infected subjects. In addition, CRT was determined in both types of samples to study Tax–CRT interaction/relationships and Tax posttranslational modifications related to HAM/TSP pathogenesis. Finally, we investigated whether Tax secreted from PBMCs of HAM/TSP patients also produced neurite retraction in neuroblastoma cells differentiated to neuronal type as Tax present in MT-2 cell culture medium.
Materials and Methods
Cell culture
Five to ten million MT-2 or K562 cells (control cell line) were cultured in 10 ml of RPMI 1640+GlutaMAX (Invitrogen, Eugene, OR) supplemented with 10% fetal bovine serum (Invitrogen) as reported by Ramírez et al.32
HAM/TSP patients, asymptomatic HTLV-1 carriers, and healthy noninfected control subjects
All the experiments were performed in compliance with relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki. The University of Chile Ethics Committee has approved the research protocol and all individuals freely gave informed written consent. EDTA-treated blood was obtained from 14 HAM/TSP patients, three asymptomatic HTLV-1 carriers, and six healthy noninfected subjects (negative control).
PBMCs isolation and culture
PBMCs were isolated from 10 ml of EDTA-treated blood samples by Ficoll-Hypaque density gradient centrifugation and then were washed three times with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 100 mM Na2HPO4, 2 mM KH2PO4, pH 7.2). We collected 2×106 PBMCs from each sample, which were cultured for 24 h in 1 ml of RPMI 1640+GlutaMAX medium supplemented with 10% fetal bovine serum. The action of CD8+ cytotoxic T lymphocytes was inhibited by 20 nM concanamycin A (Sigma-Aldrich, St Louis, MO) in order to express measurable Tax.33 In Tax and CRT kinetics experiments from PBMCs, 1 μg/ml Brefeldin A (BFA) or 1 μg/ml ionomycin (IM) and 30 ng/ml phorbol myristate acetate (PMA) were added to the culture at 6 h. The cytotoxicity of BFA and IM+PMA treatments was assayed by measuring LDH release using the Pierce LDH Cytotoxicity Assay Kit, according to the manufacturer's instructions.
Flow cytometry
Cultured MT-2 cells (300,000) were harvested and washed twice with PBS and centrifuged at 400×g for 2 min. They were then stained with fluorophore-conjugated antibodies against CD4-FITC (dilution 1:25) (BD Biosciences, San Jose, CA) and Tax-APC (dilution 1:100) prepared in Dr. Yuetsu Tanaka's Laboratory. For Tax staining, cells were treated with 100 μl of fixation/permeabilization solution (eBiosciences, San Diego, CA) for 15 min at 4°C. Matched isotype controls were used at the same concentration as the respective antibodies. We performed two-color flow cytometry in a FACS-CANTO instrument (Beckton Dickinson); WinMDI 2.9 software was used for data analysis.
Immunocytochemistry and confocal microscopy
MT-2 and K562 cells were washed four times at 37°C with PBS. Cells were deposited on glass slides at a density of 104 cells per 10 μl, allowed to dry for 2–3 h at room temperature, fixed, and permeabilized in ice-cold acetone for 8 min. Fixed cells were incubated for 40 min at 37°C with 25 μl of both monoclonal anti-Tax (Covalab, diluted 1:50) and polyclonal anti-CRT (prepared in Dr. Arturo Ferreira's laboratory, diluted 1:200). After three washings with 250 μl PBS, cells were incubated in darkness for 40 min at 37°C with 25 μl of antimouse IgG secondary antibodies conjugated with FITC (Invitrogen, diluted 1:200) and antirabbit IgG conjugated with Alexa Fluor 594 (Invitrogen, diluted 1:400). The nuclear marker TO-PRO (Invitrogen) diluted 1:400 was also included. Cells were then washed three times with 250 μl of PBS and once with MilliQ water and led dried at room temperature. Coverslips were added with 2 μl of Gel mount Aqueous mounting medium (Sigma-Aldrich Inc.). The preparations were examined under a Carl Zeiss LSM 510 Meta confocal microscope. The images were obtained with LSM 510 Image Browser, and colocalization was analyzed with ImageJ, plugin Colocalization Finder software.
Cell lysis
Cells (MT-2, K562, or PBMCs) were washed five times with PBS and the last pellet was resuspended in lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1% Triton X-100, 0.5% Nonidet P-40, 10 mM N-ethylmaleimide, 0.2 mM Na3VO4, and 0.1 mM PMSF, pH 7.5). A total of 2×106 cells per 500 μl of lysis buffer was incubated on ice for 30 min with gentle shaking. Cell debris was pelleted by centrifugation at 16,000×g for 5 min at 4°C. The lysate was kept at −20°C. Subcellular fractionation of MT-2 cells was performed according to Kfoury et al.23 to obtain three different fractions enriched in cytoplasmic, intermediate fraction (organellar membrane), and nuclear components.
Protein determination, SDS-PAGE, and western blot analysis
Protein determination of cell lysate was done using the Micro BCA Protein Assay Kit from Pierce (Pierce Biotechnology Inc., Rockford, IL) according to the manufacturer's instructions. SDS–PAGE under reducing conditions was performed with 10% polyacrylamide gels. Portions of 50 μg protein of cell lysate or 20 μl of PBMC culture medium were used. The buffer for electrotransfer to nitrocellulose membranes (Bio-Rad Laboratories Ltd., Hercules, CA) contained 25 mM Tris–HCl, 192 mM glycine, and 20% methanol, and electrotransfer was done at a total current of 600 mA at 4°C. After electrotransfer, membranes were blocked for 20 min at room temperature with 5% p/v of “Quick blocker” (Chemicon International, Temecula, CA) dissolved in TBS-T (20 mM Tris–HCl, 137 mM NaCl, 0.1% Tween-20, pH 7.6), then incubated overnight at 4°C or 2 h at room temperature with different primary antibodies at the appropriate dilution in TBS-T buffer.
