Abstract
Omics research infrastructure such as databases and bio-repositories requires effective governance to support pre-competitive research. Governance includes the use of legal agreements, such as Material Transfer Agreements (MTAs). We analyze the use of such agreements in the mouse research commons, including by two large-scale resource development projects: the International Knockout Mouse Consortium (IKMC) and International Mouse Phenotyping Consortium (IMPC). We combine an analysis of legal agreements and semi-structured interviews with 87 members of the mouse model research community to examine legal agreements in four contexts: (1) between researchers; (2) deposit into repositories; (3) distribution by repositories; and (4) exchanges between repositories, especially those that are consortium members of the IKMC and IMPC. We conclude that legal agreements for the deposit and distribution of research reagents should be kept as simple and standard as possible, especially when minimal enforcement capacity and resources exist. Simple and standardized legal agreements reduce transactional bottlenecks and facilitate the creation of a vibrant and sustainable research commons, supported by repositories and databases.
Introduction
Omics research is increasingly reliant on publicly-funded infrastructure that enables rapid and efficient sharing of data and materials. Infrastructure, such as databases and bio-repositories, requires effective governance to accomplish goals of supporting public and private research initiatives, enhancing translation of research into application, and maximizing return on public investment in research, partly through efficiencies of scale and avoidance of duplicative efforts. Here, we discuss governance mechanisms for omics infrastructure that may be thought of as a research commons in support of the pre-competitive environment. In this environment, research and development (R&D) are highly collaborative and less focused on intellectual property rights (IPRs) compared to later-stage product development. We analyze governance mechanisms that incentivize participation in the research commons, focusing on the use and structure of legal agreements, such as “conditions of use” and “material transfer agreements” (MTAs).
Databases and bio-repositories may be thought of as an integral component of research commons, a type of common pool resource. A common pool resource is available to all users on terms that encourage efficiency, equitable use, and sustainability, and that are managed by groups of varying sizes and interests (Ostrom, 1990, 1999). Our understanding of the governance of common pool resources is based on the seminal research of Nobel Laureate Elinor Ostrom on natural resource commons such as forests and fisheries (Ostrom, 1990, 2005). Ostrom's analytical framework enables the systematic study of the governance of commons and has since been applied to research commons (Dedeurwaerdere, 2010a, 2010b; Hess and Ostrom, 2006a, 2006b). The framework describes best practices for governance structures and management to achieve desired outcomes of broad-based participation and availability of data and materials for research. The notable distinction between natural resource and research commons is that the value of research commons is enhanced with both use and recontribution of new or modified data and materials, unlike natural resource commons, which may be depleted through overuse. In other words, contribution, use, and recontribution create a positive “network effect” that enhances value and sustainability of the research commons.
The core action that needs to be incentivized for a functional research commons, therefore, is sharing of data and materials, which in turn is dependent on the accepted norms and behaviors of the research community. Norms and behaviors are impacted by the heterogeneity of the community, which may include public and private sector researchers, government agencies, industry, and research institutions. A heterogeneous community creates challenges in managing differing sets of norms and behaviors about sharing versus withholding of data and materials. A concern, particularly of individual researchers, is the prevention of users benefitting from the commons without contributing to it, often referred to as the “free-rider” problem. In addition, current trends towards commercialization of research outputs from public institutions have led to a plethora of IPRs over research reagents. Indeed, the development of research commons is, in part, a reaction to commercialization policies of governments, funders, and research institutions (Caulfield et al., 2012; Popp Berman 2012).
Developing and managing research commons requires rules that (1) incentivize contribution, use, and recontribution; (2) provide a graduated system of sanctions for noncompliance; (3) provide mechanisms for conflict resolution; and (4) enable community members to participate in rule-making (Ostrom, 2005). Such rules enhance trust amongst community members and encourage participation. In Ostrom's framework, the rules-in-use to govern the commons are defined as “shared normative understandings of what a participant in a position must, must not, or may do…backed by at least a minimal sanctioning ability for noncompliance” (Ostrom and Hess, 2007:41). Rules-in-use have varied malleability and reach. They include (1) national-level laws such as those governing IP, regulatory approval processes, and animal welfare; (2) policies and guidelines for contractual arrangements, funding, and collaborative research; and (3) relatively informal community rules, norms and practices around citation, attribution, reciprocity and sharing, and form and timing of publication.
While formal laws, policies, and guidelines have previously been discussed in the context of research commons (Bubela et al., 2012), contractual arrangements, such as MTAs, require closer examination. MTAs are central mediators in exchanges of data and materials and should embody policies and guidelines for incentivizing the creation of research commons. However, MTAs have been criticized as hampering, instead of aiding, the sharing of data and materials (Schofield et al., 2009). Our empirical study examines the role of MTAs as a mechanism for establishing robust and sustainable research commons in genomics. We focus on the well-developed mouse research commons, within which mouse-related research reagents are developed and distributed to study genetic contributions to human disease. Much mouse-related research exemplifies pre-competitive research. At this stage of R&D, IPRs incur direct costs in filings and maintenance, but also add substantial transaction costs associated with the negotiation of licenses for products and processes that often have limited commercial value. IPRs also lead to duplicative research, either because knowledge of ongoing research is withheld, or because of the need to invent around proprietary products or processes. Contractual agreements within research commons, therefore, should operate to enable knowledge flows, reduce transaction costs for data and materials, and support the pre-competitive research environment (Dove et al., 2012).
Drawing on perceptions of MTAs in the research community that uses mouse models to study human diseases, we explore MTA-mediated access to mouse research reagents at three levels: (1) practical needs of individual researchers to distribute and access research reagents and associated data; (2) roles of repositories and databases in enabling access to research reagents for the research community; and (3) transfer of research reagents and data amongst repositories. The latter is necessary to facilitate international distribution of research reagents, to create mirror sites to ensure security of the resources, and to facilitate large-scale, international resource development projects.
In the context of the mouse research commons, research reagents include mouse models, mouse embryonic stem cells (mESCs), gametes, derivative cell lines, vectors, and associated genotyping and phenotyping data (Brown and Moore, 2012a, 2012b; Collins et al., 2007; Skarnes et al., 2011). As such, mice are the quintessential biomedical research tool, useful for study of gene function. Mouse models include knockout mice, wherein the function of one or more genes has been fully or partially inactivated, or made conditional. In contrast, knockin mice carry inserted gene sequences, which are often human. The importance of knockout mice as a research tool was recognized in the award of the 2007 Nobel Prize in physiology or medicine to Dr. Mario R. Capecchi, Sir Martin J. Evans, and Dr. Oliver Smithies for their early work on knockout mice.
We start with an introduction to MTAs and their negative historical impact on mouse-model research, exemplified by OncoMouse and Cre-lox technology. We then discuss policy responses to accessibility of mouse-related research reagents in the establishment of large-scale, international initiatives for their generation and distribution. These include the International Knockout Mouse Consortium (IKMC) and the International Mouse Phenotyping Consortium (IMPC). We next describe our qualitative research methods and analysis of interviews with expert informants. We focus on the role of MTAs in the distribution of research reagents (1) between researchers, (2) between researchers (as recipients and depositors of mouse materials) and repositories (as archives and distribution centers of mouse materials), and (3) between repositories and the resource-making partners of the IKMC and IMPC. We conclude with specific recommendations to address MTA-related challenges in building a robust and sustainable research commons.
MTAs: Key features
MTAs are a form of license that set terms of use and access for research reagents. They are grants of permission to use proprietary (covered by IPRs) or nonproprietary materials in the control of providers (Mirowski, 2008; Rodriguez, 2005, 2008). They range in scope and complexity from simple conditions-of-use to expansive legal agreements, requiring substantial negotiation of terms (Streitz and Bennett, 2003). They set out rights and responsibilities of the parties (providers and recipients) and include descriptions of materials to be transferred and payments or other benefits exchanged in return. MTAs place limits on the physical handling and use of materials (e.g., only for pre-clinical research or a specific research area) and generally prohibit distribution to third-party researchers (Winickoff et al., 2009). For material derived from human subjects, common limitations on the field of use reflect the specific conditions for the consent used to obtain the material (e.g., only for research into specific diseases). MTAs also limit the liability of providers through disclaimers as to quality of materials provided, ownership, and the non-infringement of IPRs used to create the material (including those of third parties). Other terms set dispute resolution mechanisms, legal jurisdiction, timelines for the provider-recipient relationship, and conditions for termination (e.g., which party may terminate, manner of termination, and/or destruction or return of the material).
Most MTAs distribute materials nonexclusively (i.e., to multiple parties) and retain the provider's rights to continue to use the material. On occasion, MTAs also contain reach-through provisions. These grant back to the provider the rights to materials derived from the original materials, including the right to use the derivative materials or receive a percentage share of royalties from derivative materials. This practice of reach-through is generally considered to be contrary to best practices for licensing of research reagents, especially those created using public funds, because it grants rights that are disproportionate to the provider's role in technological development and extend proprietary claims far beyond those granted by IPRs (Rai and Eisenberg, 2004; Streitz, 2013). For patented materials, MTAs, as contractual formulations, may set greater restrictions on use than the underlying patent claims, which are legally, geographically, and temporally bounded (Mowery and Ziedonis, 2007). MTAs may also contain clauses that delay or restrict an academic researcher's ability to publish work.
At most leading research-intensive universities, MTAs are drafted and negotiated by institutional legal counsel located within technology transfer offices (TTOs) or research services offices. These offices manage research partnerships, sponsored research, and commercialization activities such as patenting, technology licensing, and creation of spin-off companies (Fisher and Atkinson-Grosjean, 2002; Goulding et al., 2010; Miller et al., 2009). Difficulties and delays arise from this centralization and institutional approval for MTAs; it is the institution that is the party to the MTA and institutional lawyers or contracts staff therefore negotiate the terms of the agreement on behalf of researchers.
Historical issues around patents, licensing agreements, and MTAs in mouse research
MTAs for mouse research reagents have a checkered history, illustrated by the OncoMouse and Cre-lox cases (Aghion et al., 2010; Murray, 2010; Murray et al., 2009). In both cases, restrictive licensing practices stimulated research community action to improve sharing of research data and materials. In the case of the OncoMouse, Harvard University exclusively licensed to DuPont its broadly patented technology for mice genetically modified to develop cancer. The technology included the mice, embryos, gametes, vectors, cell lines derived from the mice, and the methods to produce the mice (United States (US) Patent numbers US4736866 (A) and US4736866 (B1); European Union patent numbers EP0169672 (A1) and EP0169672 (B1); Japan patent numbers JPS6181743 (A) and JPH0548093 (B2); and Canada patent number CA1341442 (C); European Patent Office, 2013). DuPont then placed sweeping restrictions on the licensing of OncoMouse technology to the research community, including restrictions on onwards transfer to third parties, onerous research reporting requirements, and reach-through claims over “a percentage share in any sales or proceeds from a product or process developed using an OncoMouse, even though the mice would not be incorporated into the end product” (Murray, 2010:362). The community responded with outrage and civil disobedience; community members continued to share cancer mouse models developed in independent research laboratories.
The situation was resolved for researchers in the United States when DuPont and the National Institutes of Health (NIH), led by Dr. Harold Varmus (2009), a Nobel prize winning mouse model and cancer researcher, signed a memorandum of understanding (MOU) that allowed academic researchers to exchange OncoMice through a simple “conditions-of-use” (CoU) agreement without reporting requirements and reach-through rights. The MOU also enabled the Jackson Laboratory (JAX) and other public repositories to distribute OncoMouse lines widely to researchers at institutions with funding agreements with the Public Health Service of the U.S. Department of Health and Human Services. Repositories notified other researchers, including those jurisdictions outside the U.S. with valid patents over OncoMouse technology, to seek a license for use with DuPont (Jackson Laboratory, 2013a).
