Abstract
Cytokines are necessary for cell communication to enable responses to external stimuli that are imperative for the survival and maintenance of homeostasis. Dysfunction of the cytokine network has detrimental effects on intra- and extracellular environments. Thus, it is critical that the expression of cytokines and the signals transmitted by cytokines to target cells are tightly regulated at numerous levels, including transcriptional and post-transcriptional levels. Here, we briefly summarize the role of AU-rich elements (AREs) in the regulation of cytokine gene expression at the post-transcriptional level and describe a role for GU-rich elements (GREs) in coordinating the regulation of cytokine signaling. GREs function as post-transcriptional regulators of proteins that control cellular activation, growth, and apoptosis. GREs and AREs work in concert to coordinate cytokine signal transduction pathways. The precise regulation of cytokine signaling is particularly important, because its dysregulation can lead to human diseases.
Introduction
Cytokines function as intercellular communication molecules, enabling cells to influence the functions of other cells to maintain normal growth, development, and homeostasis. In the immune system, cytokines play essential roles in immune cell activation, immune effector function, and maintenance of peripheral tolerance. In addition, during development, cytokines act as hormones, regulating proper growth, cellular differentiation, and appropriate programmed cell death to help coordinate the gene expression pathways during development and maturation of the organism. Dysregulation of cytokine networks can lead to adverse consequences and the development of disease. For example, autoimmune diseases involve abnormal production of cytokines. Consequently, many of the medications used to treat autoimmune diseases target cytokines or cytokine signaling. An important mechanism of cancer pathogenesis is the dysregulation of cytokines and growth factors, leading to uncontrolled cell growth. Thus, it is crucial that cytokines and cytokine signaling pathways are tightly regulated through multiple mechanisms, including transcriptional and post-transcriptional mechanisms (Seko and others 2006; Khabar and Young 2007; Friedel and others 2009; Beisang and others 2012a; Duan and others 2013).
Here, we review the role of post-transcriptional control in cytokine function, focusing on the engagement of AU-rich (AREs) and GU-rich elements (GREs) in post-transcriptional regulation of cytokine signaling. AREs and GREs are conserved sequences in the 3′untranslated region (UTR) of short-lived transcripts that encode a variety of proteins involved in cellular activation, growth, and apoptosis, including components of cytokine signal transduction pathways. Much is known about the important role of AREs in the post-transcriptional regulation of a variety of cytokines and growth factors [reviewed in Anderson (2010)]. However, the role of GREs in cytokine function is not as well understood. Our knowledge of AREs provides insights into the molecular mechanisms of post-transcriptional regulation of cytokine production (Anderson 2008) and has helped us understand how networks of genes are coordinately regulated by conserved sequences in mRNA. We discuss the potential implications of GREs in regulating a network of cytokine signaling pathway components, and we suggest that AREs and GREs regulate distinct sets of transcripts but function in concert to coordinate cytokine expression with cytokine signaling.
Post-Transcriptional Regulation of Gene Expression by AREs
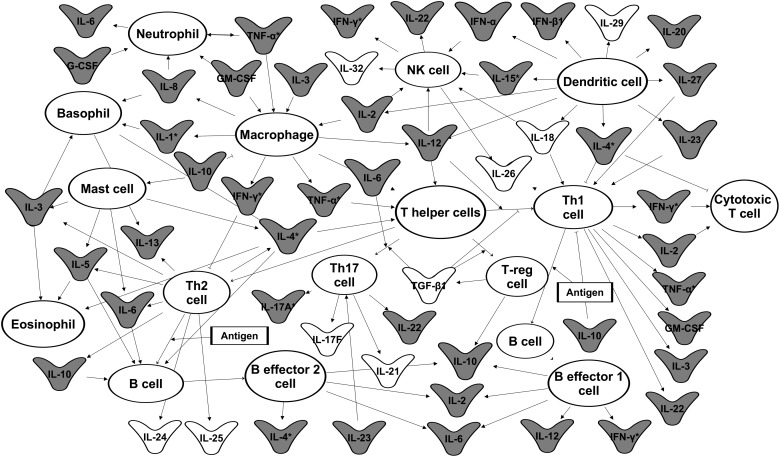
In immune cells, the production of cytokines and other early response gene proteins are induced transiently for a defined period of time after cellular activation through a preset program of gene expression. The sequential order of gene expression after immune cell activation is determined by precisely controlled gene activation programs that include transcriptional induction, transcriptional repression, and regulated mRNA stability. For example, the induction of cytokines is observed during T-cell receptor stimulation of human T lymphocytes ex vivo [reviewed in Raghavan and others (2004)]. T-cell cytokine genes such as interleukin (IL)-2, interferon (IFN)-γ, IL-4, tumor necrosis factor (TNF)-α, and so on are induced through transcriptional pulses. The transcripts become transiently stabilized, and then undergo precisely regulated degradation (Hao and Baltimore 2009). Many cytokine transcripts and other early response gene transcripts are induced as a part of gene activation programs. This is followed by degradation of the transcripts via AREs found in their 3′UTR that function to target mRNAs for rapid decay [reviewed in Khabar (2007); Schott and Stoecklin (2010); Al-Haj and Khabar (2012); Ivanov and Anderson (2013)]. Figure 1 shows a network of cytokines that are regulated by AREs in a variety of cell types. AREs function as instability elements that mediate the degradation of cytokine transcripts by ARE-binding proteins. In addition to regulating mRNA decay, ARE-binding proteins control other post-transcriptional processes such as pre-mRNA processing, transport, intracellular localization, and translation [reviewed in Stumpo and others (2010); Li and others (2012); Palanisamy and others (2012)].