The following monoclonal antibodies were used: two anti-Tax antibodies (Covalab. Cat. mab0022, diluted 1:1,000) and HTLV-1 Tax hybridoma 168A51-2 (NIH, AIDS Reagent Program, Germantown, MD; Cat. 1316, used as ascitic fluid, diluted 1:1,000), antibodies against ubiquitin (Upstate, Lake Placid, NY; Cat. 04-263, diluted 1:1,000), SUMO-1 (Upstate; Cat. 04-453, diluted 1:1,000), SUMO-2/3 (Abcam Inc., Cambridge, MA; Cat. Ab3742, diluted 1:1,000), GAPDH (Sigma-Aldrich Inc.; Cat. G8795, diluted 1:20,000), TFIIB (Santa Cruz Biotechnology Inc., Santa Cruz, CA; Cat. sc-225, diluted 1:1,000), Dynein IC1/2, cytosolic (Santa Cruz Biotechnology Inc.; Cat. sc-13524, diluted 1:500), MMP-9 (Millipore Merck, Billerica, MA; Cat. MAB3309, diluted 1:500), and polyclonal antibodies against CRT prepared in Dr. Arturo Ferreira's laboratory (diluted 1:5,000 or 1:800,000 according to the antibody titer). After washing five times (5 min each wash) with TBS-T, membranes were incubated with the appropriate secondary antibody for 1 h at room temperature.
As secondary antibodies the following were used: ImmunoPure Goat Anti-Rabbit IgG (H+L) Peroxidase Conjugated (Pierce, diluted 1:500,000), ImmunoPure Goat Anti-Mouse IgG (H+L) Peroxidase Conjugated (Pierce, diluted 1:500,000), and Donkey Anti-Mouse antibody, HRP conjugated, diluted 1:250 (SA1100, Thermo Fisher Scientific, Rockford, IL; absorbed against the sera of several animal species). After washing five times (5 min each wash) with TBS-T the reaction product was visualized using enhanced chemiluminescence SuperSignal West Femto Maximum Sensitivity Substrate Chemiluminescent substrate (Pierce). X-Ray films (CL-Xposure film, Pierce) were exposed for varying times. Control experiments without primary antibodies (only with secondary antibodies) did not give any chemiluminescent signal. For consecutive analyses with various antibodies, stripping was performed, rinsing twice for 10 min with 200 mM glycine, 3.5 mM SDS, and 10% Tween-20 at pH 2.2. Blots were then blocked and probed as described above. Quantification of blots was carried out by scanning films using the Uni-Scan-It Automated Digitizing System.
Coimmunoprecipitation studies
Portions of 500 μg of protein from cell lysates or 500 μl of PBMC culture medium were captured with either anti-Tax (Covalab), anti-CRT, anti-Dynein, or anti-MMP-9 antibodies bound to the coupling resin of the “AminoLink Plus Immobilization Kit” (Pierce). Tax and CRT antibody binding, sample application, and protein elution were done according to the manufacturer's instructions. Proteins eluted from the matrix were subjected to SDS–PAGE and/or western blot.
Mass spectrometry analysis
Excised protein bands (stained with GelCode Blue Stain Reagent, Bio-Rad) were destained, DTT and iodoacetamide treated, and proteolyzed with Trypsin. Peptides from proteolysis and extraction were concentrated using SpeedVac. Concentrated peptides were redissolved in 10 μl of 0.1% formic acid for MALDI-TOF analysis or in 30 μl of 0.1% formic acid and 5% methanol for LC-MS/MS analysis. For MALDI-TOF analysis, the dissolved peptides were mixed with CHCA matrix (10 mg/ml) in a 1:1 ratio. Spectra were acquired in a Microflex MALDI-TOF instrument (Bruker Daltonics Inc., Billerica, MA). Mass list was analyzed in Mascot, MS-Fit, and ProFound on-line databases. Additionally, experimental data were examined using the PeptideMap tool from PROWL against the Tax sequence. For LC-MS/MS analysis 20 μl of the dissolved peptides was separated on a C18 column (Jupiter-Proteo 150×1.0 mm, 4 μm, and 90 Å, Phenomenex Inc., Torrance, CA) at room temperature using the following conditions: 0–10 min 2% B, 10–70 min 2–100% B, and 70–80 min 100% B, where mobile phase A was 0.1% formic acid in water and mobile phase B was 0.085% formic acid in 80% acetonitrile. Chromatograms and spectra were recorded at positive mode on an Esquire 4000 LC-ESI IT MS/MS instrument (Bruker Daltonik GmbH, Germany). Experimental data were examined on the Mascot on-line database and on the local Mascot server against Tax (GenBank accession number BAB18052) and Env protein (GenBank accession number AAU04944) sequences.
Neurite retraction studies on SH-SY5Y cells
Neuroblastoma cells were differentiated with 10 μM all-trans-retinoic acid (Sigma-Aldrich) and 50 ng/ml of BDNF (Alomone Laboratories, Jerusalem, Israel).18 BDNF was removed from the culture medium and replaced by a mixture of DMEM-F12 Ham without fetal bovine serum 4 h previous to the addition of culture medium of PBMCs. In all experiments DMEM-F12 was replaced by PBMC culture medium, being differentiated in SH-SY5Y cells incubated up to 2 h with (1) culture medium of PBMCs from an HAM/TSP patient, (2) culture medium of PBMCs from an HAM/TSP patient with 10 μl/ml of either Tax antibody (HTLV-1 Tax Hyb 168A51-2) or a same isotype irrelevant antibody (anti-Gizzerosine), and (3) with culture medium of PBMCs from a healthy subject. Neurite retraction was followed measuring neurite length using the NIH ImageJ-1.38d program plug in NeuronJ according to Maldonado et al.18 Data collected from 200–300 neurites were used for each condition. All experiments were done in triplicate. Neurite length was expressed as the average value of (μm)/cell±SEM.