Cre-lox recombination technology originated in the private sector, in DuPont's life sciences division in 1987 (Sauer and Henderson, 1988). It enables site-specific deletions, insertions, translocations, and inversions of DNA in eukaryotic and prokaryotic cells. The system creates conditional mutants, allowing genes to be activated, suppressed, or exchanged in response to an external stimulus in specific tissues.
For over 10 years, DuPont strictly controlled access to Cre-lox mice, setting onerous terms of use such as the right to review publications in advance and reach-through royalties for products developed using the technology. While many institutions acquiesced to these demands, the Massachusetts Institute of Technology and JAX resisted. Finally, in August 1998, the NIH negotiated an MOU to allow JAX or institutions with funding agreements with the Public Health Service of the U.S. Department of Health and Human Services to distribute and share Cre-lox mice with a simple conditions-of-use agreement. According to the NIH news release: “The agreements distinguish between academic and commercial uses of the technology. DuPont has agreed to make the technology available without cost to NIH researchers and grantee institutions for noncommercial purposes. Researchers affiliated with the NIH may disseminate Cre-lox materials to other academic laboratories and investigators for academic research under a Material Transfer Agreement. The recipient not-for-profit institutions need an agreement with DuPont to further transfer the Cre-lox materials provided by the NIH. Discoveries made within the academic realm through use of the Cre-lox technology will not be subject to any payments to DuPont so long as the discovery is made outside of any benefit accruing to a commercial entity.” (NIH, 1998). Until 2007, other researchers, within and outside the US, required a license from DuPont or from Bristol-Myers Squibb (BMS) Company, which acquired DuPont in 2001 (e.g., the Harvard-BMS Cre-Lox License, Harvard University, 2013; Jackson Laboratory, 1999). As of September 26, 2007, the Cre-Lox patents and the corresponding license agreements expired in all countries except Canada (Jackson Laboratory, 2008).
Thus access to both technologies required intervention by the NIH, which recognized the importance of policies for the broad, nonrestrictive distribution of research reagents. Its own 1999 policy directs that biomedical research resources generated using public funds should be freely transferred between researchers using “…either no formal agreement, a cover letter, the Simple Letter Agreement of the Uniform Biological Materials Transfer Agreement (UBMTA), or the UBMTA itself” (NIH, 1999:72093). The Simple Letter Agreement (SLA) and UBMTA are templates for the transfer of materials developed by the NIH Office of Technology Transfer (OTT, 2012) and the Association of University Technology Managers (AUTM, 2013). For institutions signatory to the UBMTA Master Agreement, materials can be transferred upon execution of an Implementing Letter.
The AUTM and the Organisation for Economic Cooperation and Development (OECD) have also promulgated best-practice licensing guidelines for publicly funded research outputs. These include nonexclusive licensing practices and the retention of rights for the research community within the institution or more broadly (AUTM, 2007; OECD, 2006). Such policies assume added importance given the public sector dominance as generators and holders of IPRs over research reagents (e.g., animal models, DNA/RNA sequences, and stem cells) (Cook-Deegan and McCormack, 2001; Bergman and Graff, 2007). However, despite these policies and practical interventions, delays in negotiating MTAs continue to impede timely access to research reagents.
Building the mouse research commons
Beyond OncoMouse and Cre-lox technologies, the mouse research commons has long supported the generation and distribution of research reagents (Einhorn and Heimes, 2009; Schofield et al., 2009, 2010). Exchanges may be direct and small-scale amongst researchers and laboratories, or may be supported and simplified by public and private repositories for mouse reagents (Table 1; Table 2; Supplementary Table S1; supplementary tables are available online at www.liebertpub.com/omi). JAX, for example, distributes mice to both academic and industry researchers. Academic and not-for-profit researchers receive mice with a simple notification that the mice are for research use and are not for sale or transfer to third parties without permission. For industry researchers, JAX acts as a broker, distributing lines only when a license agreement has been negotiated between a donor and the industry recipient (Jackson Laboratory, 2013b, 2013c, 2013d). Other distribution models exist, with most repositories using MTAs, which may be more onerous than simple conditions of use (Table 2; Table S1). Nevertheless, despite a relatively robust sharing infrastructure supported by funding agencies and sharing policies, only approximately 35% of mouse strains are made available to the research community (Schofield et al., 2009).
Table 1.
Conditions of Deposits into Major Global Repositories of Mouse Strains
| Major Academic and Nonprofit Mouse Repositories | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Terms of Deposit | MMRRC | JAX | KOMP | TIGM | CMMR | EUMMCR | FESA | EMMA | Riken | CARD | APB |
| Material remains property of depositor(s) | Yes | Yes | KOMP owns resource materials. | Not applicable (N/A1) | Yes | N/A2 | Yes | Yes | Yes | Yes | Yes |
| Project specific deposit | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Deposit from the research community | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Evaluation committee prior to acceptance of deposit | Yes | Yes | Yes | N/A1 | No | N/A2 | Yes | Yes | Unclear | Unclear | Yes |
| Policy for depositors to allow standard terms for distribution to nonprofit researchers | Yes | Yes | Yes | N/A1 | Yes | N/A2 | Yes | Yes | Yes | Unclear | Yes |
| Depositors may limit distribution to nonprofit or academic institutions | Yes | Yes | No | N/A1 | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Attribution requirement | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Cost of deposit borne by | Depositor | Depositor | Depositor | Resource | Depositor | Resource | Resource | Resource | Resource | Unclear | Depositor and Resource |
| Incentives to deposit | Yes | Yes | Yes | N/A1 | Yes | N/A2 | Yes | Yes | Yes | Unclear | Yes |
Name and Abbreviation (left to right) (1) Mutant Mouse Regional Resource Centers (MMRRC); (2) Jackson Laboratory (JAX); (3) Knockout Mouse Project (KOMP); (4) Texas A&M Institute for Genomic Medicine (TIGM); (5) Canadian Mouse Mutant Repository (CMMR); (6) European Mouse Mutant Cell Repository (EUMMCR); (7) Harwell Frozen Embryo and Sperm Archive (FESA); (8) European Mouse Mutant Archive (EMMA); (9) Riken BRC (RIKEN BioResource Center); (10) Center for Animal Resources and Development (CARD); (11) Australian Phenome Bank (APB). (See Supplementary Table S1 for a detailed overview of repository locations, networks, and resources.)
TIGM is the creator and owner of resource materials.
The Helmholtz Zentrum Muenchen and partner Wellcome Trust Sanger Institute are the creators, providers, and resource managers.
Table 2.
Conditions of Distribution of Materials from Major Global Repositories of Mouse Strains
| Major Academic and Nonprofit Mouse Repositories | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Terms | MMRRC | JAX | KOMP | TIGM | CMMR | EUMMCR | FESA | EMMA | Riken | CARD | APB |
| Conditions of Use (CoU) and/or MTA used for distribution | CoU and MTA | CoU | MTA | MTA | CoU and MTAs | MTA | MTA | MTA | MTA | MTA | MTA |
| Project-output distribution | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes |
| Repository directly distributes materials for academic researchers | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Repository directly distributes materials for commercial/for-profit researchers | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Unclear |
| Recipient may transfer unmodified materials to third parties for academic/nonprofit research | No | No | Yes | Yes | Yes1 | Yes | Yes2 | No | No | Yes3 | Unclear |
| Recipient may transfer unmodified materials to third parties for commercial/for-profit research | No | No | No | No | No | No | No | No | No | Unclear | Unclear |
| Recipient may modify materials and transfer modifications and derivatives | Yes4 | No | Yes | Unclear | Possibly5 | Yes | Yes6 | No | No | Unclear | Unclear |
| Repository makes extended claims on modifications and derivatives generated by recipient | No | No | No7 | No | Yes8 | Yes | Yes9 | No | Unclear | Yes | Unclear |
| Repository may modify deposited materials and distribute | Yes | Yes | Possibly10 | N/A | Yes | N/A | Unclear | Unclear | Unclear | Unclear | Unclear |
| Attribution requirement | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Recontribution of materials as a condition of distribution (e.g., mice generated from ES Cells) | No | No | Yes | Yes | No | No | No | No | No | No | No |
Exceptions and special conditions: 1Except for NorCOMM materials; 2Possible for MRC Harwell's ENU DNA archive; 3Possible with permission; 4With permission, generally to nonprofit third parties; 5Possible, depending on conditions set in the deposit MTA; 6Possible for MRC Harwell's ENU DNA archive, to nonprofit third parties; 7Except for specific lines distributed on behalf of the Wellcome Trust Sanger Institute); 8Extended claims are for for-profit recipients; 9For MRC Harwell's ENU DNA archive; 10Possible because KOMP is the owner of the materials.
Partly in response to access issues, and partly to enhance efficiency and reduce costs of developing mouse models as part of individual research grants, an international consortium, the IKMC, was launched to develop a community resource. The IKMC aims to generate mutants for all protein-coding mouse genes (>20,000) using a combination of gene trapping and gene targeting in C57BL/6 mESCs. By 2012, the IKMC generated more than 17,400 embryonic stem cell clones and more than 1700 mutant mouse strains were generated from this resource by large-scale production centers, most of them conditional (Bradley et al., 2012; Brown and Moore, 2012a, 2012b). To complement and add value to the IKMC resources, a second international consortium, the IMPC, was established in 2011 for high-throughput phenotyping of the IKMC lines (Table 3). The platforms of the phenotyping pipeline aim to test, from 2011 to 2021, up to 20,000 mutant mouse lines in major adult organ systems and diseases (Brown and Moore, 2012a, 2012b). This systems-oriented effort will generate an encyclopedia of mammalian gene function. Community members may also nominate genes to be prioritized for the production of knock-out mice and phenotyping. The IMPC is still expanding to include secondary phenotypers, who will contribute additional, medium-throughput screens for more detailed analysis of preselected genes. Additional tertiary phenotyping will be the task of experienced end users who will, in networks of collaboration with the IMPC centers, access mutant embryos and identify ‘strains of interest within the primary and secondary phenotyping tiers’ (Adams et al., 2013).
Table 3.
