Abstract
Interferons (IFNs) are released by cells on exposure to various stimuli, including viruses, double-stranded RNA, and other cytokines and various polypeptides. These IFNs play important physiological and pathophysiological roles in humans. Many clinical studies have established activity for these cytokines in the treatment of several malignancies, viral syndromes, and autoimmune disorders. In this review, the regulatory effects of type I and II IFN receptors on the translation-initiation process mediated by mechanistic target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) pathways and the known mechanisms of control of mRNA translation of IFN-stimulated genes are summarized and discussed.
Introduction
The original hypothesis by Nagano and Kojima regarding the existence of “interferon” almost 60 years ago (Nagano and Kojima 1954) and the subsequent discovery of “interferon” and demonstration of its antiviral properties in 1957 by Lindenmann and Isaacs (Lindenmann and others 1957; Isaacs and Lindemann 1957; Isaacs and others 1957) dramatically transformed the fields of immunology, virology, infectious diseases, oncology, and hematology (Parmar and Platanias 2003; Borden and others 2007; Pestka 2007; Tarhini and others 2012). Extensive subsequent research studies over the last several decades led to the identification of many other cytokines and growth factors and advanced our overall understanding of the functions of the immune system.
There are 3 known types of interferon (IFNs): type I (α, β, ω, ɛ, τ), type II (γ), and type III (λ) all of which utilize distinct receptors, which are specific for each group (Darnell and others 1994; Stark and others 1998; Platanias and Fish 1999; Kotenko and Langer 2004; Pestka and others 2004; Platanias 2005a; Borden and others 2007; Pestka 2007; Donelly and Kotenko 2010; MacMicking 2012). The different type I IFNs bind to the type I IFN receptor, which is composed of 2 receptor subunits (IFNAR1 and IFNAR2) with which the tyrosine kinases Tyk2 and Jak1 are associated, respectively (Darnell and others 1994; Stark and others 1998; Platanias 2005a; Borden and others 2007; Pestka and others 2007). Similarly, the type II IFNR is also composed of 2 distinct subunits (IFNGR1 and IFNGR2) that are associated with the Jak1 and Jak2 kinases (Darnell and others 1994; Stark and others 1998; Platanias 2005a; Borden and others 2007). On the other hand, the type III cell surface IFNR includes the IFNλR1 and IL10R2 subunits that interact with Jak1 and Tyk2 (Kotenko and Langer 2004; Pestka and others 2004; Ank and others 2006). Engagement of the distinct receptors by the respective ligands results in activation of the associated Janus family kinases and subsequent engagement and activation of different combinations of Stat proteins (Darnell and others 1994; Boehm and others 1997; Stark and others 1998). After undergoing tyrosine phosphorylation by the IFNR-associated Jaks, Stat proteins move to the nucleus and activate transcription via binding to IFN-stimulated response elements and/or IFN-γ-activated sequences in the promoters of responsive genes (Darnell and others 1994; Boehm and others 1997; Stark and others 1998). Thus, IFN-inducible gene transcription is primarily regulated by Jak-Stat pathways, and such transcriptional activation of IFN-stimulated genes (ISGs) is critical for the generation of the biological effects of IFNs. There is also evidence that IFN-auxiliary pathways, such as the p38 Map kinase pathway (Uddin and others 1999, 2000; Platanias 2003a, 2003b), and protein kinase C (PKC) pathways (Uddin and others 2002; Redig and others 2009) play important and essential complementary roles in IFN-inducible gene transcription.
Activation of the Jak-Stat pathway alone is not sufficient for the generation of the biological effects of IFNs, and several other cellular signals appear to be essential for the achievement of IFN responses (Platanias 2005a, 2005b). An important step for the induction of IFN-dependent responses is the mRNA translation of ISGs, so protein products that account for biological effects can be generated (Platanias 2005a, 2005b). It is intriguing that although the signaling mechanisms required for ISG transcriptional regulation had been extensively defined for a long time, the signals needed for ISG mRNA translation were until up to recently completely unknown. As discussed next, 2 different cellular pathways, the mechanistic target of rapamycin (mTOR) pathway and the mitogen-activated protein kinase (MAPK) interacting kinase (Mnk) kinase pathway, play critical and essential roles in this process.
Cellular Pathways Required for Translation Initiation and Their Roles in IFN Signaling
Several previous studies in other systems have defined the components of the mRNA translational machinery and have established the basic principles of their functions. It should be noted that control of mRNA translation is an important adaptive response mechanism to environmental changes, stress responses, nutritional alterations, and cytokine and hormonal responses (Sheikh and Fornace 1999; Sonenberg and Hinnebusch 2009; Hershey and others 2012; Kong and Lasko 2012). The cap-dependent mRNA translation in eukaryotes requires the formation of the eukaryotic translation initiation factor 4F (eIF4F) complex on the 5′ methyl guanosine cap structure of mRNA that recruits the small ribosomal subunit (40S) to mRNA (Sheikh and Fornace 1999; Richter and Sonenberg 2005; Ma and Blenis 2009; Sonenberg and Hinnebusch 2009; Hershey and others 2012; Kong and Lasko 2012). The eIF4F complex includes eIF4E, eIF4G, and eIF4A (Richter and Sonenberg 2005; Sonenberg and Hinnebusch 2009; Hershey and others 2012). eIF4E binds to the 5′ cap and recruits eIF4G and eIF4A, while the translational repressor 4E-BP1, in its non-phosphorylated form, inhibits eIF4G binding to eIF4A (Richter and Sonenberg 2005; Sonenberg and Hinnebusch 2009; Hershey and others 2012). eIF4A, a protein with RNA helicase activity, can unwind the secondary structure of mRNA (Parsyan and others 2011). Such helicase activity can be enhanced by eukaryotic translation initiation factor 4B (eIF4B) (Grifo and others 1984; Gingras and others 1999b; Kroczynska and others 2009). The 40S ribosome subunit along with eIF2 in the GTP bound form associate with the initiator methionyl-tRNA, eIF3, eIF5, and eIF1A, forming the 43S initiation complex that associates with eIF4F and, subsequently, forms the 48S complex (Richter and Sonenberg 2005; Sonenberg and Hinnebusch 2009; Hershey and others 2012).