Statistical analysis
Statistical data analysis was performed using GraphPad Prism 5.0 and SPSS (version 13, SPSS Inc., Chicago, IL). Data were verified for Gaussian distribution using the Shapiro Wilk test. Spearman's correlation coefficient was used to evaluate relationships between Tax and CRT protein levels.
Results
Tax expression, localization, and modifications in MT-2 cells
MT-2 cells are widely used as a lymphoid line in HTLV-1 research because they contain HTLV-1 in their genome as a provirus. About 50% of these cells express Tax followed by flow cytometry as is shown in Fig. 1A. Cell fractionation yielded fractions enriched in (1) cytosolic proteins, (2) cell membrane and organelle proteins (intermediate fraction), and (3) nuclear proteins.23 Tax in western blot analysis corresponds mainly to a 71-kDa protein as shown in Fig. 1B. This band is observed only in the intermediate fraction. To evaluate the presence of modifications, antibodies against ubiquitin, SUMO-1, and SUMO-2/3 were used in western blot analysis. However, neither of them was found to be associated with the 71-kDa band (data not shown). Additionally, Tax immunoprecipitation did not enable detection of these posttranslational modifications.
FIG. 1.
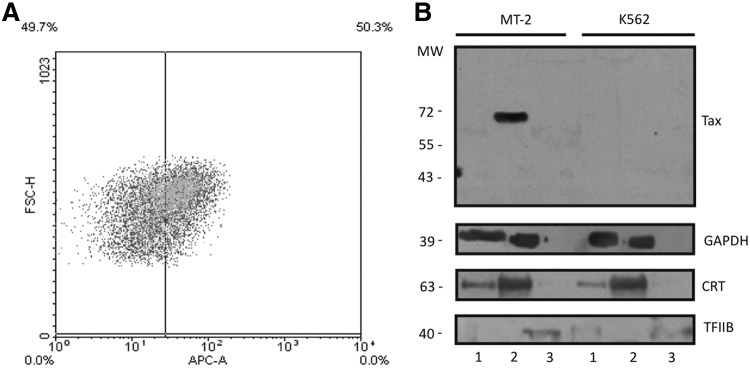
Tax expression in MT-2 cells. (A) Flow cytometry analysis of MT-2 cell culture, showing about 50% of Tax expressing cells (anti-Tax APC diluted 1:100). (B) Western blot of MT-2 or K562 cell fractionation into cytosolic (lanes 1), intermediate (lanes 2), and nuclear (lanes 3) fractions. The figure shows western blots using antibodies against Tax (Covalab, diluted 1:1,000), GAPDH (cytosolic marker, diluted 1:20,000), calreticulin (CRT) ER marker, diluted 1:5,000), and TFIIB (nuclear marker, diluted 1:1,000).
According to the literature, Tax could be fused with Env or Gag proteins in MT-2 cells as a consequence of chromosomal aberration.34,35 Glycosylated Env is an immature protein that undergoes a proteolytic processing giving rise to two glycosylated membrane proteins, gp46 and gp21. Therefore, the C-terminal end of gp21 could be fused with translated Tax (Env-Tax). Tax was submitted to immunoprecipitation, Trypsin digestion, and MALDI-TOF/ESI-IT mass spectrometry analysis. The sequences of two Tax peptides (41-HALLATCPEHQITWDPIDGR-60 and 87-VLTPPITHTTPNIPPSFLQAMR-108) and two gp21 peptides (441-EALQTGITLVALLLLVILAGPCILR-465 and 469-HLPSRVRYPHYSLINPESSL-488) were found. These results are in agreement with the previously described fusion protein between gp21 and Tax present in MT-2 cells, accounting for the difference in electrophoretic migration, discarding ubiquitin-like posttranslational modifications.
Tax, upon 2-fold concentration from MT-2 culture medium, was found only as a 40-kDa nonmodified form by western blot (unpublished results). The 71-kDa membrane-associated form was not found as expected, because it is not a secretable protein.
Tax and CRT interaction in MT-2 cells
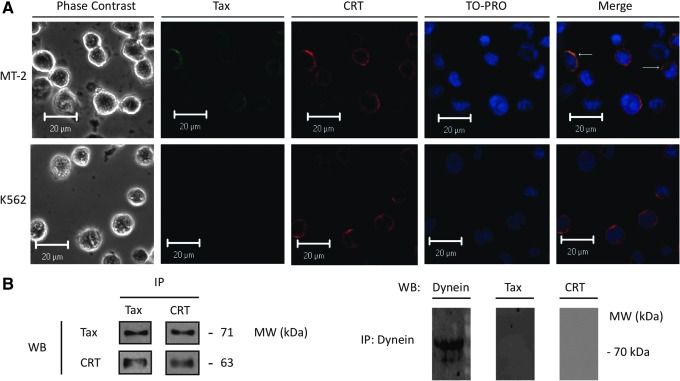
According to the literature, Tax is a secretable protein in tax-transfected cells and CRT seems to be involved in the Tax nuclear export into the cytoplasm.12,30 Consequently, CRT could participate in Tax intracellular localization, including secretion mechanisms. The interaction between Tax and CRT was determined by immunofluorescence colocalization and coimmunoprecipitation in MT-2 cells. According to confocal microscopy among a total of 500 observed cells, 57% expressed Tax protein, which agrees with previous flow cytometry data analysis (Fig. 1A). A total of 42% of Tax expressing cells showed CRT colocalization (Fig. 2A). The interaction between these proteins was confirmed by coimmunoprecipitation with either anti-Tax or anti-CRT antibodies (Fig. 2B). None of the immunoprecipitated complexes showed the presence of other irrelevant proteins (SUMO-1, SUMO-2/3, and ubiquitin). Immunoprecipitation using an irrelevant antibody (Dynein) as a negative control demonstrated the absence of Env-Tax and CRT (Fig. 2B), pointing to a specific interaction of Tax and CRT that could be involved in the secretion pattern of Tax in HTLV-1-infected cells.