Global Partners in the IKMC and IMPC Networks
| Activity Component | Partners |
|---|---|
| Production | I. Knockout Mouse Project (KOMP)* (https://www.komp.org/index.php); https://www.mousephenotype.org/martsearch_ikmc_project/aboutkomp) |
| ○ CSD partnership - Children's Hospital Oakland Research Institute*, (CHORI; US; http://www.chori.org/), the Wellcome Trust Sanger Institute* (UK; http://www.sanger.ac.uk/) and the University of California (UC) at Davis School of Veterinary Medicine* (US; http://www.vetmed.ucdavis.edu/index.cfm) | |
| ○ VelociGene division of Regeneron Pharmaceuticals (US; http://www.regeneron.com/velocigene). | |
| ○ University of Pennsylvania School of Medicine (US). | |
| ○ Samuel Lunenfeld Research Institute of Mount Sinai Hospital (SLRI, Canada; http://www.lunenfeld.ca/) | |
| II. European Conditional Mouse Mutagenesis Program (EUCOMM)* (https://www.mousephenotype.org/martsearch_ikmc_project/about/eucomm) | |
| ○ Helmholtz Zentrum München Deutsches Forschungszentrum für Gesundheit und Umwelt GmbH (formerly GSF), (Germany; http://www.helmholtz-muenchen.de/idg/) | |
| ○ University of Frankfurt, (Germany) (http://www.vonmelchner.de/); | |
| ○ Charité (Germany; http://www.ccr.charite.de/); | |
| ○ University of Technology, Dresden, (Germany; http://www.biotec.tu-dresden.de/stewart); | |
| ○ The Wellcome Trust Sanger Institute Hinxton, Cambridge (UK; http://www.sanger.ac.uk/htgt); | |
| ○ Medical Research Council (MRC), Mammalian Genetics Unit, Harwell (UK; http://www.mgu.har.mrc.ac.uk/); | |
| ○ Institut Clinique de la Souris, Strasbourg (France; http://www-mci.u-strasbg.fr/); | |
| ○ European Molecular Biology Laboratory (EMBL) Monterotondo (Italy ; http://www.embl-monterotondo.it/); | |
| ○ Consiglio Nazionale delle Ricerche, Monterotondo (Italy; http://www.emma.cnr.it/ ) | |
| EUCOMM: Tools for Functional Annotation of the Mouse Genome (EUCOMMTOOLS) is completing EUCOMM's production role in the IKMC (https://www.mousephenotype.org/martsearch_ikmc_project/about/eucommtools). Objectives include the creation of a Cre driver resource for C57Bl/6N mice produced from conditional IKMC resources and the development of novel technologies to add value to existing IKMC ES cell and mouse resources, for example, zinc-finger nuclease stimulated homologous recombination strategies in fertilized oocytes. Apart from the EUCOMM members mentioned above, EUCOMMTOOLS includes | |
| ○ Outstation European Bioinformatics Institute (EBI), Hinxton, Cambridge (UK; http://www.ebi.ac.uk/) | |
| ○ Human Genetics Unit, Edinburgh, Scotland, (UK; http://hgu.mrc.ac.uk/) | |
| ○ Universidad Miguel Hernández de Elche, Alicante, (Spain; http://www.umh.es/) | |
| III. North American Conditional Mouse Mutagenesis Project (NorCOMM* and NorCOMM2) (Canada; http://www.norcomm.org; http://www.norcomm2.org/) | |
| ○ Toronto Centre for Phenogenomics* (TCP, http://www.phenogenomics.ca/) | |
| ○ Centre for Modeling Human Disease* (CMHD, http://www.cmhd.ca/) | |
| ○ Hospital for Sick Children* (HSC or “SickKids”, http://www.sickkids.ca) | |
| ○ Mount Sinai Hospital* (http://www.mountsinai.on.ca) | |
| ○ Embryonic Stem Cell/Targeted Mutagenesis Facility, Calgary (http://www.ucalgary.ca/mousegenomics/ESCellServices) | |
| ○ Mammalian Functional Genomics Centre, Manitoba (http://www.umanitoba.ca/institutes/manitoba_institute_cell_biology/MICB/Platforms/MFG.html) | |
| ○ Manitoba Institute of Cell Biology (http://www.umanitoba.ca/institutes/manitoba_institute_cell_biology/) | |
| IV. Texas A&M Institute for Genomic Medicine (TIGM) (US; http://www.tigm.org) (US) | |
| Strain archiving and dissemination (See Table S1 (Supplementary Material) for a detailed overview of repository locations, networks and resources) | ○ KOMP Repository* (US; https://www.komp.org/index.php) is part of the Mutant Mouse Regional Resource Centers (MMRRC;* US; http://www.mmrrc.org/) and distributes KOMP targeting vectors, ES cells and mice. |
| ○ The Jackson Laboratory (JAX; US; http://research.jax.org/komp/phenotyping.html), as part of KOMP2, distributes modified (Cre-deleted) lines from the KOMP2 project that they made from KOMP ES cells. JAX returns the mouse lines for the original allele to the KOMP Repository. | |
| ○ TexasA&M Institute for Genome Medicine Mouse (TIGM) Repository (US; http://www.tigm.org/repository/) distributes TIGM gene trap ES cells and mice. | |
| ○ European Mouse Mutant Archive (EMMA) network* (http://www.emmanet.org/index.php) distributes EUCOMM mice. The European Mouse Mutant Cell Repository (EuMMCR)* (http://www.eummcr.org/) distributes EUCOMM vectors and ES cell lines. | |
| ○ Canadian Mouse Mutant Repository (CMMR)* (Canada; http://www.cmmr.ca/) distributes NorCOMM targeting vectors, ES cells and mice. | |
| ○ The Australian Phenome Bank (APB; Australia; http://pb.apf.edu.au/) is part of the Australian Phenomics Facility (APF; http://apf.anu.edu.au/), within the Australian Phenomics Network (APN; Australia; http://www.australianphenomics.org.au/http://www.australianphenomics.org.au/about-2/partners/). The APN is an IMPC partner contributing to downstream secondary phenotyping. The APB provides ENU-recessive mouse models, a sperm repository and reanimation service. | |
| ○ RIKEN BioResource Center (RIKEN BRC), Tsukuba, Ibaraki, (Japan; http://www.brc.riken.jp/inf/en/index.shtml) works with the Japan Mouse Clinic in the IMPC. | |
| Data management and dissemination | ○ International Knockout Mouse Consortium Data Coordination Center (I-DCC) supported by the European Union (IKMC Biomart Link: http://www.i-dcc.org/biomart/martview/47b5fbe7a632dfc920d0993d18e6a36a). |
| ○ The NIH-funded KOMP Data Coordination Center* (US; KOMP-DCC; https://www.mousephenotype.org/martsearch_ikmc_project/aboutkompdcc) at the Jackson Laboratory in Bar Harbor, Maine, US. | |
| ○ The Data Coordination Center Database at EBI-Hinxton* (UK; http://www.ebi.ac.uk/) supported by the JAX international Mouse Genome Informatics resource (MGI) (http://www.informatics.jax.org/mgihome/projects/aboutmgi.shtml). | |
| Phenotyping (Global map and list of “IMPC Members” - http://www.mousephenotype.org/about-impc/impc-members) |
○ The KOMP Phenotyping Pilot (K312; years 2010–2011) created, characterized, publicly archived and disseminated 312 unique mutant lines from targeted ESCs developed by the CHORI-Sanger-UC Davis and Regeneron consortium of KOMP (http://kompphenotype.org/about.php). KOMP2 is generating and phenotyping mice for over 5,000 knockout ES cell lines from EUCOMM and KOMP production. (http://commonfund.nih.gov/KOMP2/overview.aspx). KOMP2 includes JAX; the NIH-funded BaSH Consortium (Baylor College of Medicine https://www.bcm.edu/departments/molecular-and-human-genetics/bashconsortium/; The Wellcome Trust Sanger Institute and MRC Harwell Mary Lyon Centre, http://www.har.mrc.ac.uk/); and the DTCC Consortium (US; UC Davis, TCP, CHORI and Charles River Laboratories; http://www.criver.com/promo/dtcc). |
| ○ The EU-based INFRAFRONTIER project is continuing EUCOMM's work of developing infrastructure and operations in high-thoughput mouse phenotyping, archiving and distribution. It includes new scientific and government partners from Spain, Finland, Sweden, Portugal, and France, among others (https://www.infrafrontier.eu; http://www.infrafrontier.eu/partners.php/). | |
| ○ The Canadian NorCOMM2 continues NorCOMM's role in the IMPC (Canada; http://www.norcomm2.org/). It prioritizes for mouse line production and phenotyping relatively unannotated genes that are nominated by Canadian scientists and have no published existing knock-out. | |
| ○ New phenotyping partners include Model Animal Research Center (MARC), Nanjing University (China), RIKEN BioResource Center (BRC) and Japan Mouse Clinic (Japan), Korea Mouse Phenotype Consortium, and the Australian Phenomics Network (APN, including the APF and APB). In addition, the consortium is seeking industrial partners. Taconic Inc, a commercial mouse model provider, is a corporate sponsor. | |
| Funding Leads | ○ National Institutes of Health (NIH) (US; http://www.nih.gov) |
| ○ European Union Sixth Framework Programme (Life sciences, genomics and biotechnology for health; http://cordis.europa.eu/fp6/lifescihealth.htm; http://cordis.europa.eu/projects/rcn/78445_en.html) | |
| ○ Medical Research Council (MRC) (U.K.; http://www.mrc.ac.uk/index.htm) | |
| ○ Wellcome Trust (U.K.; http://www.wellcome.ac.uk/) | |
| ○ Canadian Institutes of Health Research (CIHR; Canada; http://www.cihr-irsc.gc.ca/) | |
| ○ Genome Canada (http://www.genomecanada.ca) | |
| ○ Government of Japan (Supporting the RIKEN Bioresource) | |
| ○ Government of Australia (Supporting the APN and APB) |
Asterisk denotes overlapping or continuing roles in the IKMC and the IMPC.
The IKMC and IMPC projects are broad international collaborative networks of mouse genetics centers, supported by national and regional funding bodies in North America, Europe, and Asia-Pacific (Table 3). The projects rely on an established infrastructure for archiving and sharing mouse strains and associated data (Table 3; Supplementary Table S1). For example, the IKMC mESCs are available from the Knockout Mouse Project (KOMP) Repository or the European Mouse Mutant Cell Repository (EuMMCR) (Brown and Moore, 2012b; Donahue et al., 2012). Data generated by the primary IMPC phenotyping centres are processed and disseminated by bioinformatics facilities, chiefly the NIH-funded KOMP Data Coordination Center (DCC) and the European Commission-funded International Data Coordination Center (I-DCC). These resources ensure use of common semantics for comparing and integrating imaging, text-based and numerical data produced by diverse laboratories (Mallon et al., 2012; Ringwald et al., 2011). The data are made available to the scientific community through a centralized open-access portal (https://beta.mousephenotype.org/data/search).
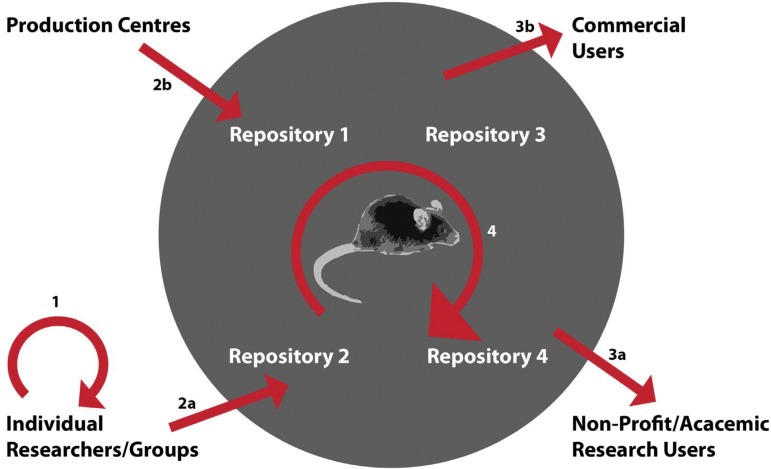
Thus, for the highly networked mouse model research community, MTAs set conditions for exchange of materials in four distinct contexts (Fig. 1): (1) simple distribution of research reagents between individual researchers and research laboratories mediated by institutional legal counsel; (2) deposit of research reagents into repositories and data into databases; (3) distribution of research reagents to the research community by repositories; and (4) distribution of research reagents amongst members of international, community resource development consortia such as the IKMC and the IMPC. We analyze each, in turn, after describing our methods.
FIG. 1.
Types of Material Transfer Agreement (MTAs) used to distribute and use mouse-related research tools in the global mouse research commons: (1) mouse resources distributed amongst researchers; (2) deposit agreements for mouse resources to repositories from (a) researchers or (b) large-scale production centers for the generation of mouse resources such as the IKMC production centers; (3) distribution agreements from the repositories to (a) nonprofit/academic users or (b) commercial users; and (4) amongst repositories for value-added activities such as phenotyping (IMPC) or mirroring of resources for distribution.
