Abstract
Previous studies have reported promising results regarding the effect of repeated virtual cue exposure therapy on nicotine dependence. This study aimed to compare the effectiveness of virtual cue exposure therapy (CET) and cognitive behavioral therapy (CBT) for nicotine dependence. Thirty subjects with nicotine dependence participated in 4 weeks of treatment with either virtual CET (n=15) or CBT (n=15). All patients were male, and none received nicotine replacement treatment during the study period. The main setting of the CET used in this study was a virtual bar. The primary foci of the CBT offered were (a) smoking cessation education, (b) withdrawal symptoms, (c) coping with high-risk situations, (d) cognitive reconstruction, and (e) stress management. Daily smoking count, level of expiratory carbon monoxide (CO), level of nicotine dependence, withdrawal symptoms, and subjective craving were examined on three occasions: week 0 (baseline), week 4 (end of treatment), and week 12 (follow-up assessment). After treatment, the daily smoking count, the expiratory CO, and nicotine dependence levels had significantly decreased. These effects continued during the entire study period. Similar changes were observed in both virtual CET and CBT groups. We found no interaction between type of therapy and time of measurement. Although the current findings are preliminary, the present study provided evidence that virtual CET is effective for the treatment of nicotine dependence at a level comparable to CBT.
Introduction
Nicotine dependence, the most prevalent and deadly substance use disorder, involves compulsive use in the face of adverse consequences and repeated cycles of abstinence and relapse.1 Of the numerous factors associated with treatment outcome, craving is considered a primary trigger for relapse.2 Additionally, environmental stimuli (context-specific stimuli or cues) that are repeatedly associated with cigarettes are known to promote compulsive smoking.3,4
Craving and cue reactivity have been conceptualized as classically conditioned responses.5 Cue exposure therapy (CET) is based on the assumption that environmental stimuli that are repeatedly associated with a drug can come to elicit conditioned responses that lead to craving and relapse.6 CET for nicotine dependence is based on the notion that prolonged and repeated nonreinforced presentation of smoking cues (conditioned stimuli) will result in a gradual diminution of craving (conditioned response) through Pavlovian extinction learning.7
CET, however, is limited in terms of the environmental cues that can be simulated and the degree to which immersion experiences can be presented. Furthermore, it has shown decreased efficacy over time.8 Thus, a virtual reality (VR) variation on cue exposure treatment (VR-CET) has been developed as an alternative to CET.9 VR is an evolving technology that produces interactive environments with stereoscopic, three-dimensional (3D) visual displays, auditory input, and immersive interaction from a first-person perspective.10 A previous study used virtual paraphernalia or avatars smoking at parties or in bars, selecting the situation to be recreated as an ad hoc virtual environment.11 Recent studies have shown the effectiveness of VR extinction for reducing cue-elicited craving, and have demonstrated that this approach is more effective in eliciting conditioned responses than are conventional methods such as traditional slides or videos.12,13
According to clinical practice guideline,14 there are two types of counseling and behavioral therapies resulting in higher success rates: (a) providing smokers with practical counseling such as problem solving/skills training/stress management, and (b) providing support and encouragement as part of treatment. Cognitive behavioral therapy (CBT) is suitable to meet these aims, and favorable outcomes and positive effects have been reported.15,16
However, no study has compared effectiveness of CBT with that of VR-CET, despite empirical evidence that has been accumulated.17–19 In this preliminary study, we aimed to compare the effectiveness of short-term VR-CET with that of CBT for subjects with nicotine dependence. We hypothesized that the effectiveness of VR-CET would be comparable to that of conventional CBT for nicotine dependence.
Materials and Methods
Participants
Thirty participants who smoked cigarettes daily and showed at least a moderate nicotine dependence level based on the Fagerström Test were enrolled. All participants were male and treatment seeking. Subjects were recruited via the smoking cessation clinic at the SMG-SNU Boramae Medical Center, and none received nicotine-replacement treatment or any other medications during the study period. Exclusion criteria included the following: known history of alcohol or drug abuse/dependence other than that involving nicotine; neurological disease or brain injury; evidence of medical illness that could manifest in psychiatric symptoms; and psychiatric disorders confirmed by the Structured Clinical Interview for DSM-IV.20 This study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Boards of the SMG-SNU Boramae Medical Center approved the study protocol, and all subjects provided written informed consent prior to participation.