FIG. 2.
Tax and CRT interaction in MT-2 cells. (A) Indirect immunofluorescence of Tax (anti-Tax Covalab diluted 1:50; secondary antibody FITC labeled, green) and CRT (anti-CRT diluted 1:200; secondary antibody Alexa Fluor 594 labeled, red)-stained MT-2 and K562 cells by confocal microscopy. Nuclei were labeled with TO-PRO (blue pseudocolor). White arrows indicate colocalization points of Tax and CRT. (B) Coimmunoprecipitation of Env-Tax and CRT from whole cells lysate of MT-2 cells. Tax and CRT were detected by western blot (WB) in both, Tax and CRT immunoprecipitated (IP), using anti-Tax NIH diluted 1:1,000 and anti-CRT diluted 1:5,000. As control, immunoprecipitation with anti-Dynein was performed. Dynein was detected by western blot using anti-Dynein diluted 1:500.
Tax expression in cultured PBMCs from infected subjects
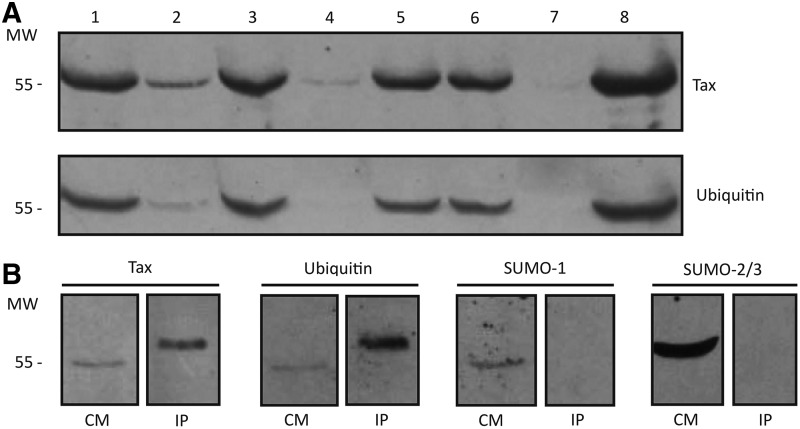
After 24 h culture, PBMCs from HTLV-1-infected patients expressed Tax protein at detectable levels by flow cytometry,7 but not by western blot. Nevertheless, secreted Tax can be followed by western blot in PBMC culture medium, which indicates Tax secretion. We did not find a significant correlation between Tax content in HAM/TSP patients and disease progression. Posttranslational modifications determined in secreted Tax from infected PBMCs by western blot of the culture medium from a set of different HAM/TSP patients and asymptomatic HTLV-1 carriers showed an immunoreactive band for Tax that matches that for ubiquitin (Fig. 3A). Tax ubiquitination was confirmed after immunoprecipitation with anti-Tax antibodies, while modifications with SUMO-1 or SUMO-2/3 were not detected in that immunoprecipitate, which agrees with a secreted protein form (Fig. 3B). Since ubiquitin's molecular weight is about 8.5 kDa, the 57-kDa form might correspond to Tax modification with two ubiquitin subunits. Differences observed in Tax electrophoretic migration in Fig. 3B are caused by serum proteins in PBMC culture medium that are absent in the immunoprecipitated sample.
FIG. 3.
Tax posttranslational modifications in peripheral blood mononuclear cell (PBMC) culture medium from HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. (A) Representative western blot for Tax identification (using antibodies against Tax, Covalab diluted 1:1,000) and ubiquitin modification in PBMC culture medium of HAM/TSP patients (lanes 1, 2, 4, 5, and 8), asymptomatic carriers (lanes 3 and 6), and healthy noninfected control (lane 7) determined by western blot. (B) Tax immunoprecipitation (IP) from PBMC culture medium (CM) and identification of Tax (using antibodies against Tax, NIH diluted 1:1,000), ubiquitin (diluted 1:1,000), SUMO-1 (diluted 1:1,000), and SUMO-2/3 (diluted 1:1,000) by western blot.
Tax and CRT secretion in PBMCs of HAM/TSP patients
CRT was found in the PBMC culture medium of HAM/TSP patients as a broad band (ranging from 87 to 70 kDa) and a discrete band at 56 kDa (Fig. 4A). To understand the secretion mechanisms of CRT and Tax protein, PBMCs of three patients were incubated with either Brefeldin A or with ionomycin (IM) and phorbol myristate acetate (PMA). The former inhibits protein transport from the ER to the Golgi; the latter—IM and PMA—increase protein secretion. Cytotoxicity assays measured as LDH release, expressed as mean value±SD, showed the following values: for BFA treatment 0.210±0.012, for IM+PMA treatment 0.176±0.085, and for the control condition 0.237±0.023 after 12 h culture. No significant differences among the three conditions were found. The results obtained agree with a canonical secretion mechanism for both proteins in HTLV-1-infected PBMCs: Brefeldin A reduced the secretion of both Tax and CRT proteins, while IM and PMA increased their secretion levels (Fig. 4B).
FIG. 4.