Signaling to the translation initiation machinery by IFNs is significant for the control of IFN-protein synthesis and the generation of IFN-dependent biological effects, as outlined next. While many signaling pathways intersect to control protein synthesis and expression, the mTOR/S6K and MEK/ERK pathways appear to be the key and essential players. Previous work in other systems has established that members of these 2 pathways have been found in close proximity to the translation initiation cap complex that can enable phosphorylation of multiple substrates (Mendoza and others 2011). For instance, activated mTORC1 interacts with the eIF3 complex, and such an interaction correlates with phosphorylation of S6K1 at Thr389 and subsequently, the release of S6K from the eIF3 complex (Holz and others 2005). Other studies have shown that non-phosphorylated ribosomal S6 kinase 1 (RSK1) can also interact with 4E-BP1 in a cell-type specific manner (Kroczynska and others 2011), underscoring the functional relevance of the interactions between MAPK and mTOR pathways in the control of mRNA translation (Mendoza and others 2011).
PI3K/Akt/mTOR Pathway
After the original reports that the phosphatidylinositol-3-kinase (PI3K) is activated by IFNs (Uddin and others 1995, 1997), several studies established that such activation is important for regulation of the AKT kinase and its downstream mediators mTOR, S6K, rpS6, and 4E-BP1 (Lekmine and others 2003; Thyrell and others 2004; Kaur and others 2007, 2008a, 2008b; Panaretakis and others 2008; Goncharova and others 2010). The AKT/mTOR pathway is essential for mRNA translation of genes in various systems (Hay and Sonenberg 2004; Platanias 2005a; Zoncu and others 2011; Laplante and Sabatini 2012). It should be noted that, in addition to controlling mTOR signals for mRNA translation, the PI3K pathway plays an important role in mediating IFN-dependent gene transcription (Uddin and others 1997; Kaur and others 2008b). There is also some recent evidence implicating AKT in transcriptional activation of ISGs via phosphorylation of EMSY, a transcriptional repressor of ISGs (Ezell and others 2012).
Due to such important regulatory functions, the PI3K/AKT pathway is essential for type I IFN-induced antiviral activity (Kaur and others 2008a, 2008b), underscoring the relevance and importance of the pathway in IFN signaling. Other studies have also suggested an important role for the PI3K pathway as an upstream mTOR regulator in the induction of antiviral responses by another type I IFN, IFN-ω (Seo and others 2011). However, mTOR has been also implicated in the generation of IFN-antiviral responses against hepatitis C virus (HCV) in certain systems, independently of PI3K and Akt (Matsumoto and others 2009), while there is also recent evidence that the HCV can promote autophagy by inhibiting the TSC-mTORC1 cascade (Huang and others 2013). Recently emerging evidence suggests that there are differences in the regulation of mTOR signals for mRNA translation of ISGs in the IFN system, as compared with growth factors and pro-oncogenic signals (Kaur and others 2012). Next. we discuss in detail what is known these days about the function of unique effectors downstream of IFN-activated Akt/mTOR.
Regulation of IFN-Dependent mRNA Translation and Protein Synthesis by 4E-BP1 Phosphorylation
The translational repressor 4E-BP1 is a key mTORC1 effector in IFN signaling (Kaur and others 2007). It has been established by extensive previous work in other systems that 4E-BP1 functions as a translational repressor. 4E-BP1 binds constitutively to eIF4E and competitively inhibits the binding of eIF4G to eIF4E and formation of the eIF4F complex (Richter and Sonenberg 2005; Platanias 2005a). On phosphorylation by mTORC1, 4E-BP1 is released from the mRNA cap complex, enabling initiation of translation (Richter and Sonenberg 2005; Platanias 2005a). Previous work has shown that 4E-BP1 is phosphorylated on Thr37/Thr46, Ser65, and Thr70 in response to a variety of stimuli and cellular receptor ligands (Richter and Sonenberg 2005; Platanias 2005a). Similarly, type I and II IFNs induce phosphorylation on these sites, resulting in de-activation of the 4E-BP1, to enable initiation of mRNA translation (Lekmine and others 2003, 2004; Thyrell and others 2004; Kaur and others 2007) (Table 1). Induction of key IFN-α- or IFN-γ-inducible proteins are enhanced in 4E-BP1 (4E-BP1−/−) knockout mouse embryonic fibroblast cells (MEFs), as compared with MEFs usually expressing 4E-BP1 (Kaur and others 2007). The importance of mTOR-mediated phosphorylation of 4E-BP1 in the generation of type I IFN-induced biological responses has been shown both in in vitro studies (Kaur and others 2007) and more recently, in in vivo studies (Burke and others 2011) using mice with targeted disruption of 4E-BP1. The in vivo studies demonstrated that 4E-BP1−/− mice were more sensitive to the antiviral effects of IFNβ against coxsackie virus B3, a known cause of myocarditis (Burke and others 2011).