Procedure
Before the experimental intervention, data on demographic characteristics, medical history, and smoking behavior (daily smoking count and typical locations for smoking) were collected from participants. All subjects with nicotine dependence participated in four weekly treatment sessions under one of two conditions: VR-CET (n=15) or CBT (n=15). Both treatments were conducted once a week for 4 weeks. After the end of each session, the researchers administered the Fagerström Test for Nicotine Dependence (FTND), Questionnaire of Smoking Urges (QSU), and expiratory carbon monoxide (CO) level to each subject. Participants who completed the four-session treatment visited the clinic on a monthly basis as a follow-up interview and engaged in simple counseling. Questionnaires and rating scales were completed on three occasions: week 0 (baseline), week 4 (end of treatment), and week 12 (follow-up assessment). Subjects in the two groups were demographically matched (Table 1).
Table 1.
Demographic and Clinical Characteristics in Study Subjects
| VR-CET (n=15) | CBT (n=15) | |||
|---|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | t | p |
| Age (years) | 30.73 (6.60) | 33.07 (5.47) | −1.05 | 0.30 |
| Daily smoking counts | 20.67 (4.17) | 20.33 (4.41) | 0.21 | 0.83 |
| Expiratory CO | 13.07 (3.61) | 13.73 (3.63) | −0.50 | 0.62 |
| Fagerström Test | 4.87 (1.06) | 5.80 (1.15) | −2.31* | 0.03* |
| Minnesota nicotine withdrawal | 9.93 (3.53) | 7.20 (4.06) | 1.96 | 0.06 |
| Smoking urges | 108.47 (28.02) | 112.13 (49.09) | −0.25 | 0.80 |
p<0.05. SD, standard deviation; VR-CET, virtual reality-cue exposure therapy; CBT, cognitive behavioral therapy; CO, carbon monoxide.
Measures
Expiratory CO concentration
The expiratory CO concentration is the CO level remaining in the alveoli after smoking. We used a Micro CO Meter (Cardinal Health, Chatham, UK), which is a hand held battery operated device used to measure the concentration of CO on the breath and calculates the percentage of carboxyhaemoglobin (%COHb) in the blood. Daily smoking counts and expiratory CO levels were monitored to determine whether participants smoked during the study. Smoking cessation success or failure was determined based on information provided by the subjects at weeks 4 and 12, and verified by expiratory CO levels ≤6.
Nicotine dependence level
The FTND was used to measure the participants' dependence on nicotine.21 This measure, which consists of six questions, is strongly correlated with pulse rate, body temperature, and cotinine levels, the primary metabolite of nicotine. Scores range from 0 to 10; scores of 3–6 are considered to reflect a moderate level of nicotine dependence, and those higher than 7 are considered to reflect a severe level. Participants with at least a moderate dependence (scores of 4 and higher) were included in this study.
Withdrawal symptoms
The Minnesota Nicotine Withdrawal Scale (MNWS) is a 15-item self-report questionnaire used to assess withdrawal symptoms. It has been validated in multiple studies and demonstrated sensitivity to abstinence symptom and good reliability and validity.22
Subjective craving
A 32-item self-report version of the QSU was administrated to assess subjective craving.23 A two-factor solution best described the item structure of the QSU. The first factor consisted of a strong desire and intention to smoke and a perception that smoking is rewarding for active smokers. The second consisted of an anticipation of relief from negative affect associated with an urgent desire to smoke.
Intervention
Condition 1: VR-CET
Participants allocated to the VR-CET group experienced four serial VR cue exposure environments in a 3D surround screen projection room equipped with real time psychophysiological response monitoring. The primary focus of the VR used in this study was a “virtual bar” because bars are the most frequent smoking environment according to the results of a previous survey.17 Only those participants who reported that their urge to smoke was most powerful in situations involving alcohol were assigned to this condition. The treatment procedure was as follows. Researchers configured the four different VR environments (a, neutral cue; b, smoking-related objects; c, social situation related with smoking; d, neutral cue). Participants were exposed to this series of environments for approximately 25 minutes per visit. After each environment ended, participants were asked to check the severity of nicotine craving using a mouse button. The detailed information about VR-CET, including a screenshot of the virtual bar, was described in the previous report.17
Condition 2: CBT
In a systemized smoking cessation clinic, psychosocial treatments commonly utilized are based on the CBT approach. In this study, four sessions of the CBT protocol were used, which researchers reconstructed within the framework of adequate treatment based on the previous literature.24,25 The primary foci of the CBT offered were (a) smoking cessation education, (b) withdrawal symptoms, (c) coping with high-risk situations, (d) cognitive reconstruction, and (e) stress management. Participants received individual CBT from a psychiatrist at a smoking cessation clinic in the SMG-SNU Boramae Medical Center, and none received nicotine replacement treatment or any other medication during the study period.