Methods
Analysis of MTAs
We identified the repositories engaged in the IMPC and IKMC consortia. We compiled MTAs and other legal instruments as available online used by eleven repositories for deposit and distribution. We characterized the nature of the terms for both deposit and distribution (Table 1; Table 2), noting that each repository used multiple MTAs and other legal instruments depending on the provenance of the material being distributed (Table 2; Supplementary Table S1). Our analysis noted standard terms, including: the transferred material remains the property of the donor but may be distributed by the repository as a service to the community; liabilities and warranties concerning the quality of the material and underlying IPRs; the hazardous/experimental nature of the materials; prohibition against use in human subjects; use in compliance with all applicable laws, and regulations; and execution clauses. However, below we discuss only those terms that either enhance or impede the flow of materials within the research commons (Table 1; Table 2).
Semi-structured Interviews
We conducted 87 semi-structured interviews with members of the mouse model research community (Table 4). The interview guides were informed by research on the legal, technical, social, and ethical issues of developing community resources for animal-model genomics. The guides were reviewed for depth and breadth of coverage by experts in the Canadian mouse genomics community. While interview guides were specific to the stakeholder groups, the central theme was the sustainable development and use of federated, high-impact resources for mouse-model genomics. The guides explored professional backgrounds of participants, ongoing research, collaborations, role in and awareness of the IMPC and/or IKMC, funding sources and challenges, and experiences with materials sharing and transfer. The Research Ethics Office of the University of Alberta approved the study.
Table 4.
Interview Participants
| Interviewees | IKMC (N=36) (Years: 2007–2008) | IMPC (N=51) (Years: 2012–2013) |
|---|---|---|
| Animal facility managers* | – | 4 |
| Consortium directors | 6 | 10 |
| Database managers | 3 | 5 |
| Funders | 1 | 6 |
| Industry representatives | – | 2 |
| Repository managers | 1 | 7 |
| Resource makers | 18 | – |
| Technology transfer offices | – | 8 |
| Users of mouse models | 7 | 9 |
Animal facility managers are not represented in the data in this report.
We spoke with consortium directors, resource developers, managers of repositories, animal facilities and databases, funders, TTO representatives, commercial reagent suppliers, and academic researchers (Table 4). We invited participants on the basis of their publication record and institutional affiliations. Of our 87 interviews, two consortium directors and a repository manager were repeat participants, invited to speak on their roles both in the IKMC and IMPC. We collected data in two phases: (1) 36 interviews in 2007–2008 during the early stages of the IKMC; and (2) 51 interviews in 2012–2013 during the early stages of the IMPC. The use of data across a 5-year period permitted a longitudinal, inter-project comparison of MTA-related issues.
Interviews were 45 to 60 minutes long. We addressed participants' questions about our aims and methods by telephone, e-mail, and in-person interviews. We asked: (1) mouse-model users to describe their experiences of accessing and sharing mouse research tools, with a focus on IP and MTAs; awareness and views of IKMC and IMPC resources; and data and materials sharing plans and activities; (2) consortium directors to describe strategies of coordinating communications and transnational flows of tools and outputs; (3) resource makers about their rationale for contributing to the IKMC effort, their view of the utility of the resource, and challenges in creating the resources; and sustainability models; (4) funders to describe incentives and enforcement mechanisms for best practices around publicly funded research tools and results; (5) manager-developers of repositories and databases to discuss infrastructure and capacity, funding sources and challenges, practices around receiving and distributing resources, and current use and potential improvements of relevant instruments, particularly MTAs; (6) TTOs to describe their institutional policies and practices around seeking IPRs on research output, licensing in and out of research materials, and monitoring and enforcing MTAs; (7) industry participants who were commercial suppliers of mouse materials to describe their corporate products, market operations, IP policies, and their experiences in working with the IKMC and IMPC; and (8) animal facility managers and staff to discuss the practicalities of mouse colony management to assist us in understanding the context of mouse model research.
Analysis of interview transcripts
AM stripped interview transcripts of identifiers and read the transcripts through to obtain a preliminary overview of the dominant themes and issues. We then coded the data using standard qualitative methods. We read the transcripts and coded for initial themes using the ‘constant comparison’ method (Charmaz, 2006; Corbin and Strauss, 2008). This method involved an iterative reading and comparison between the transcripts to identify relationships between interviews and themes that required further exploration in subsequent interviews. As new codes emerged, previous transcripts were re-analyzed to incorporate the new codes. We used NVivo 10© (QSR International) qualitative analysis software to organize and code the data. In the first phase of coding, AM segmented the transcripts into broad ‘open’ codes (e.g., ‘MTA negotiations’; ‘MTA process’; ‘NIH UBMTA’). AM then compared and related these open codes to each other and integrated them into ‘axial’ codes that captured specific actions, interactions, and consequences (e.g., ‘Delayed Transfers and TTO-Researcher Frictions’; ‘MTA negotiations and delays’; ‘NIH UBMTA–effects of deviation’). In the next stage, TB (the Principal Investigator; PI) reviewed AM's codes and selected those codes relevant to the current topic for further analysis and inclusion in the results (e.g., ‘MTAs and exchange of materials within international resource development consortia’).
Our qualitative analysis was guided by relevant peer-reviewed and gray literature (conference proceedings, news, and legal and policy documents). These readings enabled us to gain a greater understanding of the social and professional contexts in which our participants operated. As we coded, we verified the selected quotes to ensure that they retained their original meaning. Further, we sought to ensure that ‘saturation’ had been achieved, meaning that we had iteratively coded the data until no new codes emerged. To further validate our analysis, we discussed our findings with specific informants who suggested how we could further nuance and refine the analysis. In the Results section, we focus on quotes on MTAs and data or materials sharing. We use square parentheses to indicate concealed identities or inserted explanations.
Our study has certain limitations. Being a qualitative study, it offers narrative richness, but due to a smaller sample size compared to quantitative methods such as a survey, our analysis may not be widely generalizable. Additionally, while we followed standard practices to validate our findings, qualitative analyses offer an inherently subjective selection and interpretation of illustrative quotations to address research questions.
Results
We order the results according to the increasing complexity of relationships around MTAs (1) between researchers; (2) deposit into repositories; (3) distribution by repositories; and (4) exchanges between repositories, especially those that are consortium members of the IKMC and IMPC. Our participants consistently reported problems of restrictive clauses, varying content, high volume of requests for MTAs, extended negotiations, and resulting delayed transfer of materials. Identified solutions included use of the simplest agreement method possible and compliance with best-practice licensing strategies, such as the use of the UBMTA and SLA, standing reciprocal MTAs, and simple ‘conditions-of-use’ for academic research.
MTAs used in exchanges between researchers
Academic researchers associated MTAs with significant delays, which were problematic when considered within research funding and productivity timeframes. Researchers felt that the delays were unacceptable because most of the research was firmly within the pre-competitive research environment and of no commercial value.
The vast majority of MTAs are a complete waste of time, and they delay research for really no commercial benefit to anyone. And this is based on 20 years of working as a mouse geneticist. Somebody like me is funded by grant applications. So any delay, I never ever get that time back. [Researcher/End-User #1]
A focus on commercialization, specifically patenting, diminished the value of the resources supplied for research because required data were withheld.
I was working with [Scientist] who did stem cell research in [United Kingdom (UK) University] and was very involved in commercialization. I found it frustrating because you couldn't get the information you needed out of him because it was in a patent application. So, while he's asking me to use his ES-cells [Embryonic Stem cells] to go down differentiation routes towards neural differentiation, he wouldn't tell me what was in the media or any of the genetic background of the cells or anything because they were all in the process of being patented. [Resource Maker #2]
Academic researchers suggested that the process of sharing materials with noncollaborators was more complicated than with collaborators, because the latter posed no immediate competition.
So about half the time I'm contacted for this special mouse line [name], distributed under an MTA from [Canadian University], which has a breeding colony. I forward the request to the [Canadian University], who vets the request to ensure that the people who want the mice are just duplicating an experiment that had already been done…When we give the mice to collaborators the process is really more to do with health status or it involves exchange of information, of serology status of the animals between our facility and the receiving facility. [Researcher/End-User #4]
Researchers felt the need to control the timing and conditions for the distribution of materials because of the effort and expense of making mouse lines “in-house”. They were more likely to share materials when the distributors ascertained the absence of direct competition or post-publication. Such considerations necessitated the use of MTAs that restrict onwards distribution and field of use.
Sometimes we're still using those mouse lines for our own research and we would like to know who is going to be using it to see whether they're potential competition…It takes so long to prepare those lines, to generate those lines and it's so expensive that we're sure that we have some ownership of the results, right, that we can have some control what's going on. We want to make sure that there is no overlap to what we are doing or if there is I mean we're doing it in a collaboration. Even if they are going to compete, we always provide the mouse lines, but as long as they have been published before. [Researcher/End-User #8]
Material transfers from industry were especially problematic because of reach-through claims, delayed transfers, and high transaction costs.
The worst example of this we had was when we were trying to import an Alzheimer's model from [Company]. Our university technology transfer office had spent SIX months [emphasis in recording] of my 3-year grant. So that's time that's gone. Finally my university would not allow me to import the mice. They were right because these mice had been produced by an academic lab in North America who then sold it to [Company], and the [Company] allowed people to have these mice. But if you breed the mice, and this is what I as a geneticist wanted to do, then the progeny animals become owned by [Company] along with any IP. [Researcher/End-User #1]
Onerous terms in MTAs made researchers consider alternative research strategies to meet deadlines, serve the interests of junior laboratory members, and sidestep IPRs.
Getting the mice has become so onerous in the last 5 years, I am looking for alternatives. It is going to be a year and a half long thing and if you're a graduate student and you have to get out with your PhD in a timely fashion we're going to look for another way. Maybe it's not as good as getting the mouse but it's an alternative that we can actually do in a reasonable timeframe. [Researcher/End-User #5]
The reason we started working with FLP recombination [FLP-FRT, a recombination technology, analogous to Cre-Lox (Schlake and Bode, 1994)] is I tried to work with Cre and DuPont sent me this utterly aggressive absurd MTA which basically said that anything I did with the Cre coding sequence was DuPont property and that I would have no rights under any circumstances. I said okay I'm going to work with FLP because in those days no one knew if Cre was going to be better than FLP or not. [Researcher/End-User #7]
Consistently, academic researchers reported friction with technology transfer offices over extended MTA negotiations and delayed transfers. Researchers felt that TTOs caused unnecessary interference in the academic culture of “gifting” materials to colleagues. Researchers criticized their TTOs for being overly risk-averse, which, when combined with an inadequate grasp of scientific method and academic culture, impeded the ability to share research reagents. Indeed, academics felt that the focus of TTOs on commercialization of research outputs, including research tools, was inconsistent with the mission of universities to generate and disseminate knowledge, while appropriately seeking IPRs over inventions closer to clinical or practical application. The TTOs were overly focused on potential commercial gains, which were unlikely to arise from pre-competitive research tools. This was especially the case when the exchange was between researchers at academic institutions. Thus the lengthy timelines and complex negotiations merely interfered with academic research that was highly unlikely to result in the generation of revenues for the institution. The delays caused many researchers to bypass their TTOs and formally negotiated inter-organizational MTAs.
If it will take a year or six months to straighten out the MTA, I will send things before they're signed which is completely not what you're supposed to do. I just e-mail people and ask them and some require MTAs and some don't. I imagine in every case probably their institute would require an MTA if they knew. [Researcher/End-User #5]
TTOs, for their part, saw themselves as facilitating translational science and serving innovation systems by linking researchers, industry, and healthcare organizations. TTO representatives expressed frustration with researchers who avoided formal transfer routes.
We rely on the PIs to call us and say, hey I need something, which is a lot of them. But some sign the thing like they're not supposed to. First of all I think there's a legal obligation that they need to come to us. [TTO #3]
TTO representatives felt that sharing of data and materials are greatly facilitated through use of the NIH UBMTA. Unfortunately, to the frustration of many representatives of TTOs and funding agencies, local practices commonly deviated from the standards set by the NIH.