Table 1.
Elements of Cellular Pathways That Regulate Translation-Initiation in the Interferon System
| Initiation regulators | Kinase | Site and sequence | Function | References |
|---|---|---|---|---|
| 4E-BP1 | mTOR, RSK1 | Thr37/T46 | Repressor of mRNA translation and negative regulator of IFN-antiviral responses. Dissociation from eIF4E enables mRNA translation to proceed, leading to induction of expression of certain ISG products | Lekmine and others (2003); Lekmine and others (2004); Thyrell and others (2004); Kaur and others (2007); Burke and others (2011); Kroczynska and others (2011) |
| YSTT*PGGTLFSTT*PGG | ||||
| Thr70 | ||||
| NSPVTKT*PPR | ||||
| Ser65 | ||||
| LMECRNS*PVT | ||||
| eIF4E | MNKs | Ser209 | Requirement for mRNA expression of certain ISGs and generation of IFN-growth inhibitory responses | Joshi and others (2009); Joshi and others (2011); Mehrotra and others (2013) |
| TATKSGS*TTK | ||||
| eIF4B | S6K1, RSK1 | S422 | Increase interaction with eIF3A, ATPase activity, and anti-leukemic effects of IFNs | Kroczynska and others (2011) |
| ERSRTGS*ESS | ||||
| PDCD4 | S6K1, RSK | S67 | Suppressive effects on mRNA translation of certain ISGs. Negative regulation on IFN-antileukemic responses | Kroczynska and others (2012) |
| RRLRKNS*SRD | ||||
| rpS6 | S6K1, S6K2, RSK | S235/S236 | Not well-defined role in mRNA translation | Lekmine and others (2003); Lekmine and others (2004); Kaur and others (2008a); Goncharova and others (2010) |
| AKRRRLS*S*LRA, | ||||
| S240/S244, S247 | ||||
| LSSLRAS*TSKS*ESS*QK |
Indicated phosphorylation sites and sequences are for human proteins.
eIF4B, eukaryotic translation initiation factor 4B; eIF4F, eukaryotic translation initiation factor 4F; IFN, interferon; mTOR, mechanistic target of rapamycin; RSK, ribosomal S6 kinase; MNK, MAPK interacting kinase.
It should be noted that the Thr37/Thr46 phosphorylation of 4E-BP1 is not always blocked by rapamycin, suggesting that a rapamycin-insensitive kinase may contribute to this event (Gingras and others 1999a). In a previous work (Kroczynska and others 2011), we have shown that the non-active form of RSK1 interacts with 4E-BP1. Induction of phosphorylation/activation of RSK1 by IFN-λ results in its dissociation from 4E-BP1, while pharmacological blocking of the RSK1 activity or down-regulation of RSK1 using RNAi results in blocking the phosphorylation of 4E-BP1 on Thr37/Thr46 (Kroczynska and others 2011). Such IFN-λ-dependent activation of RSK1 and phosphorylation/deactivation of 4E-BP1 is essential for translational up-regulation of p21 WAF/CIP1, suggesting a mechanism for the generation of growth-inhibitory responses (Kroczynska and others 2011).
IFN Signaling and eIF4B Phosphorylation
eIF4B is a regulator of the helicase activity of eIF4A (Bi and others 2000). These 2 proteins unwind complex secondary structures that are associated with the 5′-UTR of some mRNAs, to enable the 40S ribosomal subunit to scan for the start codon (Jaramillo and others 1991; Richter and Sonenberg 2005). Ser422 of eIF4B is phosphorylated by S6K, and this phosphorylation promotes its association with the eIF3 complex (Méthot and others 1996; Raught and others 2004; Shahbazian and others 2006). We have provided evidence that eIF4B is phosphorylated on Ser422 in an IFN-α and IFN-γ-inducible manner, suggesting its involvement in IFN signaling (Kroczynska and others 2009) and raising questions on which kinase accounts for such phosphorylation. Earlier work had shown that eIF4B is not a substrate for the kinase activity of protein kinase R (PKR) in vitro (Berry and others 1985). Our studies demonstrated that such phosphorylation is cell-type dependent, mediated by either S6K or RSK depending on the cellular context (Kroczynska and others 2009) (Table 1). This results in enhanced interaction of eIF4B with eIF3A promoting eIF4B-associated ATPase activity (Kroczynska and others 2009). We have also found that mTOR-mediated signals regulating S6K/eIF4B or ERK-mediated signals regulating RSK/eIF4B are necessary for optimal mRNA translation of ISGs. siRNA-mediated knockdown of either eIF4B or eIF3A was found to result in decreased ISG15 protein expression, further supporting the significance of both translation initiation factors in IFNs responses (Kroczynska and others 2009). Importantly, the RSK1/eIF4B cascade is necessary for the growth-suppressing activity of IFN-α on KT-1-derived leukemic progenitors (Kroczynska and others 2009). Altogether, these studies have suggested an important role for eIF4B phosphorylation and its upstream regulatory effectors, S6K and RSK, in the generation of IFN responses. Notably, some antiviral agents inhibit S6K activity (Petkovic and others 2013), underscoring the complexity of the regulatory networks required for optimal responses and raising questions on whether different antiviral agents that could be theoretically used in combination with IFNs may ameliorate their effects.