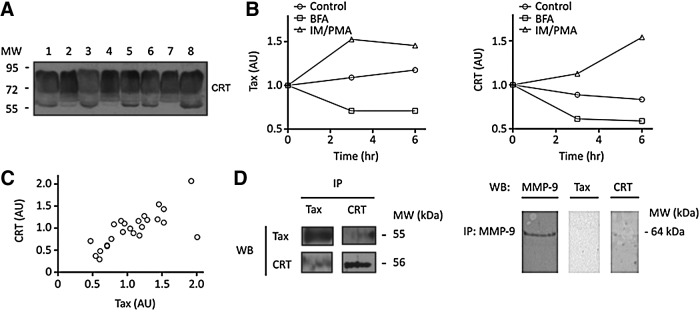
Tax and CRT secretion from PBMCs of HAM/TSP patients. (A) Representative western blot for CRT secreted from PBMC samples of HAM/TSP patients (lanes 1, 2, 4, 5, and 8), asymptomatic carriers (lanes 3 and 6), and healthy noninfected control (lane 7) determined in PBMC culture medium by western blot (anti-CRT, diluted 1:5,000). (B) Tax and CRT kinetics release from PBMCs of a representative HAM/TSP patient. Cells were cultured in control condition (Control) and determinations were made after 16 h of PBMC culture in the presence of Brefeldin A (BFA) or ionomycin/PMA (IM/PMA). Proteins were determined by western blot during 24 h of culture (anti-Tax NIH diluted 1:1,000 using as secondary antibody a donkey antimouse 1:250 absorbed against the sera of several animal species; anti-CRT diluted 1:800,000). Data are expressed in arbitrary units (AU) of pixel intensity corresponding to Tax and CRT normalized with respect to the initial value. (C) Direct relationship between Tax and 56-kDa CRT (AU) released levels from PBMC culture medium of HAM/TSP patients shown in (B). A significant positive correlation of 0.766 was found according to Spearman's correlation (p<0.05, 24 data of XY pairs). (D) Coimmunoprecipitation of Tax and CRT from PBMC culture medium. Tax and CRT were detected by western blot (WB) in both Tax and CRT immunoprecipitations (IP). As control, immunoprecipitation with anti-MMP-9 was performed. MMP-9 was detected by western blot using anti-MMP-9 diluted 1:500.
A positive correlation (Spearman's correlation coefficient of 0.8) between Tax protein and the 56-kDa band of CRT was found in PBMC culture medium of 14 HAM/TSP patients and three HTLV-1 carriers studied (data not shown). A similar positive correlation was determined between Tax and CRT during the time course of secretion of PBMCs cultured with Brefeldin A or IM/PMA for the three patients under study (Fig. 4C). Immunoprecipitation with either anti-Tax or anti-CRT antibodies from PBMC culture medium indicated coprecipitation of Tax and CRT (Fig. 4D). Immunoprecipitated complexes did not show other irrelevant proteins such as SUMO-1 and SUMO-2/3. Immunoprecipitation with an irrelevant antibody (MMP-9, secretable protein), used as negative control, evidenced the absence of Tax and CRT (Fig. 4D), confirming the specific interaction between these proteins. All these results suggest a common vesicular transport for secretion of both proteins.
Effect of secreted Tax from human PBMCs on neurite retraction of SH-SY5Y cells
Figure 5 shows the effect of PBMC culture medium of an HAM/TSP patient on differentiated neuroblastoma cells, SH-SY5Y cells, using as control the culture medium of a healthy subject. Neurite retraction (neurite shortening) was observed with secreted medium from PBMCs of the HAM/TSP patient. Inclusion of anti-Tax antibody prevented the retraction effect. The presence of an irrelevant antibody did not modify the neurite shortening produced by PBMC culture medium of the HAM/TSP patient (data not shown).
FIG. 5.
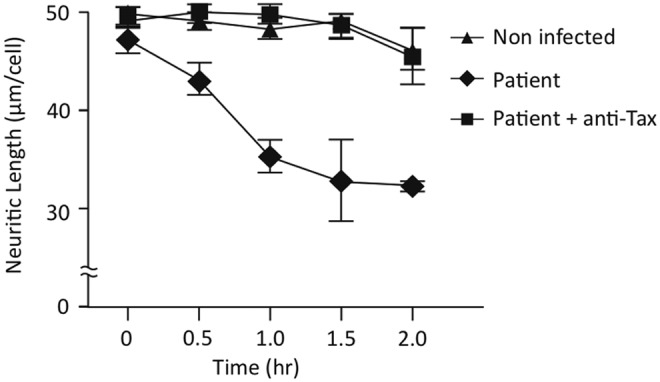
Effect of PBMC culture medium on SH-SY5Y neurite length. Neurite length, expressed as (μm/cell±SEM collected from 200–300 neurites for each condition, was measured using the NIH ImageJ-1.38d program plug in neuronJ. Differentiated SH-SY5Y cells were incubated with PBMC culture medium of a noninfected subject (healthy subject), of an HAM/TSP patient, and the same culture of the HAM/TSP patient including anti-Tax (HTLV-1 Tax Hyb 168A51-2). After 30 min of treatment, significant differences between the HAM/TSP sample and the two control conditions were found (p<0.05).