If the MTA language does adhere to the UBMTA then it doesn't take any time—there's no negotiation required. [TTO #1]
Some tech transfer offices give you an MTA with all sorts of constricting language. And sometimes institutions sign them and it perpetuates the idea that they can continue to do that. I say “don't call this UBMTA because it's not. It's something else that you rebranded for your institution.” [Funder #2]
TTOs also raised the issue of ability to negotiate terms of MTAs versus ability to monitor the terms. TTOs have limited resources to monitor the terms of use of materials and development milestones once they are sent to the recipient. This negates the value of complex licensing agreements, which require enforcement of terms.
The challenges of monitoring compliance are the lack of systems to do so. Right now, the only way to monitor compliance is to have a database with the time points for monitoring compliance for the duration of agreement. If you have a 3 year MTA, one should follow up in 3 years to make sure the material is no longer being used. For licensed agreements [with commercial users], there needs to be follow-up on payments. All this should be in one database so that any individual could see, get reminders about what's happening. It's impossible for any contract research or tech transfer office to manage that volume of contracts without a well-structured database. [TTO #1]
Finally, TTO representatives acknowledged that any licensing terms for research tools should be commensurate with their potential commercial value. Indeed, some TTOs, such as at the University of British Columbia (UBC, 2009, 2013), have a policy not to seek IPRs over mouse models and instead encourage their submission to a repository such as JAX. Representatives of TTOs sensibly recognized that most mouse-related research reagents were neither unique nor commercially valuable, a notable exception being the VelocImmune™ technology of Regeneron Pharmaceuticals (New York and New Jersey, US), which is a mouse model for producing monoclonal antibodies (Valenzuela et al., 2003). Therefore, most institutions licensed mouse models nonexclusively and without reach-through terms, if at all.
Almost all of these tools now, be it a mouse or reagent are done nonexclusively. Fifteen years ago when these mouse models were super unique, you could license to somebody exclusively. Now if the university is sending the company some mice you won't get a reach through. [TTO #3]
Regeneron's immune model for making humanized antibodies obviously that's made a lot of money for them but I think overall if you are just going to knockout a gene and make a mouse I don't think people see that as a very profitable way to make money by putting in licenses that are restrictive to the use of it. [Industry representative #1]
MTAs for the deposit of materials into bio-repositories
As pillars of the mouse research commons, repositories have implemented policies for data and materials sharing and have attempted to incentivize best practices within the research community. Repositories vary in structure in (1) accepting deposits from the research community following expert review of criteria, such as quality, novelty, and potential interest from the research community; (2) accepting deposits of reagents resulting from a specific project, such as the KOMP; and (3) not accepting deposits but distributing only those reagents generated by the repository-associated production project/facility (Table 1). Repository managers described bio-repositories as supporting open access to publicly generated research reagents, a stated policy of funding agencies. Those repositories that accepted deposits provided: “a big advantage for the small labs. They don't have to set up their own cryopreservation facility, we will do the quality control and we will also do distribution for laboratories [Repository Manager #1].” Repositories additionally relieved depositors of the burden of distribution, which included the negotiation of distribution agreements, such as MTAs, “with which researchers are not always familiar” [Repository Manager #2].
Researchers, however, felt that the financial and time costs of depositing materials in bio-repositories were disincentives. Indeed, most deposit MTAs (with some exceptions such as the Harwell Frozen Embryo and Sperm Archive (FESA), part of the European Mouse Mutant Archive (EMMA) network), specified that the donor bore the cost for the deposit into the repository (Table 1; FESA, 2013).
I've been asked by JAX to, you know, open access to other researchers and we do provide mouse lines to other researchers when they request. But I haven't had really the time and the resources to look at the JAX because…I have to pay to deposit mice for other people to use. That's a complication for me. [Researcher/End-User #8]
Repository managers and consortium directors stated that increasing deposit of, and therefore access to, materials needed a shift in culture, supported by funding agencies such as the NIH and the Wellcome Trust, which already have policies to that effect. In addition, journals could require evidence of deposit, such as a repository number, prior to publication. This could mean repositories have to accept pre-published strains. However, not all repositories accept pre-published strains. Repositories that accepted deposits from the community generally had policies encouraging depositors to agree to unrestricted donation, including standard simple conditions for distribution to nonprofit or academic institutions (Table 1). Conversely, highly variant MTAs hampered deposit of materials and created an administrative burden for repositories. This is because MTAs for the deposit of materials generally dictate the terms under which those materials may be distributed for noncommercial and/or commercial research. A standard deposit MTA simplifies the onwards distribution of materials, the core business of repositories.
We have a standard MTA that we encourage depositors to sign but we don't enforce this. It's not unusual for depositors to want us to agree to their MTA. So we have several variants of MTAs for the stocks we hold in the archive and this creates an administrative burden for us. [Repository Manager #1]
However, most deposit MTAs enabled depositors to restrict distribution to for-profit entities or for commercial use (Table 1) and, in special circumstances, to designate the terms for onwards distribution for all users (Table 2).
MTAs for the distribution of reagents by bio-repositories
Resource makers as well as directors and managers of repositories agreed that access should be made as simple as possible for nonprofit and academic institutions. For example, JAX distributes materials to academic researchers with notification of two simple conditions: that the mice are for research use and are not for sale or transfer to third parties without permission. Other repositories have similar policies to enhance distribution and uptake of resources (Table 2; Supplementary Table S1).
For the NorCOMM resource [North American Conditional Mouse Mutagenesis Project; http://www.norcomm.org/index.htm] there's two avenues to request. For nonprofit investigators we have worked out a Conditions of Use without formal MTA's. [Repository Manager #3]
BayGenomics [Stryke et al., 2003] was an NIH-funded program to generate a large gene trap resource. There were no MTAs on those reagents and [the resource] gave away cells to the research community. Lots of papers came from that resource in terms of assigning function to gene in mice. [Resource Maker #5]
Some repositories use a blend of conditions of use and MTAs, depending on the deposit agreement.
For 13 years the Mutant Mouse Regional Resource Center [MMRRC] has taken in mutant mice people have created in their laboratories, archived them, and then distributed them to the academic research community. In some cases there are MTAs. In some cases it is just a simple online conditions of use statements that they need to check often. It has not hindered anyone to access the lines. [Consortium Director #5]
Other repositories, because of funding and institutional requirements, use more complex MTAs, which range from those based on the UBMTA to more complex standard-form MTAs (Table 2; Supplementary Table S1). These MTAs are designed to promote the distribution of materials as well as the sustainability of the repositories. For example, the KOMP repository, the official archive and distribution centre for KOMP at University of California (UC), Davis, uses an MTA based closely on the UBMTA, which has not been “a major hurdle at least from the KOMP perspective” [Consortium Director #5].
Standard terms that enhance the commons include attribution of the resource or the donor in publications. Such attribution is important for the long-term sustainability of repositories, because it is a metric for utility of the resource and directs new users to repositories, as users may want either to reproduce mouse-model research or conduct novel research on the same model. Ensuring attribution, however, requires building goodwill with the user community, since monitoring and enforcement are impractical.
We always ask for the originators to be acknowledged but we are not policing that particular aspect of mouse usage. I don't think we can really. It's all based on goodwill…we don't need much, we are not asking for co-authorship, just say in the acknowledgements that the mouse was sourced from [Repository] or was generated as part of [Consortium project], that's all. It would be nice to take that a step forward and give a link to a website from where the mouse could be sourced…Like the extremely useful IMSR, the International Mouse Strain Resource [http://www.findmice.org] [Repository Manager #1]
Generally there are differential access arrangements with noncommercial researchers in academic institutions and commercial researchers (Table 2; Supplementary Table S1). For example, while JAX distributes freely to academic researchers, it acts as a broker between depositors and commercial users. Other repositories directly distribute to commercial entities or prohibit distribution to commercial entities entirely (Table 2; Supplementary Table S1). The KOMP MTA is unusual in its broad applicability, including “research directed toward the discovery, development or commercialization of therapeutic and diagnostic products…[of research institutions of commercial entities]…whether or not resulting in patentable inventions and whether or not published” (Knockout Mouse Project (KOMP), Repository, 2013; Table 2; Supplementary Table S1). It only excludes contract research defined as “fee-for-service conducted for the benefit of a third party.” In contrast, the MTA used by repositories to distribute resources of the European Conditional Mouse Mutagenesis Program (EUCOMM), for example, allows distribution only to noncommercial researchers.
The MTA issue with the EUCOMM mice is that they can't be distributed to the private sector…we were never able to come up with an MTA that's agreeable across EUCOMM and KOMP. [Funder #1]
Industry can take the NIH KOMP mice but not EUCOMM mice…Most of the resources made from the NIH resources are not a problem because the way that project started out, is that there was a mandate from NIH that these resources would be made available under very simple MTA. Unfortunately, when EUCOMM started, it's that the MTA almost became an afterthought…and they were multiple countries, it was a European initiative. So the MTA is much more restricted. [Consortium Director #1]
EUCOMM accounts for nearly half of the anticipated IKMC resources and over 44% of the ES cells currently available, excluding the Texas A&M Institute for Genomic Medicine (TIGM) gene trap resource (Bradley et al., 2012). Inability to access IKMC resources generated by EUCOMM has frustrated industry representatives, which may have a negative impact on the long-term sustainability of repositories. Without industry support and users, funding of infrastructure to support the mouse research commons rests with public funders. This hampers the development of business plans for repositories based on engagement with both noncommercial researchers and industry.
Someone from [Pharmaceutical Company] asked me whether I could help him connect with EUCOMM because he had been sending multiple emails asking for clones a couple genes of interest. At first they said, “We're working on putting in place a process to provide these for profit” then they stopped writing. He was frustrated with the lack of response. Then I followed up on my end and they said that the IP is so complicated that there's no way at this point they can provide access to full profit. So that door seems to be closed at least for now. [Industry Representative #1]
The current MTA for the EUCOMM ES cells prohibits [distribution to] for-profit organizations [for use in] in house research program and develop drugs or treatments. Until that is resolved we can do all the industry outreach we want, we can get them as excited as we want. It's important to have industry engaged, but until the MTA issues are resolved, we can't actually facilitate industry goals because they can't access those lines. [Repository Manager #3]
There are, however, legal and structural differences between European countries and the United States that complicate industry relationships in the former. These complications are magnified with the development and then distribution of high-throughput resources such as those of the IKMC because of the complexity in methods and processes required to construct the resource and the need to aggregate the associated IPRs within the pipeline. It is very difficult to identify all underlying IPRs to negotiate rights upfront (ex ante). The risk is therefore of submarine patents held mainly by small biotechnology companies or research institutions where rights to use and associated royalties may need to be negotiated once the valuable resource has already been created and is ready for distribution (ex post). Restricting use by distributing only to noncommercial entities, therefore, reduces the risk of patent infringement litigation, because it reduces the value of the resource and the amount of royalties that could be claimed by a submarine patent holder.
There are background IP issues in the tools we are using, which might complicate the situation. Who is going to protect us, the academic world, if a company tries to sue us? Some companies wait until there is something going on of great value and then they may come out of their holes. [Consortium Director #2]
In the United States, the contract from the NIH to develop the KOMP resource was structured to shield the resource developers from patent infringement litigation. The powerful legal tool most commonly used in defense contracts, known as “Authorization and Consent” under the Federal Acquisition Regulations, immunizes Federal Contractors from patent infringement suits (Lavenue, 1995; US Government, 2013). It enables Federal contractors to utilize any invention patented in the United States and substitutes the federal government as defendant in the unlikely event a patentee still wishes to pursue a claim of infringement.