IFN-Dependent Phosphorylation and Degradation of the Translation Initiation Inhibitor PDCD4
Previous studies have shown that PDCD4 binds eIF4A and can be a substrate for AGC kinases (Palamarchuk and others 2005; Frescas and Pagano 2008; Lankat-Buttgereit and Göke 2009). PDCD4 binds eIF4A via both its MA3 domains and competes with the MA3 domain of eIF4G for eIF4A binding (Frescas and Pagano 2008; Suzuki and others 2008; Lankat-Buttgereit and Göke 2009). In 2006, the Pagano group demonstrated for the first time that PDCD4 is phosphorylated by S6K1 at Ser67, resulting in the recruitment of SCF βTRCP ubiquitin ligase complex and the subsequent degradation of PDCD4, relieving the negative effects of the protein on translation-initiation (Dorrello and others 2006). In the IFN system, it has been shown that the activity of S6K is required for phosphorylation of PDCD4 in MEFs, while RSK1 is the kinase responsible for the phosphorylation of PDCD4 in U266 and KT1 human hematopoietic cells (Kroczynska and others 2012) (Table 1). Degradation of the phosphorylated PDCD4 facilitates IFN-induced eIF4A activity and binding to eIF4G to promote mRNA translation of ISG15, p21, and Schlafen5 (SLFN5) (Kroczynska and others 2012), all of which play important roles in the generation of IFN responses (Katsoulidis and others 2009, 2010; Joshi and others 2010; Borden and Williams 2011; Schoggins and Rice 2011; Wang and Fish 2012; Mavrommatis and others 2013). Moreover, engagement of the ERK/RSK/PDCD4 pathway is required for the suppressive effects of IFN-α on normal CD34+ hematopoietic precursors and induction of type I IFN antileukemic effects in vitro (Kroczynska and others 2012).
Phosphorylation of Ribosomal Protein S6
rpS6, a component of the 40S ribosomal subunit, was the first identified member of the family of S6K substrates (Flotow and Thomas 1992). S6K1 and S6K2 are required for full control of phosphorylation of rpS6, yet such targeting is predominantly carried out by S6K2 (Pende and others 2004). Furthermore, phosphorylation of rpS6 at Ser235/Ser236 can be still detected in cells lacking both of these kinases, and such residual phosphorylation of these sites is carried out by the MEK/ERK pathway, via RSK engagement (Roux and others 2007). It has been shown in vitro that phosphorylated rpS6 binds with a greater affinity to mRNA and the 5′-cap (Jefferies and others 1997). Both type I and type II IFN signaling via mTOR leads to activation of S6K and, subsequently, phosphorylation of rpS6 (Lekmine and others 2003, 2004; Goncharova and others 2010) (Table 1). Remarkably, in contrast to what is seen in response to growth factor receptors and serum, IFN-induced phosphorylation of rpS6 requires the activity of both mTORC1 and mTORC2 complexes, which is consistent with IFN-inducible mTORC2-dependent engagement of AKT and subsequent activation of mTORC1 and S6K (Kaur and others 2012). Despite the recent advances in the field, the precise biochemical role of rpS6 in the mRNA translation in response to not only IFN, but also other cytokines and stimuli, still remains to be defined, and additional work will be necessary in the future.
MEK/ERK Pathway
Previous work has established that Ras and the ERK/MAPK pathway are activated by growth factors, hormones, cytokines, neurotransmitters, and phorbol esters (Schaeffer and Weber 1999; Kolch 2005; Rosner and others 2006; Rozengurt 2007). The activation occurs frequently through engagement of the receptor with tyrosine kinase activity and G protein-coupled receptors (Rozengurt 2007). The components of this pathway are Ras and the protein kinases Raf, MEK, and ERK (Carriere and others 2008; Mendoza and others 2011). The activated form of ERK phosphorylates p90 RSK, and this pathway mediates signals that promote cell survival (Anjum and Blenis 2008; Carriere and others 2008; Mendoza and others 2011). Previous work has established that the ERK pathway is involved in the regulation of ISG transcription (David and others 1995). Recent evidence indicates that IFN-mediated activation of ERK1/2 plays an essential role in RSK-dependent phosphorylation of eukaryotic translation factor 4B (eIF4B) (Kroczynska and others 2009), and activation of the Mnk1 and 2 (Joshi and others 2009), ultimately controlling the phosphorylation of the eukaryotic cap-binding protein eIF4E on serine 209 (Joshi and others 2009, 2011; Mehrotra and others 2013).