Discussion
Tax regulation and interaction with cellular proteins and transcriptional complexes in HTLV-1-infected cells are widely accepted as essential factors in virus replication and disease progression.14 Posttranslational modifications of Tax modulate its transcriptional activities and intracellular localization.36 Tax protein has been detected in the nucleus and in three extranuclear compartments: around the microtubule organizer center, colocalizing with the centrosome and associated with the Golgi complex, and also in the region of the virological synapse.23,37 Tax localization in both the nucleus and cytoplasm is important for activation of the NF-κB pathway.24 The canonical NF-κB pathway requires NEMO/IKK-γ, which acts as a platform for recruitment of activators and inhibitors of the IKK complex (effector subunits IKK-α and IKK-β).24 K63-linked polyubiquitination at lysines K4 to K8 of Tax promotes the targeting of Tax and NEMO to perinuclear spots associated with the Golgi complex. After their interaction with IKK-α and IKK-β, nuclear translocation of RelA occurs.24,26,27
In HAM/TSP patients, the mechanisms related to extracellular Tax on disease occurrence and progression remain to be elucidated. In this pathology, the extracellular Tax action has been considered relevant mostly because of the following evidence: (1) about 40% of patients with paraparesis are seronegative for HTLV-1 but exhibit a truncated virus form with the Tax gene incorporated10 and (2) the neurite shortening (retraction) effect of secreted Tax from culture medium of MT-2 cells on differentiated human neuroblastoma cells.18 In this in vitro model of the disease, we also found an increase in CdK5 activity that could be involved in extracellular Tax signaling. The participation of this viral protein was confirmed by the blocking effect of anti-Tax antibodies.18
Cell-free Tax detected in cerebrospinal fluid of HAM/TSP patients comes from secretion of infected cells, mainly CD4+ T cells.17 Increasing evidence suggests an extracellular axonal effect of Tax protein related to the occurrence and progression of HAM/TSP.13,18,30,38 Infected lymphocytes are initially in the CNS regions including periventricular areas of the blood–brain barrier, the subarachnoid space, and the thoraxic and lumbar region.39 Brain endothelial cells that express the receptors for HTLV-1 (GLUT-1, Neuropilin-1, and HSPG) can be infected, producing a dysfunction of the brain–blood barrier, increasing its permeability, thus facilitating lymphocyte passage through the endothelial monolayer cells.8
We identified ubiquitinated Tax protein in the culture medium of PBMCs isolated from HTLV-1 carriers and HAM/TSP patients (Fig. 3). Isolated Tax showed the presence of at least two ubiquitin units deduced from a molecular weight of 57 kDa. This modification should correspond to K63-linked ubiquitination since K48-linked ubiquitination is related to proteasomal degradation.40 The lack of SUMO groups corroborated that Tax corresponds to a secreted protein form since this modification is considered a nuclear retention signal.21,22 To our knowledge, this is the first evidence of ubiquitinated Tax secretion from infected lymphocytes of HAM/TSP patients and HTLV-1 carriers. Furthermore, CRT levels in culture medium are associated with Tax extracellular levels, which would point to a common secretion mechanism as shown in Fig. 4.
CRT, a classical ER resident protein, could participate in the destination of Tax protein to the canonical secretory pathway.30 This can be explained by at least two putative secretory signals in Tax, allowing the secretion of both Tax and CRT as we have observed.11,13 In the current study, CRT secretion in culture medium of PBMCs supports the growing evidence that CRT is not confined in the ER as initially described by Michalak in 1992.41,42 CRT has been found in cytoplasm, nucleus, Golgi, plasma membrane, and even extracellularly in many cell types and tissues, despite of the C-terminal ER localization signal (KDEL) and the lack of secretory signals.41,42 In Hep2 cells, Tax leads to a redistribution of CRT from the nucleus to the cytoplasm.31 Some authors suggest a cleavage of the ER retention signal or interaction with another protein masking the KDEL sequence.41 Tax interaction with CRT in HTLV-1-infected lymphocytes might be involved in ER retention signal masking. Moreover, Tax interaction with various proteins might form complexes involved in the regulated secretion pathway. Tax could act as a carrier protein for CRT trafficking via secretion vesicles in HTLV-1-infected lymphocytes.13 Thus, Tax and CRT could be cosecreted, which would imply a new mechanism of CRT and Tax secretion. Our results show a positive correlation between Tax and the 56-kDa CRT levels in PBMC culture medium of HAM/TSP patients and also coincide with the similar secretion of both proteins.
CRT is widely accepted as a multifunctional protein and current information describes some extracellular effects of CRT, such as promoting wound healing, cell survival, and participating in immune responses against cancer cells.41,42,43 Thus, CRT in extracellular environments favors human health. HTLV-1 has evolved along with humans since ancient times and has a variety of immunological escape and host survival mechanisms.44,45 Consequently, the higher levels of secreted CRT would have beneficial effects on HTLV-1-infected subjects, providing survival mechanisms that would counteract the deleterious effects of Tax.
Our studies on Tax posttranslational modifications in the HTLV-1-infected MT-2 cell line discard the modifications with ubiquitin-like proteins; however, Tax was detected as a fusion protein with gp21—a membrane HTLV-1 glycoprotein.34,35,46 Previous studies showed an interaction between Tax and CRT near the nuclear membrane of the BHK-21 cell line cotransfected with tax and crt genes.30 These authors identified CRT as a nuclear export receptor for Tax, suggesting a control checkpoint for nuclear export, including an additional complexity level to the Tax intracellular location. We found this interaction in MT-2 cells of the cytoplasmic region, probably in ER structures where CRT preferentially locates and interacts with different proteins during folding and quality control of secreted proteins.47 In these infected cells, we demonstrated that the Tax–CRT interaction occurred by immune colocalization and coimmunoprecipitation, similar to that reported in transfected cells with both tax and crt genes.30
The steady state of K63-linked ubiquitination of Tax seems to depend on Ubc13, an E2 ubiquitin-conjugating enzyme, while the ubiquitin-specific peptidase USP20 would act as a ubiquitin-editing enzyme.24,26,48,49 On the other hand, this peptidase was detected in Tax transfected HEK293T cells, with reduced activity, as well as in several HTLV-1 transformed cells such as MT-1, MT-2, MT-4, and AT-2.48 We detected a ubiquitinated Tax form only in infected human PBMCs, probably indicating that peptidase might be less active or absent in these cells.
In conclusion, our study reveals differences between the Tax secreted from HTLV-1-infected T-lymphocytes—which becomes ubiquitinated—and that from MT-2 cells. Tax secretion occurs via the classical ER–Golgi pathway, where CRT may be involved in a cosecretion mechanism due to the extracellular interaction of Tax and CRT. The similar effects of the 40-kDa Tax present in supernatants of MT-2 cells18 and the 57-kDa Tax form in HAM/TSP PBMC supernatants indicate the ubiquitin-independent neurotoxic effect of this secretable viral protein.
Sequence Data
Accession numbers: HTLV-1 Tax, BAB18052; HTLV-1 envelope glycoprotein, AAU04944.