Fortunately, the NIH has the authority to grant some waivers to grantees to sort of protect them from litigation. Because we've decided that those materials should be available to commercial entities, we've extended those waivers to our grantees, but the Europeans funders are not able to do that apparently. So we still haven't resolved that, which is a big thing for us. We would prefer that the material produced in Europe be available to all parties for the sake of research. You can't get into drug development unless you involve commercial parties. [Consortium Director #4]
A further discrepancy between the KOMP and EUCOMM MTAs is a “reach-through” clause, which is contrary to internationally recognized licensing best practices.
The EUCOMM-compliant MTA used by a production centre must include a liability clause that indemnifies EuCOMM (not a problem), must deposit modifications to a public repository (also not a problem), and entails the production centre's institution to enforce reach-through on all modifications it distributes that provides a lifetime, royalty-free license for academic and teaching purposes on behalf of Sanger [The Wellcome Trust Sanger Institute; WTSI] and Helmholtz (the original ‘owner’ of the ES cells) [Consortium Director #3]
Ironically, the “reach-through” clause is intended as an extension of the practice of retention of rights for noncommercial research purposes and could therefore be considered as enhancing the pre-competitive research environment and the mouse research commons. Nevertheless, the same criticism applies to all reach-through clauses—that they extend rights beyond what may fairly be attributed to the inventor/originator of the materials. The clause entitles the resource originator (the legal entity behind the repository for the EUCOMM resource, the Helmholtz Zentrum Munchen) to a worldwide, nonexclusive, royalty-free, sublicensable and fully paid-up license to use, for noncommercial and teaching purposes, any IPRs that arise from the recipient's use of the EUCOMM material. In other words, if the recipient develops a drug based on its research using EUCOMM material, then it must grant a license to the Helmholtz Zentrum Munchen on the terms specified in the EUCOMM MTA. However, the ability of repositories to monitor and then enforce this clause is questionable, and its complexity and presence may serve as a disincentive for potential users.
Differences in MTAs create administrative complexities for repositories that distribute different resources under different terms. For example,
The EUCOMM MTA does not allow any supply from our project to for-profit. The KOMP MTA allows [our project] to distribute to for-profit but requires the requester to pay US$15000 per clone to Regeneron through the KOMP repository; not a simple or clear process. [Consortium Director #3]
On the practical side, rather than substantive content of MTAs, participants described the problems from the use of multiple MTAs between repository and receiver. They suggested that a possible solution involves one blanket MTA to facilitate material flows.
Timelines for academic institutions versus industrial institutions can be very different. The complaint was that it takes sometimes too long as it can take months from the order until the receipt of the clone. [Industry Representative #1]
Every time KOMP distributes a line to an investigator, their institutional official has to sign an MTA. They can download it from the website. It is already signed by our institutional official but what the receiver needs to do is have their institutional official sign it and send it in, and then we can fulfill an order. But a second investigator totally unrelated to the first but at the same institution says, “my institution has already signed an MTA, I do not want to have to have to sign it the second time.” So the fix is that if that [receiving] institution has already signed it there is no need to wait around to have that institutional official sign a second time. [Consortium Director #5]
MTAs and exchange of materials within international resource development consortia
Thus far, we have discussed MTAs amongst researchers, and the flow of materials between researchers/resource generators and repositories. However, MTAs also play a role within consortia and remain problematic in the context of legal interoperability, discussed above when MTAs attach to transferred materials and must then be adhered to for onwards distribution by a partner repository to end-users. IMPC directors agreed that standard MTAs could optimize the movement of materials and associated data between consortium members. However, consortium directors described operational challenges resulting from variable MTAs drafted in jurisdictions within Europe, North America, Australia, and Asia. They ascribed disagreements over MTAs to the risk-aversive approach of institutional legal personnel, whom they viewed as distanced from the realities of the consortium's operational needs.
We now have to deal with potentially thousands of MTAs, which almost on its own would kill the system…But all the MTA issues are not about people trying to make money or trying to turn a profit. But the institutions have a fear of responsibility and liability. The materials transfer officers and the legal groups in these other countries are afraid that they'll leave something out and then they're going to lose their jobs. [Consortium Director #1]
The EUCOMM MTA is a particular challenge because there are so many institutions involved…You unnecessarily weigh too many people in on a project and all their institutions and then you have to agree on MTAs which is going to be really a challenge…Once you get into institutional differences with different tech transfer offices, legal departments and whatnot, you run into roadblocks from people who are not [as] committed to a project as a PI might be. [Consortium Director #4]
Participants agreed that project MTAs need standard terms for overall efficiency. MTA volumes and related processing labor could be reduced by standardization and single agreements for multiple transfers between consortium members. Participants differed, however, in their views on the challenges of coordination of materials distribution and phenotyping efforts in the IMPC. Some interviewees believed that distribution challenges were adequately addressed, others felt varying MTAs added a layer of difficulty to growing demands on the capacity of repositories to hold ES cells and distribute them to phenotyping centers. However, at least amongst consortium members, distribution challenges seemed more related to practical challenges of managing a global resource rather than MTAs.
So far distribution doesn't seem to be a major problem. We're almost years into the project and we've been tracking that, and it seems for our centres it's working fairly well…[Consortium Director #4]
The main nonscientific challenge for building resources on the scale of the IMPC endeavour probably boils down to coordination…to make sure that everyone is consistently collecting data in the same way, in an interchangeable way, handing that data the same way using the same types of assays and procedures and processes, cataloguing it, uploading it to open resources and to assure that is all happening in a coordinated way so we're not needlessly duplicating efforts. [Funder #3]
Discussion
Legal agreements, including MTAs, play a central role in the governance of research commons in support of pre-competitive mouse model research. Such agreements are key tools in incentivizing participation in research commons, and, if appropriately managed, may encourage participation and enhance the availability of data and materials for research. Legal agreements may enhance the efficiency and sustainability of commons by facilitating core actions of contribution, use, and recontribution of research materials and data, creating the required “network effect” (Bubela et al., 2012; Schofield, et al., 2010). In this manner, legal agreements should be supportive of funding agency policies for data and materials sharing and should foster community norms to enhance the commons. Indeed, funders and journals play a pivotal role in incentivizing, monitoring, and enforcing policies embodied in legal agreements that enhance the commons (Box 1). Unfortunately, as shown in our empirical study, legal agreements may instead cause frictional drag within the system, requiring significant work-arounds for transactional bottlenecks (Merges, 2011).
Box 1. Governance of Research Commons: Key Lessons from Materials Sharing in Mouse Genomics.
• Research infrastructure, such as databases and bio-repositories, requires effective governance to support data and materials sharing within pre-competitive public and private research initiatives that enhances the translation of research into application.
• A myriad of data and materials sharing policies exists, largely promulgated by public funding agencies, but implementation and enforcement of such policies remains problematic.
• Material Transfer Agreements (MTAs) are central mediators in exchanges of data and materials and should embody policies and guidelines that incentivize data and materials sharing and foster related community norms.
• Complex MTAs and protracted negotiations by institutional actors, including Technology Transfer Offices, frustrate the sharing of materials and data amongst researchers.
• Standard and simple agreements, such as the National Institutes of Health Universal Biological Materials Transfer Agreement or the Simple Letter Agreement, decrease the administrative burden for repositories, institutions, and researchers alike.
• Simplified procedures, such as those that enable the distribution of reagents to multiple researchers within an institution, also reduce transactional bottlenecks.
• Reach-through rights are contrary to licensing best practices because they increase transaction costs by attaching obligations to derivatives and may even require a recipient institution to enforce terms for the benefit of the originator of materials and data.
• In general, it is contrary to best practices and good governance of omics resources to insert terms in legal agreements that create transactional bottlenecks and that are unlikely to be monitored or enforced.
• Established large-scale resource generation projects, repositories, and databases have the opportunity to develop governance mechanisms, including standardized and simplified MTAs, that implement the data and materials sharing policies of key funding agencies, concurrently building capacity in research management with partner or recipient institutions.
At the level of the community, complex MTAs and protracted negotiations by institutional actors, including TTOs, frustrated the attempts of researchers to share materials with colleagues. Community norms for sharing were strong in the mouse model community but were balanced against concerns about free-riders and academic/publication priority. Researcher criticisms of MTA processes resonate with the statement of a group of leading North American universities: “a specific MTA is not required when our investigators and their research colleagues elsewhere are exchanging nonhazardous or nonhuman biological materials for in vitro research use” (University of British Columbia, 2009). The UBC University Industry Liaison Office recommends the use of MTAs only in specific circumstances, such as when the giver requires an assurance of acknowledgment in publications or when there are concerns as about confidentiality, revenue from commercialization, and the involvement of third party rights in the material (University of British Columbia, 2013). TTOs recommended the use of standard agreements, such as the NIH UBMTA or the SLA to enhance administrative efficiency.
Biological repositories and databases also enhance efficiency in the storage and dissemination of materials and data, respectively. Repositories relieve the depositor of the burden of sharing through a single deposit and management of onwards distribution to third parties. These resources therefore represent essential infrastructure supportive of the research commons, and their role is simplified through simple deposit agreements that enable distribution on standard terms, especially to the nonprofit or academic research sector. Most repositories enable some variation in deposit terms, most notably, restrictions on onwards distribution to for-profit entities. Further variability in deposit terms, however, creates legal interoperability issues since terms of deposit attach to terms for distribution. It is therefore crucial to develop community norms for simplified terms of deposit, which are easy to use for creators of research reagents.
Terms should be carefully analyzed with respect to their ability to enhance the commons. For example, terms that request attribution of the originator may incentivize deposit, since the depositor is credited as the originator of the resource. This is in line with community citation norms for academic publication, and attribution could be used by institutions and funders as a performance metric in the same manner as a publication citation. Acknowledgement of the source repository directs other researchers to the repository and enhances visibility and profile, both important factors in sustainability models for repositories. However, attribution may be problematic in some circumstances if the obligation reaches through to derivative products, leading to a phenomenon called “attribution stacking.” This phenomenon occurs when the blending of datasets or materials, especially those licensed in a similar way, raises dilemmas about the extent of citations required at the point of use or re-use of the blended sets (Ball, 2012; Korn and Oppenheim, 2011).
One disincentive for deposits from the research community, however, is that the cost of the deposit is most commonly borne by the researcher. Funding agencies could incentivize deposit, therefore, by requiring specific line items in budget covering the cost of deposit for resources generated using public funds. Some repositories provide additional incentives. For example, the KOMP Repository's “Sharing Plan” for academic researchers offers them up to 50% refunds on purchases of ES cells and chimeras. These refunds are conditional on the users sending KOMP “at least two germline-confirmed, heterozygous mutant male mice on a congenic C57BL/6N genetic background” generated from KOMP ES cells and chimeras. The mice must be repatriated within 18 months to a year of purchase of the original ES cells and chimeras, respectively (Knockout Mouse Project (KOMP) Repository, 2013). The EMMA network offers free cryopreservation of mouse embryos and sperm via its funded resources such as the Harwell FESA (FESA, 2013). The RIKEN Bioresource (RIKEN BRC) in Japan is unusual in covering shipping costs and offering depositors credits on future purchases from the resource (RIKEN Bioresource, 2013).
In terms of distribution of research reagents by repositories, whether these be generated by individual researchers or by large-scale resource production centers, standard and simplified legal agreements decrease the administrative burden for repositories, research institutions, and researchers alike. Simplified legal agreements facilitate distribution to the research community, as well as the transfer or materials between repositories and within research consortia like the IMPC. Many authors have advocated for the use of simple “conditions of use” as alternatives to MTAs, such as those used by JAX and the Canadian Mouse Mutant Repository (CMMR) (Bubela et al., 2012; Schofield et al., 2009). Alternatively, the terms of MTAs should be as simple as possible, such as the KOMP MTA based on the UBMTA. In addition, standing reciprocal agreements for multiple inter-institutional transfers may enhance distribution. For example, the Harvard Stem Cell Institute (HSCI) enhanced distribution by using one agreement for multiple transfers to a receiver (McCormick et al., 2009). In practical terms, standard electronic MTAs are most amenable to rapid processing.