Phosphorylation of eIF4E by Mnk Kinases and Its Significance in Generation of IFN Antiproliferative Responses
The Mnk1 and Mnk2 directly phosphorylate eIF4E on Ser209 (Waskiewicz and others 1997; Joshi and others 2010; Joshi and Platanias 2012). eIF4E is an oncogene with important roles in post-transcriptional gene regulation (Carroll and Borden 2013). The role of eIF4E phosphorylation on serine 209 in the control of translation is still not well understood, but there is earlier evidence that Mnk kinases are recruited to the translation initiation complex by binding to eIF4G (Dobrikov and others 2011). Both type I and II IFNs induce the activation of Mnk1 and phosphorylation of its effector eIF4E via engagement of Mek/Erk (Joshi and others 2009, 2011) (Fig. 1). Mnk kinases are important for mRNA translation of ISGs during the regulation of normal hematopoiesis by type I and II IFNs (Joshi and others 2009, 2011). In addition, it was recently shown that the induction of Mnk kinase activity is required for the suppressive effects of type I IFNs on malignant hematopoietic progenitors from patients with Polycythemia Vera (PV) and generation of the antineoplastic effects in Jak2V617F-transformed cells (Mehrotra and others 2013). Other recent studies have shown that IFNs induce up-regulation of Sprouty (Spry) proteins 1, 2, and 4, in an Mnk-dependent manner and that these proteins exhibit suppressive effects on IFN-dependent activation of the p38 Map kinase and transcriptional regulation of ISGs (Sharma and others 2012). Importantly, knockdown of Spry proteins enhanced the antileukemic effects of type I IFNs and promoted IFN responses on malignant progenitors from patients with Polycythemia Vera (Sharma and others 2012). Thus, beyond effects on mRNA translation of ISGs, Mnk kinases appear to mediate IFN responses, in part, by modulating IFN-dependent gene transcription via their effects on Spry-p38 MAPK signaling (Sharma and others 2012). Altogether, there is now extensive evidence that Mnk kinases are important for IFNR-inducible mRNA translation and generation of the effects of IFNs on normal and malignant hematopoiesis.
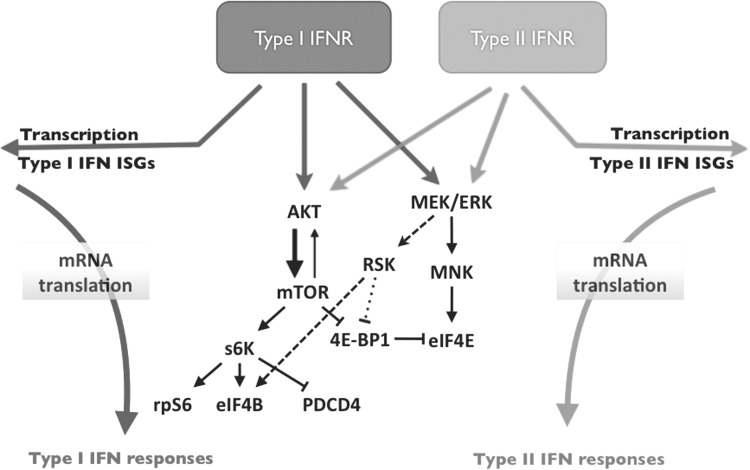
FIG. 1.
Proposed model for the regulation of mRNA translation for type I and type II IFN-dependent ISGs by the AKT/mTOR and MEK/ERK-dependent pathways. IFN, interferon; mTOR, mechanistic target of rapamycin; ISGs, IFN-stimulated genes.
Conclusions
IFNs generate their biological effects by inducing the expression of ISGs and their protein products. Activation of the AKT/mTOR pathway results in the engagement of various effectors phosphorylation that control cap-dependent mRNA translation and regulate the translation of ISGs. The MEK/ERK MAPK pathway plays an important role in modulating the translation of ISGs by controlling IFN-mediated phosphorylation of eIF4E via Mnk kinases, as well as eIF4B, and PDCD4 via RSK in certain cell types. Although there have been major advances in recent years in the field of mRNA translation of ISGs, a substantial amount of additional work will be required in the future to complete our overall understanding of the mechanisms of translational regulation and control of the expression of ISGs in the IFN system. Better understanding of these pathways and mechanisms may well provide the basis for approaches that could ultimately enhance the clinical efficiency of IFNs and advance our overall understanding of the mechanisms of innate immunity.
Author Disclosure Statement
No competing financial interests exist.