Statistical analysis
All statistical analyses were conducted with SPSS v17.0 (SPSS Inc., Chicago, IL). A repeated measures analysis of variance (ANOVA) was used to analyze changes in the target variables as a function of the treatment condition. Bonferroni post hoc correction was used to compare mean differences between measurements taken at different times. Chi-square tests were used to analyze differences in the success and failure rates under both conditions. Statistical significance was set at 0.05 (two-tailed).
Results
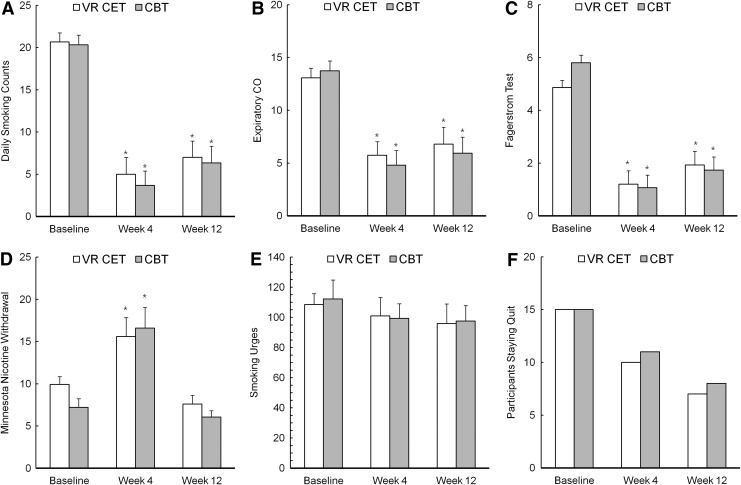
Repeated measures ANOVAs revealed significant reductions in daily smoking count, expiratory CO levels, and level of nicotine dependence as the study proceeded. As shown in Table 2, we found main effects for time of measurement (week 0, 4, or 12) on daily smoking count, expiratory CO levels, and level of nicotine dependence, and post hoc analysis confirmed that every score recorded at week 4 (end of treatment) was significantly lower than its baseline counterpart (p=0.00). No significant interaction between measurement time and treatment condition was observed (Table 2). Moreover, subjective cravings decreased as the study proceeded, although these differences were not statistically significant. With regard to nicotine withdrawal symptoms, we found a main effect of MNWS scores but no significant interaction between treatment group and time of measurement. Post hoc pairwise comparisons confirmed the significant increase in withdrawal symptoms at the end of 4 weeks of treatment (p=0.00), but these symptoms decreased to below baseline levels after 12 weeks (p=0.05). After 4 weeks, 10 out of 15 participants in the VR-CET group succeeded in maintaining their nonsmoking status; as did 11 of the participants in CBT group. Until the research was completed (week 12), seven of the participants in the VR-CET group were successful in quitting, and eight of the participants in the CBT group maintained their abstinence. Chi-square analyses revealed no significant differences between the groups in the distribution of participants who had successfully stopped smoking (week 4: χ2=0.16, p=0.69; week 12: χ2=0.13, p=0.71; Fig. 1).
Table 2.
Analysis of the Smoking Related Variables at Baseline, Week 4, and Week 12 in the VR-CET and CBT Groups
| F | p | Post hoc (Bonferonni) | |
|---|---|---|---|
| Daily smoking counts | |||
| Timea | 110.17 | 0.00** | Week 0>week 4, week12 |
| Conditionb | 0.16 | 0.70 | Week 4<week 12 |
| Time×condition | 0.09 | 0.91 | |
| Expiratory CO | |||
| Time | 54.82 | 0.00** | Week 0>week 4, week 12 |
| Condition | 0.06 | 0.81 | |
| Time×Condition | 0.58 | 0.57 | |
| Fagerström test | |||
| Time | 84.39 | 0.00** | Week 0>week 4, week 12 |
| Condition | 0.17 | 0.68 | Week 4<week 12 |
| Time×Condition | 1.68 | 0.20 | |
| Minnesota nicotine withdrawal | |||
| Time | 29.13 | 0.00** | Week 4>week 0, week 12 |
| Condition | 0.45 | 0.51 | Week 0>week 12 |
| Time×Condition | 1.09 | 0.34 | |
| Smoking urges | |||
| Time | 2.55 | 0.09 | |
| Condition | 0.01 | 0.93 | — |
| Time×Condition | 0.10 | 0.91 | |
p<0.05; **p<0.01.
Time: week 0 (baseline), week 4 (end of treatment), week 12 (follow-up assessment).
Condition: VR-CET, CBT.
In the post hoc column, significant pairs only listed.