Acknowledgments
We thank Professor Claudio Telha for his valuable input when reviewing the manuscript. We are grateful to Fondecyt, which supported this study (Grant Fondecyt 108-0396), to Project Mecesup UCH 0115, which allowed the use of the Mass Spectrometry Instrument, to the doctoral thesis supported by CONICYT no. 24090150, and to the master thesis supported by CONICYT no. 22110639. We also thank the NIH AIDS Reagent Program for the HTLV-1 Tax hybridoma.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, and Gallo RC: Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 1980;77:7415–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida M, Miyoshi I, and Hinuma Y: Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA 1982;79:2031–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartier L, Cea JG, Vergara C, Araya F, and Born P: Clinical and neuropathological study of six patients with spastic paraparesis associated with HTLV-1: An axomyelinic degeneration of the central nervous system. J Neuropathol Exp Neurol 1997;56:403–413 [DOI] [PubMed] [Google Scholar]
- 4.Cartier L, Vergara C, and Valenzuela MA: Immunohistochemistry of degenerative changes in the central nervous system in spastic paraparesis associated to human T lymphotropic virus type I (HTLV-1). Rev Med Chil 2007;135:1139–1146 [DOI] [PubMed] [Google Scholar]
- 5.Yamano Y, Takenouchi N, Li H, Tomaru U, Yao K, Grant C, Maric D, and Jacobson S: Virus-induces dysfunction of CD4+CD25+ T cells in patients with HTLV-1-associated neuroimmunological disease. J Clin Invest 2005;115:1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toulza F, Heaps A, Tanaka Y, Taylor GP, and Bangham CR: High frequency of CD4+Foxp3+ cells in HTLV-1 infection: Inverse correlation with HTLV-1-specific CTL response. Blood 2008;111:5047–5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberti C, Cartier L, Valenzuela MA, Puente J, Tanaka Y, and Ramirez E: Molecular and clinical effects of betamethasone in human T-cell lymphotropic virus type-1-associated myelopathy/tropical spastic paraparesis patients. J Med Virol 2011;83:1641–1649 [DOI] [PubMed] [Google Scholar]
- 8.Afonso PV, Ozden S, Cumont MC, et al. : Alteration of blood–brain barrier integrity by retroviral infection. PLoS Pathog 2008;4:e1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant C, Barmak K, Alefantis T, Yao J, Jacobson S, and Wigdahl B: Human T cell leukemia virus type I and neurologic disease: Events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. Cell Physiol 2002;190:133–159 [DOI] [PubMed] [Google Scholar]
- 10.Ramírez E, Fernández J, Cartier L, Villota C, and Ríos M: Defective human T-cell lymphotropic virus type I (HTLV-1) provirus in seronegative tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) patients. Virus Res 2003;91:231–239 [DOI] [PubMed] [Google Scholar]
- 11.Alefantis T, Mostoller K, Jain P, Harhaj E, Grant C, and Wigdahl B: Secretion of the human T cell leukemia virus type I transactivator protein tax. J Biol Chem 2005;280:17353–17362 [DOI] [PubMed] [Google Scholar]
- 12.Alefantis T, Jain P, Ahuja J, Mostoller K, and Wigdahl B: HTLV-1 Tax nucleocytoplasmic shuttling, interaction with the secretory pathway, extracellular signaling, and implications for neurologic disease. J Biomed Sci 2005;12:961–974 [DOI] [PubMed] [Google Scholar]
- 13.Jain P, Mostoller K, Flaig KE, et al. : Identification of human T cell leukemia virus type 1 tax amino acid signals and cellular factors involved in secretion of the viral oncoprotein. J Biol Chem 2007;282:34581–34593 [DOI] [PubMed] [Google Scholar]
- 14.Boxus M, Twizere JC, Legros S, Dewulf JF, Kettmann R, and Willems L: The HTLV-1 Tax interactome. Retrovirology 2008;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodewick J, Lamsoul I, Polania A, et al. : Acetylation of the human T-cell leukemia virus type 1 Tax oncoprotein by p300 promotes activation of the NF-kappaB pathway. Virology 2009;386:68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodewick J, Lamsoul I, and Bex F: Move or die: The fate of the Tax oncoprotein of HTLV-1. Viruses 2011;3:829–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cartier L. and Ramírez E: Presence of HTLV-1 Tax protein in cerebrospinal fluid from HAM/TSP patients. Arch Virol 2005;150:743–753 [DOI] [PubMed] [Google Scholar]
- 18.Maldonado H, Ramirez E, Utreras E, et al. : Inhibition of cyclin-dependent kinase 5 but not of glycogen synthase kinase 3-β prevents neurite retraction and tau hyperphosphorylation caused by secretable products of human T-cell leukemia virus type I-infected lymphocytes. J Neurosci Res 2011;89:1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durkin S, Ward M, Fryrear K, and Semmes O: Site-specific phosphorylation differentiates active from inactive forms of the human T-cell leukemia virus type 1 Tax oncoprotein. J Biol Chem 2006;281:31705–31712 [DOI] [PubMed] [Google Scholar]
- 20.Peloponese JM, Jr, Iha H, Yedavalli VR, et al. : Ubiquination of human T-cell leukemia virus type 1 Tax modulates its activity. J Virol 2004;78:11686–11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamsoul I, Lodewick J, Lebrun S, et al. : Exclusive ubiquination and SUMOylation on overlapping lysine residues mediate NF-kB activation by the human T-cell leukemia virus Tax oncoprotein. Mol Cell Biol 2005;25:10391–10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasr R, Chiari E, El-Sabban M, et al. : Tax ubiquitination and SUMOylation control critical cytoplasmic and nuclear steps of NF-kB activation. Blood 2006;107:4021–4029 [DOI] [PubMed] [Google Scholar]