References
- Anjum R, Blenis J. 2008. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 9(10):747–758 [DOI] [PubMed] [Google Scholar]
- Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. 2006. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 80(9):4501–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, Knutson GS, Lasky SR, Munemitsu SM, Samuel CE. 1985. Mechanism of interferon action. Purification and substrate specificities of the double-stranded RNA-dependent protein kinase from untreated and interferon-treated mouse fibroblasts. J Biol Chem 260(20):11240–11247 [PubMed] [Google Scholar]
- Bi X, Ren J, Goss DJ. 2000. Wheat germ translation initiation factor eIF4B affects eIF4A and eIFiso4F helicase activity by increasing the ATP binding affinity of eIF4A. Biochemistry 39(19):5758–5765 [DOI] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, Howard JC. 1997. Cellular responses to interferon-gamma. Annu Rev Immunol 15:749–795 [DOI] [PubMed] [Google Scholar]
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. 2007. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 6(12):975–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC, Williams BR. 2011. Interferon-stimulated genes and their protein products: what and how? J Interferon Cytokine Res 31(1):1–4 [DOI] [PubMed] [Google Scholar]
- Burke JD, Sonenberg N, Platanias LC, Fish EN. 2011. Antiviral effects of interferon-β are enhanced in the absence of the translational suppressor 4E-BP1 in myocarditis induced by Coxsackievirus B3. Antivir Ther 16(4):577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere A, Ray H, Blenis J, Roux PP. 2008. The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci 13:4258–4275 [DOI] [PubMed] [Google Scholar]
- Carroll M, Borden KL. 2013. The oncogene eIF4E: using biochemical insights to target cancer. J Interferon Cytokine Res 33(5):227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE, Jr., Kerr IM, Stark GR. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264(5164):1415–1421 [DOI] [PubMed] [Google Scholar]
- David M, Petricoin E, 3rd, Benjamin C, Pine R, Weber MJ, Larner AC. 1995. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science 269(5231):1721–1723 [DOI] [PubMed] [Google Scholar]
- Dobrikov M, Dobrikova E, Shveygert M, Gromeier M. 2011. Phosphorylation of eukaryotic translation initiation factor 4G1 (eIF4G1) by protein kinase C{alpha} regulates eIF4G1 binding to Mnk1. Mol Cell Biol 31(14):2947–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly RP, Kotenko SV. 2010. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 30(8):555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. 2006. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314(5798):467–471 [DOI] [PubMed] [Google Scholar]
- Ezell SA, Polytarchou C, Hatziapostolou M, Guo A, Sanidas I, Bihani T, Comb MJ, Sourvinos G, Tsichlis PN. 2012. The protein kinase Akt1 regulates the interferon response through phosphorylation of the transcriptional repressor EMSY. Proc Natl Acad Sci U S A 109(10):E613–E621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotow H, Thomas G. 1992. Substrate recognition determinants of the mitogen-activated 70K S6 kinase from rat liver. J Biol Chem 267(5):3074–3078 [PubMed] [Google Scholar]
- Frescas D, Pagano M. 2008. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8(6):438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. 1999a. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13(11):1422–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. 1999b. EIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation Annu Rev Biochem, 68:913–963 [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Lim PN, Chisolm A, Fogle HW, 3rd, Taylor JH, Goncharov DA, Eszterhas A, Panettieri RA, Jr., Krymskaya VP. 2010. Interferons modulate mitogen-induced protein synthesis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 299(1):L25–L35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifo JA, Abramson RD, Satler CA, Merrick WC. 1984. RNA-stimulated ATPase activity of eukaryotic initiation factors J Biol Chem 259(13):8648–8654 [PubMed] [Google Scholar]
- Hay N, Sonenberg N. 2004. Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945 [DOI] [PubMed] [Google Scholar]
- Hershey JW, Sonenberg N, Mathews MB. 2012. Principles of translational control: an overview. Cold Spring Harb Perspect Biol 4(12): pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. 2005. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123(4):569–580 [DOI] [PubMed] [Google Scholar]
- Huang H, Kang R, Wang J, Luo G, Yang W, Zhao Z. 2013. Hepatitis C virus inhibits AKT-tuberous sclerosis complex (TSC), the mechanistic target of rapamycin (MTOR) pathway, through endoplasmic reticulum stress to induce autophagy. Autophagy 9(2):175–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J. 1957. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 147(927):258–267 [DOI] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J, Valentine RC. 1957. Virus interference. II. Some properties of interferon. Proc R Soc Lond B Biol Sci 147(927):268–273 [DOI] [PubMed] [Google Scholar]
- Jaramillo M, Dever TE, Merrick WC, Sonenberg N. 1991. RNA unwinding in translation: assembly of helicase complex intermediates comprising eukaryotic initiation factors eIF-4F and eIF-4B. Mol Cell Biol 11(12):5992–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. 1997. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J 16(12):3693–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kaur S, Kroczynska B, Platanias LC. 2010. Mechanisms of mRNA translation of interferon stimulated genes. Cytokine 52(1–2):123–127 [DOI] [PubMed] [Google Scholar]