The most controversial issues raised by our interviewees were distribution by repositories to for-profit entities, reach-through rights, and restrictions on the use of materials, such as the breeding of mice. On the latter issue, Schofield et al. (2009) recommended that researchers be free to breed mice received from repositories or engage in similar activities to derive novel reagents. In terms of distribution to for-profit entities, JAX serves as a model, acting as a broker for the distribution of reagents to industry once an agreement is in place between the depositor and the industry end-user (Einhorn and Heimes, 2009). Other repositories that distribute production-center resources, such as KOMP, also enable distribution to industry. This will likely enhance the sustainability of the resource, which may generate a source of revenue through differential pricing between nonprofit and for-profit entities. Establishing a viable business model is essential for the long-term viability of all repositories, which may not be able to rely on public funds in the long term. In contrast, restrictions over repositories to distribute EUCOMM resources to industry may jeopardize the long-term viability of the resource.
Reach-through rights have been recognized in a number of policy statements as being contrary to best practices for licensing (AUTM, 2007; OECD 2006). We argue that reach-through is an inappropriate practice for all actors, including publicly-funded repositories. Reach-through rights increase transaction costs by attaching obligations to derivatives and may even require a recipient institution to enforce terms for the benefit of the resource originator. Such complexity delays the negotiation of MTAs. Further, in practical terms, repositories and academic institutions, in particular, have limited resources to monitor and enforce MTAs. Indeed, searches of legal databases indicate that globally there is virtually no litigation (a sign of enforcement) of MTAs. Therefore, it is contrary to best practices and contrary to good governance of the commons to insert terms that create transactional bottlenecks and that are unlikely to ever be monitored or enforced.
Finally, however, we acknowledge that the international nature of large-scale bioresources adds a layer of complexity to the negotiation of legal agreements, with different contracting cultures coming into play. Different IP rules in different jurisdictions may impose some terms, and may prevent distribution to for-profit entities. The NIH has a long history of stepping in to negotiate transactional blockages, evidenced by our discussion of Cre-lox and OncoMouse, for NIH-supported researchers. It has an arsenal of tools ranging from Acquisition and Consent in the Federal Acquisition Regulations for government contractors (Lavenue 1995; US Government, 2013), in this case, the production centers for mouse reagents to March-in rights under Bayh-Dole (Thambisetty, 2007:262) and compulsory licensing provisions (Maybarduk and Rimmington, 2009; Reichman, 2009). While a detailed analysis of these mechanisms is beyond the scope of this article, it is important to note that absence of such mechanisms in other jurisdictions may set limits on the terms of distribution for resources, especially to industry, if repositories are concerned about underlying IP used in the generation of large-scale resources.
Conclusion
Legal agreements are the embodiment of policies to support the pre-competitive research environment in “omics” fields. Effective design and management of legal agreements, therefore, are essential for the efficient governance of the research commons that includes essential infrastructure such as databases and biorepositories. Our study of the mouse research commons indicates that legal agreements, especially MTAs, the deposit and distribution of research reagents should be kept as simple and standardized as possible, especially since minimal capacity and resources exist to enforce the terms of MTAs. Standardized MTAs and simplified procedures, such as those that enable the distribution of reagents to multiple researchers within an institution, reduce transactional bottlenecks and facilitate the creation of a vibrant and sustainable research commons.
Consortia, such as the IKMC and IMPC, and established repositories, such as JAX, present an opportunity to develop governance mechanisms, including standardized and simplified MTAs, that implement the data and materials sharing policies of key funding agencies. These organisations engaged in the research commons have the opportunity to lead by example, concurrently building capacity in research management with partner or recipient institutions, especially in academia.
The mouse research community represents not only a model for research into human diseases, but also a model for the effective governance of research reagents. Biobanks for human tissue and associated databases confront many of the same issues, with the added layer of complexity of donor consent. Lessons may, nevertheless, be taken from the mouse research commons in the avoidance of bottlenecks that unnecessarily complicate the flow of data and materials in the pre-competitive research environment. Future research should focus on the continued uptake and effectiveness of data and materials sharing mechanisms within the mouse commons and their applicability to other model organism and human research communities.
Supplementary Material
Abbreviations Used in Article
- AUTM
Association of University Technology Managers
- BMS
Bristol-Myers Squibb
- CMMR
Canadian Mouse Mutant Repository
- CoU
conditions-of-use
- EMMA
European Mouse Mutant Archive
- EUCOMM
European Conditional Mouse Mutagenesis Program
- EuMMCR
European Mouse Mutant Cell Repository
- FESA
Harwell Frozen Embryo and Sperm Archive
- HSCI
Harvard Stem Cell Institute
- I-DCC
International Data Coordination Center
- IKMC
International Knockout Mouse Consortium
- IMPC
International Mouse Phenotyping Consortium
- IMSR
International Mouse Strain Resource
- IPRs
intellectual property rights
- JAX
Jackson Laboratory
- KOMP
Knockout Mouse Project
- KOMP DCC
KOMP Data Coordination Center
- mESC
mouse embryonic stem cells
- MMRRC
Mutant Mouse Regional Resource Center
- MOU
memorandum of understanding
- MRC
Medical Research Council
- MTAs
material transfer agreements
- NIH
National Institutes of Health
- NIH OTT
NIH Office of Technology Transfer
- NorCOMM
North American Conditional Mouse Mutagenesis Project
- OECD
Organisation for Economic Co-operation and Development
- R&D
research and development
- RIKEN BRC
RIKEN (Rikagaku Kenkyūjo) Bioresource
- SLA
Simple Letter Agreement
- TIGM
Texas A&M Institute for Genomic Medicine
- TTO
technology transfer office
- UBC
University of British Columbia
- UBMTA
Uniform Biological Material Transfer Agreement
- UC
University of California
- UK
United Kingdom
- US
United States of America
- WTSI
Wellcome Trust Sanger Institute
Abbreviations Used in Tables 1–4 and Supplementary Table S1
- APB
Australian Phenome Bank
- APF
Australian Phenome Facility
- APN
Australian Phenomics Network
- BaSH Consortium
Baylor College of Medicine; The Wellcome Trust Sanger Institute and MRC Harwell Mary Lyon Centre.
- CARD
Center for Animal Resources and Development
- CFI
Canada Foundation for Innovation
- CHORI
Children's Hospital Oakland Research Institute
- CIHR
Canadian Institutes of Health Research
- CMHD
Centre for Modeling Human Disease
- CMMR
Canadian Mouse Mutant Repository
- CoU
conditions-of-use
- CRC
Charles River Canada
- CSD Partnership
Children's Hospital Oakland Research Institute (CHORI), the Wellcome Trust Sanger Institute (WTSI) and the University of California (UC) at Davis School of Veterinary Medicine
- DTCC Consortium
UC Davis, TCP, CHORI and Charles River Laboratories
- EBI
European Bioinformatics Institute
- EMBL
European Molecular Biology Laboratory
- EMMA
European Mouse Mutant Archive
- ES cells
embryonic stem cells
- EuCOMM
European Conditional Mouse Mutagenesis Program
- EUCOMMTOOLS
EUCOMM Tools for Functional Annotation of the Mouse Genome
- EuMMCR
European Mouse Mutant Cell Repository
- FESA
Harwell Frozen Embryo and Sperm Archive
- FIMRe
Federation of International Mouse Resources
- GC
Genome Canada
- HSC
Hospital for Sick Children Research Institute (‘SickKids’)
- ICSC
Informatics, Coordination and Service Center
- I-DCC
International Data Coordination Center
- IKMC
International Knockout Mouse Consortium
- IMPC
International Mouse Phenotyping Consortium
- IMSR
International Mouse Strain Resource
- JAX
Jackson Laboratory
- KFDA
Korea Food and Drug Administration
- KOMP
Knockout Mouse Project
- KOMP DCC
KOMP Data Coordination Center
- MARC
Model Animal Research Center
- mESC
mouse embryonic stem cells
- MGI
Mouse Genome Informatics
- MMRRC
Mutant Mouse Regional Resource Center
- MOU
memorandum of understanding
- MRC
Medical Research Council
- MTAs
material transfer agreements
- NHMRC
National Health and Medical Research Council
- NIFDC
National Institutes for Food And Drug Control, China
- NIH
National Institutes of Health
- NorCOMM
North American Conditional Mouse Mutagenesis Project
- OIT
Ontario Innovation Trust
- RIKEN BRC
RIKEN (Rikagaku Kenkyūjo) Bioresource
- SLA
Simple Letter Agreement
- SLRI
Samuel Lunenfeld Research Institute
- TCP
Toronto Centre for Phenogenomics
- TIGM
Texas A&M Institute for Genomic Medicine
- UC
University of California
- UK
United Kingdom
- US
United States of America
- WTSI
Wellcome Trust Sanger Institute
Acknowledgments
We thank Rhiannon Noble, Lesley Dacks, Noelle Orton, and Camille Ryan for assistance with background research. We thank the interviewees who took time out of their busy schedules for interviews and for insightful comments on the manuscript. We thank Dr. Lauryl Nutter and Dr. Ann Flenniken for expert input and research support. Dr. Mishra's postdoctoral fellowship is funded by the NorCOMM2 Project funded by Genome Canada. Dr. Bubela's research is supported by the NorCOMM2 Project funded by Genome Canada, and the Ontario Genome Institute (Co-Lead Investigators: Colin McKerlie and Steve Brown) as well as the Canadian Stem Cell Network.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Adams D, Baldock R, Bhattacharya S, et al. (2013). Bloomsbury report on mouse embryo phenotyping: Recommendations from the IMPC workshop on embryonic lethal screening. Dis Models Mech 6, 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghion P, Dewatripont M, Kolev J, Murray F, and Stern S. (2010). Innovation and open science: The public and private sectors in the process of innovation: Theory and evidence from the mouse genetics revolution. Am Econ Rev Papers Proc 100, 153–158 [Google Scholar]
- Association of University Technology Managers, (2007). In the Public Interest: Nine Points to Consider in Licensing University Technology. https://www.autm.net/Nine_Points_to_Consider.htm Accessed January23, 2014
- Association of University Technology Managers (2013) Uniform Biological Materials Transfer Agreement (UBMTA). http://www.autm.net/Technology_Transfer_Resources/8395.htm Accessed January23, 2014
- Ball A. (2012). How to License Research Data. Edinburgh: Digital Curation Centre; http://www.dcc.ac.uk/resources/how-guides Accessed January23, 2014 [Google Scholar]
- Bergman K, and Graff GD. (2007). The global stem cell patent landscape: Implications for efficient technology transfer and commercial development. Nature Biotechnol 25, 419–424 [DOI] [PubMed] [Google Scholar]
- Bradley A, Anastassiadis K, Ayadi A, et al. (2012). The mammalian gene function resource: The international knockout mouse consortium. Mammal Genome 23, 580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, and Moore MW. (2012a). The International Mouse Phenotyping Consortium: Past and future perspectives on mouse phenotyping. Mammal Genome 23, 632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, and Moore MW. (2012b). Towards an encyclopaedia of mammalian gene function: The International Mouse Phenotyping Consortium. Dis Models Mech 5, 289–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubela T, Schofield PN, Ryan CD, Adams R, and Einhorn D. (2012). Managing intellectual property to promote pre-competitive research: The mouse as a model for constructing a robust research commons. J Law Inform Sci 22, 98–121 [Google Scholar]