FIG. 1.
Changes in smoking-related variables at baseline, week 4, and week 12 in the VR-CET and CBT groups. VR-CET, virtual reality-cue exposure therapy; CBT, cognitive behavioral therapy; CO, carbon monoxide; *p<0.05 significant mean difference compared to baseline.
Discussion
To our knowledge, the present study is the first to compare the effectiveness of VR-CET and CBT for treating nicotine dependence. In this study, both therapies were offered in a short-term format, and weekly changes under the two conditions were compared. We found that significant decreases in daily smoking counts, levels of nicotine dependence, and expiratory CO levels of both groups were observed after 4 weeks, and these changes continued until the study terminated (week 12). Second, significant increases in nicotine withdrawal symptoms of both groups were reported after 4 weeks of therapy, but these values returned to baseline levels at week 12. Finally, two-thirds of the participants succeeded in maintaining their nonsmoking status at the end of treatment (week 4), and almost half the participants maintained their abstinence until the research was completed. Similar trends were observed among those in both groups.
Despite the existence of empirically validated psychological and pharmacological treatments for nicotine dependence,26 high relapse rates underscore the need to develop more effective strategies for maintaining abstinence. Our results show that both VR-CET and CBT are effective treatment modalities for nicotine dependence. Even more importantly, VR-CET was associated with a significant treatment effect that was similar to the effect of CBT.
Of the treatments used for smoking cessation, CBT is typically selected in practice. CBT focuses on cognitive restructuring, education, self-monitoring, and practical coping strategies aimed at successful smoking cessation. Due to the nature of the approach, CBT devotes a large amount of time to education during the early stage of treatment. However, this sometimes reduces the immediacy of the treatment and poses barriers to early engagement. Ensuring the expertise of practitioners remains challenge and time–cost considerations are also present.
In this context, VR-CET has emerged as a new treatment for smoking cessation. This approach involves repeated exposure to nicotine-related cues with the goal of reducing cue reactivity via extinction. Actual cue exposure is the core treatment element of CET, and no techniques of active exposure took place in CBT, thus establishing a clear difference between the two approaches.27 It is easier to validate the contents in VR-CET, and the administration procedure is simple as well. Although previous studies have noted the limitations of this approach related to the restricted variety of stimuli presented and individual differences in cue reactivity,28 virtual reality makes it possible to recreate the complexity of a smoking environment. This may increase realism and enhance participants' motivation by using scientifically and ecologically validated contexts and cues. This is reflected in the previous research that patients favored CET with regard to usefulness and practicability of treatment contents.27 These factors may render treatment more effective.
On the other hand, no significant changes in subjective cravings were found. This result may be explained by the following considerations. First, the intervention performed in this study was short term. Duration of intervention may not be sufficient to achieve extinction through repeated exposures in the CET model or for new coping skills to become habitual in the CBT model. Second, a selection bias may have affected the results for the VR-CET condition. As mentioned earlier, those who experienced the strongest urge to smoke in drinking situations were assigned to the VR-CET group, and the only smoking cue used for this group was alcohol related. It would be expected that the urge to smoke would decrease in a drinking situation. However, generalization to other situations would be surprising. Future research should identify and test a variety of smoking cues and situations.
One important concern in the study is the absence of a control group of smokers not involved in VR or CBT treatment, which may lead to the question of whether this change was due to time course or the real effect of the treatment itself. However, we tried to exclude the effect of time course indirectly. Previous research revealed that specific types of counseling and behavioral treatment yielded a statistically significant increase in abstinence rate relative to no contact (untreated control condition).14
Some limitations of the present study should be pointed out, such as the small sample size and the inclusion of only male participants. A possible bias might be the motivation of the subjects involved in the study, which could have been higher than subjects saying that they wanted to stop without a strong motivation. These factors limit the generalizability of the results. However, we included only drug-naive participants. It is important to recruit a homogeneous sample to control for confounding factors such as medication and sex.
Conclusions
Although the underlying therapeutic mechanisms differed, the two treatments examined here were both effective for smoking cessation. This result may suggest the potential of constituting a new treatment modality. Future research is needed to evaluate the possible combined effect of both treatments and to explore the possibility of combining VR-CET incorporating a variety of smoking-related stimuli with components of CBT.