- Joshi S, Kaur S, Redig AJ, Goldsborough K, David K, Ueda T, Watanabe-Fukunaga R, Baker DP, Fish EN, Fukunaga R, Platanias LC. 2009. Type I interferon (IFN)-dependent activation of Mnk1 and its role in the generation of growth inhibitory responses. Proc Natl Acad Sci U S A 106(29):12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Platanias LC. 2012. Mnk kinases in cytokine signaling and regulation of cytokine responses. Biomol Concepts 3(2):127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Sharma B, Kaur S, Majchrzak B, Ueda T, Fukunaga R, Verma AK, Fish EN, Platanias LC. 2011. Essential role for Mnk kinases in type II interferon (IFNgamma) signaling and its suppressive effects on normal hematopoiesis. J Biol Chem 286(8):6017–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoulidis E, Carayol N, Woodard J, Konieczna I, Majchrzak-Kita B, Jordan A, Sassano A, Eklund EA, Fish EN, Platanias LC. 2009. Role of Schlafen 2 (SLFN2) in the generation of interferon alpha-induced growth inhibitory responses. J Biol Chem 284(37):25051–25064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoulidis E, Mavrommatis E, Woodard J, Shields MA, Sassano A, Carayol N, Sawicki KT, Munshi HG, Platanias LC. 2010. Role of interferon {alpha} (IFN{alpha})-inducible Schlafen-5 in regulation of anchorage-independent growth and invasion of malignant melanoma cells. J Biol Chem 285(51):40333–40341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Lal L, Sassano A, Majchrzak-Kita B, Srikanth M, Baker DP, Petroulakis E, Hay N, Sonenberg N, Fish EN, Platanias LC. 2007. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. J Biol Chem 282(3):1757–1768 [DOI] [PubMed] [Google Scholar]
- Kaur S, Sassano A, Dolniak B, Joshi S, Majchrzak-Kita B, Baker DP, Hay N, Fish EN, Platanias LC. 2008a. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci U S A 105(12):4808–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Sassano A, Joseph AM, Majchrzak-Kita B, Eklund EA, Verma A, Brachmann SM, Fish EN, Platanias LC. 2008b. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J Immunol 181(10):7316–7323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Sassano A, Majchrzak-Kita B, Baker DP, Su B, Fish EN, Platanias LC. 2012. Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses. Proc Natl Acad Sci U S A 109(20):7723–7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W. 2005. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6(11):827–837 [DOI] [PubMed] [Google Scholar]
- Kong J, Lasko P. 2012. Translational control in cellular and developmental processes. Nat Rev Genet 3(6):383–394 [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Langer JA. 2004. Full house: 12 receptors for 27 cytokines. Int Immunopharmacol 4(5):593–608 [DOI] [PubMed] [Google Scholar]
- Kroczynska B, Joshi S, Eklund EA, Verma A, Kotenko SV, Fish EN, Platanias LC. 2011. Regulatory effects of ribosomal S6 kinase 1 (RSK1) in IFNλ signaling. J Biol Chem 286(2):1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczynska B, Kaur S, Katsoulidis E, Majchrzak-Kita B, Sassano A, Kozma SC, Fish EN, Platanias LC. 2009. Interferon-dependent engagement of eukaryotic initiation factor 4B via S6 kinase (S6K)- and ribosomal protein S6K-mediated signals. Mol Cell Biol 29(10):2865–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczynska B, Sharma B, Eklund EA, Fish EN, Platanias LC. 2012. Regulatory effects of programmed cell death 4 (PDCD4) protein in interferon (IFN)-stimulated gene expression and generation of type I IFN responses. Mol Cell Biol 32(14):2809–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Göke R. 2009. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell 101(6):309–317 [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell 149(2):274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekmine F, Sassano A, Uddin S, Smith J, Majchrzak B, Brachmann SM, Hay N, Fish EN, Platanias LC. 2004. Interferon-gamma engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp Cell Res 295(1):173–182 [DOI] [PubMed] [Google Scholar]
- Lekmine F, Uddin S, Sassano A, Parmar S, Brachmann SM, Majchrzak B, Sonenberg N, Hay N, Fish EN, Platanias LC. 2003. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J Biol Chem 278(30):27772–27780 [DOI] [PubMed] [Google Scholar]
- Lindenmann J, Burke DC, Isaacs A. 1957. Studies on the production, mode of action and properties of interferon. Br J Exp Pathol 38(5):551–562 [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. 2009. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10(5):307–318 [DOI] [PubMed] [Google Scholar]
- MacMicking JD. 2012. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol 12(5):367–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Ichikawa T, Nakao K, Miyaaki H, Hirano K, Fujimito M, Akiyama M, Miuma S, Ozawa E, Shibata H, Takeshita S, Yamasaki H, Ikeda M, Kato N, Eguchi K. 2009. Interferon-alpha-induced mTOR activation is an anti-hepatitis C virus signal via the phosphatidylinositol 3-kinase-Akt-independent pathway. J Gastroenterol 44(8):856–863 [DOI] [PubMed] [Google Scholar]
- Mavrommatis E, Fish EN, Platanias LC. 2013. The schlafen family of proteins and their regulation by interferons. J Interferon Cytokine Res 33(4):206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra S, Sharma B, Joshi S, Kroczynska B, Majchrzak B, Stein BL, McMahon B, Altman JK, Licht JD, Baker DP, Eklund EA, Wickrema A, Verma A, Fish EN, Platanias LC. 2013. Essential role for the Mnk-pathway in the inhibitory effects of Type I interferons on myeloproliferative neoplasm (MPN) precursors. J Biol Chem 288(33):23814–23822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. 2011. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36(6):320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méthot N, Song MS, Sonenberg N. 1996. A region rich in aspartic acid, arginine, tyrosine, and glycine (DRYG) mediates eukaryotic initiation factor 4B (eIF4B) self-association and interaction with eIF3. Mol Cell Biol 16(10):5328–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano Y, Kojima Y. 1954. Pouvoir immunisant du virus vaccinal inactivé par des rayons ultraviolets. C R Seances Soc Biol Fil 148(19–20):1700–1702 [PubMed] [Google Scholar]
- Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y. 2005. Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res 65(24):11282–11286 [DOI] [PubMed] [Google Scholar]
- Panaretakis T, Hjortsberg L, Tamm KP, Björklund AC, Joseph B, Grandér D. 2008. Interferon alpha induces nucleus-independent apoptosis by activating extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase downstream of phosphatidylinositol 3-kinase and mammalian target of rapamycin. Mol Biol Cell 19(1):41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar S, Platanias LC. 2003. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol 15(6):431–439 [DOI] [PubMed] [Google Scholar]
- Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, Sonenberg N. 2011. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol 12(4):235–245 [DOI] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. 2004. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol 24(8):3112–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. 2007. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem 282(28):20047–20051 [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Walter MR. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol Rev 202:8–32 [DOI] [PubMed] [Google Scholar]
- Petković F, Blaževski J, Momčilović M, Timotijević G, Zocca MB, Mijatović S, Maksimović-Ivanić D, Mangano K, Fagone P, Stošić-Grujičić S, Nicoletti F, Miljković D. 2013. Saquinavir-NO inhibits S6 kinase activity, impairs secretion of the encephalytogenic cytokines interleukin-17 and interferon-gamma and ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol 259(1–2):55–65 [DOI] [PubMed] [Google Scholar]
- Platanias LC. 2003a. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol Ther 98(2):129–142 [DOI] [PubMed] [Google Scholar]
- Platanias LC. 2003b. Map kinase signaling pathways and hematologic malignancies. Blood 101(12):4667–4679 [DOI] [PubMed] [Google Scholar]
- Platanias LC. 2005a. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5(5):375–386 [DOI] [PubMed] [Google Scholar]
- Platanias LC. 2005b. Introduction: interferon signals: what is classical and what is nonclassical? J Interferon Cytokine Res 25(12):732. [DOI] [PubMed] [Google Scholar]
- Platanias LC, Fish EN. 1999. Signaling pathways activated by interferons. Exp Hematol 27(11):1583–1592 [DOI] [PubMed] [Google Scholar]
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW. 2004. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J 23(8):1761–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redig AJ, Sassano A, Majchrzak-Kita B, Katsoulidis E, Liu H, Altman JK, Fish EN, Wickrema A, Platanias LC. 2009. Activation of protein kinase C{eta} by type I interferons. J Biol Chem 284(16):10301–10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Sonenberg N. 2005. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433(7025):477–480 [DOI] [PubMed] [Google Scholar]
- Rosner D, Stoneman V, Littlewood T, McCarthy N, Figg N, Wang Y, Tellides G, Bennett M. 2006. Interferon-gamma induces Fas trafficking and sensitization to apoptosis in vascular smooth muscle cells via a PI 3′K- and Akt-dependent mechanism. Am J Pathol 168(6):2054–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. 2007. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem 282(19):14056–14064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. 2007. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213(3):589–602 [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 19(4):2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Rice CM. 2011. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol 1(6):519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Kim M, Choi M, Kim S, Park K, Oh I, Chung S, Suh H, Hong S, Park S. 2011. Possible role of phosphoinositide-3-kinase in Mx1 protein translation and antiviral activity of interferon-omega-stimulated HeLa cells. Pharmacology 87(3–4):224–231 [DOI] [PubMed] [Google Scholar]
- Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N. 2006. The mTOR/PI 3′K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J 25(12):2781–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Joshi S, Sassano A, Majchrzak B, Kaur S, Aggarwal P, Nabet B, Bulic M, Stein BL, McMahon B, Baker DP, Fukunaga R, Altman JK, Licht JD, Fish EN, Platanias LC. 2012. Sprouty proteins are negative regulators of interferon (IFN) signaling and IFN-inducible biological responses. J Biol Chem 287(50):42352–42360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh MS, Fornace AJ., Jr.1999. Regulation of translation initiation following stress. Oncogene 18(45):6121–6128 [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136(4):731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. 1998. How cells respond to interferons. Annu Rev Biochem 67:227–264 [DOI] [PubMed] [Google Scholar]
- Suzuki C, Garces RG, Edmonds KA, Hiller S, Hyberts SG, Marintchev A, Wagner G. 2008. PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc Natl Acad Sci U S A 105(9):3274–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhini AA, Gogas H, Kirkwood JM. 2012. IFN-α in the treatment of melanoma. J Immunol 189(8):3789–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyrell L, Hjortsberg L, Arulampalam V, Panaretakis T, Uhles S, Dagnell M, Zhivotovsky B, Leibiger I, Grandér D, Pokrovskaja K. 2004. Interferon alpha-induced apoptosis in tumor cells is mediated through the phosphoinositide 3-kinase/mammalian target of rapamycin signaling pathway. J Biol Chem 279(23):24152–24162 [DOI] [PubMed] [Google Scholar]
- Uddin S, Fish EN, Sher DA, Gardziola C, White MF, Platanias LC. 1997. Activation of the phosphatidylinositol 3-kinase serine kinase by IFN-alpha. J Immunol 158(5):2390–2397 [PubMed] [Google Scholar]
- Uddin S, Lekmine F, Sharma N, Majchrzak B, Mayer I, Young PR, Bokoch GM, Fish EN, Platanias LC. 2000. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon alpha-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J Biol Chem 275(36):27634–27640 [DOI] [PubMed] [Google Scholar]
- Uddin S, Majchrzak B, Woodson J, Arunkumar P, Alsayed Y, Pine R, Young PR, Fish EN, Platanias LC. 1999. Activation of the p38 mitogen-activated protein kinase by type I interferons. J Biol Chem 274(42):30127–30131 [DOI] [PubMed] [Google Scholar]
- Uddin S, Sassano A, Deb DK, Verma A, Majchrzak B, Rahman A, Malik AB, Fish EN, Platanias LC. 2002. Protein kinase C-delta (PKC-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol Chem 277(17):14408–14416 [DOI] [PubMed] [Google Scholar]
- Uddin S, Yenush L, Sun XJ, Sweet ME, White MF, Platanias LC. 1995. Interferon-alpha engages the insulin receptor substrate-1 to associate with the phosphatidylinositol 3'-kinase. J Biol Chem 270(27):15938–15941 [DOI] [PubMed] [Google Scholar]
- Wang BX, Fish EN. 2012. The yin and yang of viruses and interferons. Trends Immunol 33(4):190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 16(8):1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]