- Caulfield T, Harmon SH, and Joly Y. (2012). Open science versus commercialization: A modern research conflict?. Genome Med 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmaz K. (2006). Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. Sage Publications, Wiltshire, UK [Google Scholar]
- Collins FS, Rossant J, and Wurst W. (2007). A mouse for all reasons. Cell 128, 9–13 [DOI] [PubMed] [Google Scholar]
- Cook-Deegan RM, and McCormack SJ. (2001). Patents, secrecy, and DNA. Science 293, 217. [DOI] [PubMed] [Google Scholar]
- Corbin J, and Strauss A. (2008). Basics of Qualitative Research: Grounded Theory Procedures and Techniques, 3rded Sage Publications, Wiltshire, UK [Google Scholar]
- Dedeurwaerdere T. (2010a). Global microbial commons: Institutional challenges for the global exchange and distribution of microorganisms in the life sciences. Res Microbiol 161, 407–413 [DOI] [PubMed] [Google Scholar]
- Dedeurwaerdere T. (2010b). Self-governance and international regulation of the global microbial commons: Introduction to the special issue on the microbial commons. Intl J Commons 4, 390–403 [Google Scholar]
- Donahue LR, Hrabe de Angelis M, Hagn M, et al. (2012). Centralized mouse repositories. Mammal Genome 23, 559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove ES, Faraj SA, Kolker E, and Ozdemir V. (2012). Designing a post-genomics knowledge ecosystem to translate pharmacogenomics into public health action. Genome Med 4, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn D, and Heimes R. (2009). Creating a mouse academic research commons. Nature Biotechnol 27, 890–891 [DOI] [PubMed] [Google Scholar]
- European Patent Office (2013). US4736866 (A)–1988-04-12– Transgenic non-human mammals. http://worldwide.espacenet.com/publicationDetails/biblio?CC=US&NR=4736866A&KC=A&FT=D Accessed January23, 2014
- Fisher D, and Atkinson-Grosjean J. (2002). Brokers on the boundary: Academy-industry liaison in Canadian universities. Higher Education 44, 449–467 [Google Scholar]
- Goulding R, Marden E, Manion R, and Levy E. (2010). Alternative intellectual property for genomics and the activity of technology transfer offices: Emerging directions in research. Boston U J Sc Technol Law 16, 194–230 [Google Scholar]
- Harvard University (2013). The Harvard-BMS Cre-Lox License: Non-Commercial Research License Agreement. http://otd.harvard.edu/technologies/materialtransfer/cre-lox/harvard/ Accessed January23, 2014
- Harwell Frozen Embryo and Sperm Archive (2013) Guidelines. http://www.har.mrc.ac.uk/services/frozen-embryo-and-sperm-archive/fesa-guidelines Accessed January23, 2014
- Hess C, and Ostrom E. (2006a). Introduction: An Overview of the Knowledge Commons. In: Hess C, and Ostrom E. (Eds). Understanding Knowledge as a Commons: From Theory to Practice. MIT Press, Cambridge, MA: http://mitpress.mit.edu/books/chapters/0262083574intro1.pdf Accessed January23, 2014 [Google Scholar]
- Hess C, and Ostrom E. (2006b). A framework for analysing the microbiological commons. Intl Soc Sci J 58, 335–349 [Google Scholar]
- Jackson Laboratory (1999). NIH, Jackson Laboratory and DuPont Pharmaceuticals sign Cre-lox technology use agreements. JAX® Notes Issue 476, Winter 1999. http://jaxmice.jax.org/jaxnotes/archive/476.pdf Accessed January23, 2014
- Jackson Laboratory (2008). Cre-lox Patents Expire. JAX® Notes Issue 508, Winter 2008. http://jaxmice.jax.org/jaxnotes/508/508j.html Accessed January23, 2014
- Jackson Laboratory (2013a). Licenses for strains designated as “OncoMouse®”1,2,3’ http://jaxmice.jax.org/licensing/ONCO.htm Accessed January23, 2014
- Jackson Laboratory (2013b). Conditions of use. http://jaxmice.jax.org/cou/index.html Accessed January23, 2014
- Jackson Laboratory (2013c). Policy on licensing and use restriction. http://jaxmice.jax.org/orders/note1.html Accessed January23, 2014
- Jackson Laboratory (2013d). Licenses for strains using “TET-System” technology. http://jaxmice.jax.org/licensing/TET.htm Accessed January23, 2014
- Knockout Mouse Project Repository (2013). Download MTAs. https://www.komp.org/mta.php Accessed January23, 2014
- Korn N, and Oppenheim C. (2011) Licensing Open Data: A Practical Guide. http://discovery.ac.uk/files/pdf/Licensing_Open_Data_A_Practical_Guide.pdf Accessed January23, 2014
- Lavenue LM. (1995). Patent Infringement against the United States and Government Contractors under 28 U.S.C. 1498 in the United States Court of Federal Claims. J Intellectual Property Law 2, 389 [Google Scholar]
- Mallon AM, Iyer V, Melvin D, et al. (2012). Accessing data from the International Mouse Phenotyping Consortium: State of the art and future plans. Mammal Genome 23, 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybarduk P, and Rimmington S. (2009). Compulsory licenses: A tool to improve global access to the HPV vaccine? Am J Law Med 35, 323–350 [DOI] [PubMed] [Google Scholar]
- McCormick JB, Owen-Smith J, and Scott CT. (2009). Distribution of human embryonic stem cell lines: Who, when, and where. Cell Stem Cell 4, 107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merges RP. (2011). Justifying Intellectual Property. Harvard University Press, Cambridge, MA [Google Scholar]
- Miller FA, Sanders CB, and Lehoux P. (2009). Imagining value, imagining users: Academic technology transfer for health innovation. Soc Sci Med 68, 1481–1488 [DOI] [PubMed] [Google Scholar]
- Mirowski P. (2008). Livin’ with the MTA. Minerva 46, 317–342 [Google Scholar]
- Mowery D, and Ziedonis A. (2007). Academic patents and materials transfer agreements: Substitutes or complements? J Technol Transfer 32, 157–172 [Google Scholar]
- Murray F. (2010). The oncomouse that roared: Hybrid exchange strategies as a source of distinction at the boundary of overlapping institutions. Am J Sociol 116, 341–388 [Google Scholar]
- Murray F, Aghion P, Dewatripont M, Kolev J, and Stern S. (2009). Of Mice and Academics: Examining the Effect of Openness on Innovation. (Working Paper No 14819, National Bureau of Economic Research) http://www.nber.org/papers/w14819 Accessed January23, 2014
- National Institutes of Health (1998). NIH, Jackson Laboratory and DuPont Pharmaceuticals Sign Cre-Lox Technology Use Agreement. http://www.nih.gov/news/pr/aug98/od-21.htm Accessed January23, 2014
- National Institutes of Health (1999). Principles and Guidelines for Recipients of NIH Research Grants and Contracts on Obtaining and Disseminating Biomedical Research Resources. Federal Register 64, 72090–72096http://grants.nih.gov/grants/intell-property_64FR72090.pdf Accessed January23, 2014 [Google Scholar]
- National Institutes of Health Office of Technology Transfer (2012). United States Public Health Service Technology Transfer Policy Manual Chapter No. 503A: NIH Simple Letter Agreement (SLA). http://www.ott.nih.gov/sites/default/files/documents/policy/pdfs/503-a-policy.pdf Accessed January23, 2014
- Organisation for Economic Co-operation and Development (2006). Guidelines for the Licensing of Genetic Inventions. http://www.oecd.org/dataoecd/39/38/36198812.pdf Accessed January23, 2014
- Ostrom E. (1990). Governing the Commons: The Evolution of Institutions for Collective Action. Cambridge University Press, New York, NY [Google Scholar]
- Ostrom E. (1999). Revisiting the commons: Local lessons, global challenges. Science 284, 278–282 [DOI] [PubMed] [Google Scholar]
- Ostrom E. (2005). Understanding Institutional Diversity. Princeton University Press, Princeton, NJ [Google Scholar]
- Ostrom E, and Hess C. (2007). A framework for analyzing the knowledge commons. In: Hess C, and Ostrom E. (Eds). Understanding Knowledge as a Commons: From Theory to Practice. MIT Press, Cambridge, MA [Google Scholar]
- Popp Berman E. (2012). Creating the Market University: How Academic Science Became an Economic Engine. Princeton University Press, Princeton, NJ [Google Scholar]
- Rai AK, and Eisenberg RS. (2004). Proprietary Considerations. In: Lanza R, et al. (Eds) Handbook of Stem Cells (Vol. 2) 793–798 [Google Scholar]
- Reichman JH. (2009). Comment: Compulsory licensing of patented pharmaceutical inventions: Evaluating the options. J Law Med Ethics 37, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIKEN Bioresource (2013). Experimental Animal Division—Strain Deposition. http://www.brc.riken.jp/lab/animal/en/depo.shtml Accessed January23, 2014
- Ringwald M, Iyer V, Mason JC, et al. (2011). The IKMC web portal: A central point of entry to data and resources from the International Knockout Mouse Consortium. Nucleic Acids Res 39, D849–D855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez V. (2005). Material transfer agreements: Open science vs. proprietary claims. Nature Biotechnol 23, 489–491 [DOI] [PubMed] [Google Scholar]
- Rodriguez V. (2008). Governance of material transfer agreements. Technol Soc 30, 122–128 [Google Scholar]
- Sauer B, and Henderson N. (1988). Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci 85, 5166–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlake T, and Bode J. (1994). Use of mutated FLP Recognition Target (FRT) sites for the exchange of expression cassettes at defined chromosomal loci. Biochemistry 33, 12746–12751 [DOI] [PubMed] [Google Scholar]
- Schofield PN, Bubela T, Weaver T, et al. (2009). Post-publication sharing of data and tools. Nature 461, 171–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PN, Eppig J, Huala E, et al. (2010). Sustaining the data and bioresource commons. Science 330, 592–593 [DOI] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, et al. (2011). A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitz WD. (2013). MTA Guiding Principles: Best Practices in Non-Profit to Non-Profit Transfers of Published Research Materials. www.autm.net/AM/Template.cfm?Section=Proposed_MTAs&Template=/CM/ContentDisplay.cfm&ContentID=7549 Accessed January23, 2014
- Streitz WD, and Bennett AB. (2003). Material transfer agreements: A University perspective. Plant Physiol 133, 10–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryke D, Kawamoto M, Huang CC, et al. (2003). BayGenomics: A resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res 31, 278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty S. (2007). The institutional nature of the patent system: Implications for bioethical decision-making. In: Lenk C, Hoppe N. and Andorno R. (eds.) Ethics and Law of Intellectual Property. Ashgate; Chapter 13, pp 247–267 [Google Scholar]
- University of British Columbia (2009). Material Transfer Agreements. http://www.uilo.ubc.ca/sites/research.ubc.ca/files/uploads/documents/UILO/Simplified_MTA_Procedures7081.pdf Accessed January23, 2014
- University of British Columbia (2013) Material Transfer Agreements. http://www.uilo.ubc.ca/uilo/industry-engagement/partnering/types/mta Accessed January23, 2014
- US Government (2013). Title 48: Federal Acquisition Regulations System, Part 52—Solicitation Provisions and Contract Clauses, Subpart 52.2—Text of Provisions and Clauses, 52.227-1 Authorization and Consent. https://acquisition.gov/far/current/pdf/FAR.pdf Accessed January23, 2014
- Valenzuela DM, Murphy AJ, Frendewey D, et al. (2003). High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nature Biotechnol 21, 652–659 [DOI] [PubMed] [Google Scholar]
- Varmus H. (2009). The Art and Politics of Science. WW Norton, New York: [PubMed] [Google Scholar]
- Winickoff DE, Saha K, and Graff G. (2009). Opening stem cell research and development: A policy proposal for the management of data, intellectual property, and ethics. Yale J Health Policy Law Ethics 9, 52–127 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.