Acknowledgment
This work was supported by a grant from the SNUH Research Fund (30-2011-0210).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization (2009) WHO report on the global tobacco epidemic, 2009: implementing smoke-free environments. Geneva, Switzerland; WHO [Google Scholar]
- 2.Carter BL, Tiffany ST. Meta‐analysis of cue‐reactivity in addiction research. Addiction 1999; 94:327–340 [PubMed] [Google Scholar]
- 3.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacological Reviews 2002; 54:1–42 [DOI] [PubMed] [Google Scholar]
- 4.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacology Biochemistry & Behavior 2002; 71:517–529 [DOI] [PubMed] [Google Scholar]
- 5.Conklin CA, Tiffany ST. Applying extinction research and theory to cue‐exposure addiction treatments. Addiction 2002; 97:155–167 [DOI] [PubMed] [Google Scholar]
- 6.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology 2013;226:659–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantak K, Nic Dhonnchadha B. Pharmacological enhancement of drug cue extinction learning: translational challenges. Annals of the New York Academy of Sciences 2011; 1216:122–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenberg C. (2012) Virtual reality in psychological, medical and pedagogical applications. New York: InTech [Google Scholar]
- 9.Martin T, LaRowe S, Malcolm R. Progress in cue exposure therapy for the treatment of addictive disorders: a review update. Open Addiction Journal 2010; 3:92–101 [Google Scholar]
- 10.Coiffet P, Burdea GC. (2003) Virtual reality technology. Hoboken, NJ: Wiley Interscience [Google Scholar]
- 11.Traylor AC, Bordnick PS, Carter BL. Using virtual reality to assess young adult smokers' attention to cues. CyberPsychology & Behavior 2009; 12:373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann SB, Sayette MA. Smoking cues in a virtual world provoke craving in cigarette smokers. Psychology of Addictive Behaviors 2006; 20:484. [DOI] [PubMed] [Google Scholar]
- 13.Giroux I, Faucher-Gravel A, St-Hilaire A, et al. Gambling exposure in virtual reality and modification of urge to gamble. CyberPsychology, Behavior, & Social Networking 2013; 16:224–231 [DOI] [PubMed] [Google Scholar]
- 14.Fiore M. (2008) Treating tobacco use and dependence: 2008 update: clinical practice guideline. Washington, DC: US Department of Health and Human Services [Google Scholar]
- 15.Nides M, Leischow S, Sarna L, et al. Maximizing smoking cessation in clinical practice: pharmacologic and behavioral interventions. Preventive Cardiology 2007; 10:23–30 [DOI] [PubMed] [Google Scholar]
- 16.Reid RD, Quinlan B, Riley DL, et al. Smoking cessation: lessons learned from clinical trial evidence. Current Opinion in Cardiology 2007; 22:280–285 [DOI] [PubMed] [Google Scholar]
- 17.Choi J-S, Park S, Lee J-Y, et al. The effect of repeated virtual nicotine cue exposure therapy on the psychophysiological responses: a preliminary study. Psychiatry Investigation 2011; 8:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard B, Turcotte V, Bouchard S, et al. Crushing virtual cigarettes reduces tobacco addiction and treatment discontinuation. CyberPsychology & Behavior 2009; 12:477–483 [DOI] [PubMed] [Google Scholar]
- 19.Moon J, Lee J-H. Cue exposure treatment in a virtual environment to reduce nicotine craving: a functional MRI study. CyberPsychology & Behavior 2009; 12:43–45 [DOI] [PubMed] [Google Scholar]
- 20.First MB, Gibbon M. (1997) User's guide for the structured clinical interview for DSM-IV axis I disorders: SCID-1 clinician version. Washington DC: American Psychiatric Press [Google Scholar]
- 21.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction 1991; 86:1119–1127 [DOI] [PubMed] [Google Scholar]
- 22.Hughes JR, Gust SW, Skoog K, et al. Symptoms of tobacco withdrawal: a replication and extension. Archives of General Psychiatry 1991; 48:52. [DOI] [PubMed] [Google Scholar]
- 23.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction 1991; 86:1467–1476 [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association Work Group on Nicotine Dependence Practice guideline for the treatment of patients with nicotine dependence. American Journal of Psychiatry 1996; 153:1–31 [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Wright FD. (2011) Cognitive therapy of substance abuse. New York: Guilford Press [Google Scholar]
- 26.Fiore M, Bailey W, Cohen S, et al. Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services. Public Health Service; 2000:00-0032 [Google Scholar]
- 27.Loeber S, Croissant B, Heinz A, et al. Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. British Journal of Clinical Psychology 2006; 45:515–529 [DOI] [PubMed] [Google Scholar]
- 28.García-Rodríguez O, Pericot-Valverde I, Gutiérrez-Maldonado J, et al. Validation of smoking-related virtual environments for cue exposure therapy. Addictive Behaviors 2012; 37:703–708 [DOI] [PubMed] [Google Scholar]