- 23.Kfoury Y, Nasr R, Favre-Bonvin A, et al. : Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene 2008;27:1665–1676 [DOI] [PubMed] [Google Scholar]
- 24.Kfoury Y, Nasr R, Journo C, Mahieux R, Pique C, and Bazarbachi A: The multifaceted oncoprotein Tax: Subcellular localization, posttranslational modifications, and NF-kB activation. Adv Cancer Res 2012;113:85–120 [DOI] [PubMed] [Google Scholar]
- 25.Jeang KT: Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: Cellular signaling through NF-kappa B. Cytokine Growth Factor Rev 2001;12:207–217 [DOI] [PubMed] [Google Scholar]
- 26.Journo C, Bonnet A, Favre-Bonvin A, et al. : HTLV-2 Tax-mediated NF-κB activation involves a mechanism independent of Tax conjugation to ubiquitin and SUMO. J Virol 2013;87:1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnet A, Randrianarison-Huetz V, Nzounza P, et al. : Low nuclear body formation and tax SUMOylation do not prevent NF-kappaB promoter activation. Retrovirology 2012;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alefantis T, Barmak K, Harhaj EW, Grant C, and Wigdahl B: Characterization of a nuclear export signal within the human T cell leukemia virus type I transactivator protein Tax. J Biol Chem 2003;278:21814–21822 [DOI] [PubMed] [Google Scholar]
- 29.Tsuji T, Sheehy N, Gautier VW, Hayakawa H, Sawa H, and Hall WW: The nuclear import of the human T lymphotropic virus type I (HTLV-1) tax protein is carrier- and energy-independent. J Biol Chem 2007;282:13875–13883 [DOI] [PubMed] [Google Scholar]
- 30.Alefantis T, Flaig KE, Wigdahl B, and Jain P: Interaction of HTLV-1 Tax protein with calreticulin: Implications for Tax nuclear export and secretion. Biomed Pharmacother 2007;61:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avesani F, Romanelli MG, Turci M, et al. : Association of HTLV Tax proteins with TAK1-binding protein 2 and RelA in calreticulin-containing cytoplasmic structures participates in Tax-mediated NF-κB activation. Virology 2010;408:39–48 [DOI] [PubMed] [Google Scholar]
- 32.Ramírez E, Cartier L, and Flores R: In vitro cytoskeleton changes of mouse neurons induced by purified HTLV-1, and PBMC from HAM/TSP patients and HTLV-1 carriers. Arch Virol 2004;149:2307–2317 [DOI] [PubMed] [Google Scholar]
- 33.Hanon E, Hall S, Taylor GP, et al. : Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-1) is prevented by cytotoxic T lymphocytes. Blood 2000;95:1386–1392 [PubMed] [Google Scholar]
- 34.Miwa M, Shimotohno K, Hoshino H, Fujino M, and Sugimura T: Detection of pX proteins in human T-cell leukemia virus (HTLV)-infected cells by using antibody against peptide deduced from sequences of X-IV DNA of HTLV-1 and Xc DNA of HTLV-1I proviruses. Gann 1984;75:752–755 [PubMed] [Google Scholar]
- 35.Takeuchi K, Kobayashi N, Nam SH, Yamamoto N, and Hatanaka M: Molecular cloning of cDNA encoding gp68 of adult T-cell leukaemia-associated antigen: Evidence for expression of the pX IV region of human T-cell leukaemia virus. J Gen Virol 1985;66:1825–1829 [DOI] [PubMed] [Google Scholar]
- 36.Journo C, Douceron E, and Mahieux R: HTLV-1 gene regulation: Because size matters, transcription is not enough. Future Microbiol 2009;4:425–440 [DOI] [PubMed] [Google Scholar]
- 37.Nejmeddine M, Barnard A, Tanaka Y, Taylor G, and Bangham C: Human T lymphotropic virus type 1, Tax protein triggers microtubule reorientation in the virological synapse. J Biol Chem 2005;280:29653–29660 [DOI] [PubMed] [Google Scholar]
- 38.Irish BP, Khan ZK, Jain P, et al. : Molecular mechanisms of neurodegenerative diseases induced by human retroviruses: A review. Am J Infect Dis 2009;5:231–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lepoutre V, Jain P, Quann K, Wigdahl B, and Khan KK: Role of resident CNS cell populations in HTLV-1-associated neuroinflammatory disease. Front Biosci 2009;14:1152–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao G: NF-kappaB activation: Tax sumoylation is out, but what about Tax ubiquitination? Retrovirology 2012;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold LI, Eggleton P, Sweetwyne MT, et al. : Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J 2010;24:665–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang WA, Groenendyk J, and Michalak M: Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–846 [DOI] [PubMed] [Google Scholar]
- 43.Ferreira V, Molina MC, Schwaeble W, Lemus D, and Ferreira A: Does Trypanosoma cruzi calreticulin modulate the complement system and angiogenesis? Trends Parasitol 2005;21:169–174 [DOI] [PubMed] [Google Scholar]
- 44.Li HC, Fujiyoshi T, Lou H, et al. : The presence of ancient human T-cell lymphotropic virus type I provirus DNA in an Andean mummy. Nat Med 1999;5:1428–1432 [DOI] [PubMed] [Google Scholar]
- 45.Gallo RC: Human retroviruses after 20 years: A perspective from the past and prospects for their future control. Immunol Rev 2002;185:236–265 [DOI] [PubMed] [Google Scholar]
- 46.Sugamura K, Fujii M, Ueda S, and Hinuma Y: Identification of a glycoprotein, gp21, of adult T cell leukemia virus by monoclonal antibody. J Immunol 1984;132:3180–3184 [PubMed] [Google Scholar]
- 47.Michalak M, Corbett EF, Mesaeli N, Nakamura K, and Opas M: Calreticulin: One protein, one gene, many functions. Biochem J 1999;344(Pt 2):281–292 [PMC free article] [PubMed] [Google Scholar]
- 48.Yasunaga J, Lin FC, Lu X, and Jeang KT: Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J Virol 2011;85:6212–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shembade N, Harhaj NS, Yamamoto M, Akira S, and Harhaj EW: The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-kappaB activation. J Virol 2007;81:13735–13742 [DOI] [PMC free article] [PubMed] [Google Scholar]