FIG. 1.
The role of cytokines in mediating communication between immune cells. Transcripts in gray are cytokine transcripts that contain AU-rich elements (AREs) in their 3′ untranslated regions (UTRs). Transcripts labeled with an asterisk (*) contain GU-rich elements (GREs) in their 3′UTRs. This network diagram was built using Ingenuity Pathway Assistant Software.
The regulation of mRNA stability by AREs is a complex process that involves the concerted actions of heterogeneous ARE-containing transcripts interacting with many different ARE-binding proteins. Not all AREs exhibit equivalent destabilizing functions. AREs have been classified into 3 classes based on sequence characteristics and decay kinetics (Chen and Shyu 1995). In this classification system, class I AREs contain 1–3 copies of scattered AUUUA motifs with a nearby U-rich region and cause synchronous deadenylation. Class II AREs are composed of at least 2 overlapping copies of the (U/A)AUUUA(U/A) nonamer in a U-rich region and cause asynchronous deadenylation. Many cytokine transcripts contain class II AREs. Finally, class III AREs contain U-rich regions and other poorly defined features and cause synchronous deadenylation. Certain Class III AREs are similar to GREs (discussed later), as the class III AREs and GREs can be U-rich and can serve as binding sites for ARE and GRE-binding proteins. More recently, a database of ARE-containing transcripts from mice, rats, and humans was created (Bakheet and others 2006; Halees and others 2008). This database groups AREs into 5 clusters based on the number of overlapping AUUUA pentamers found in the 3′UTR of a transcript with clusters 1–5 having 5 to 1 overlapping AUUUA pentamers, respectively. Cluster 1 AREs are enriched in secreted proteins, such as cytokines, involved in the growth of hematopoietic and immune cells, while the other ARE clusters are found in a diverse set of transcripts. Overall, ARE-containing transcripts represent ∼5–8% of the transcriptome (Bakheet and others 2001).
AREs function to regulate mRNA decay through their interactions with ARE-binding proteins that function to stabilize mRNA or target it for degradation by interacting with the cellular mRNA decay machinery. Numerous ARE-binding proteins expressed in a variety of cell types that are involved in mRNA turnover regulation have been described [reviewed in Stumpo and others (2009); Gratacos and Brewer (2010); von Roretz and others (2011); Li and others (2012)]. In addition, microRNAs may regulate mRNA stability (Guo and others 2010), often interacting with ARE-binding proteins to direct mRNA degradation [reviewed in Moore and others (2011); van Kouwenhove and others (2011); Palanisamy and others (2012); Whelan and others (2012)]. Several ARE-binding proteins, including ZFP36 (also known as TTP), BRF1, BRF2, KSRP, and HNRNPD (AUF1), promote transcript destabilization by recruiting cellular deadenylases and enzymes involved in both 5′→3′ and 3′→5′ mRNA decay, whereas other ARE-binding proteins, such as ELAVL1 (also known as HuR) and ELAVL4, function to stabilize target transcripts [reviewed in Turner and Hodson (2012b); Bronicki and Jasmin (2013); Brooks and Blackshear (2013); Wu and others (2013)].
mRNA molecules move through different cellular compartments within messenger ribonucleoprotein complexes, in dynamic association with RNA-binding proteins (RBPs) that bind to conserved cis-elements shared by subsets of transcripts (Turner and Hodson 2012a). The association of specific RBPs with conserved regulatory cis-elements shared by subsets of transcripts coordinates the fate of these bound transcripts through post-transcriptional processes such as translation, intracellular localization, storage, or mRNA decay (Keene 2007). The coordinately regulated targets of a given RBP can be used to define post-transcriptional regulatory networks after cellular activation. RNA-immunoprecipitation (RNA-IP) experiments, by which proteins are immunoprecipitated from cellular lysates using an antibody against a given RBP and the identity of co-purified mRNA is determined, have provided insights into post-transcriptional networks of coordinately regulated ARE-containing transcripts. The target transcripts of several ARE-binding proteins, including ZFP36 (Emmons and others 2008; Stoecklin and others 2008), ELAVL1 (Lopez de Silanes and others 2004; Mukherjee and others 2009; Fan and others 2011), KSRP (Winzen and others 2007), and TIA-1 (Lopez de Silanes and others 2005), have been identified through this technique, and represent distinct but overlapping sets of transcripts. Targets of ARE-binding proteins include transcripts encoding early response proteins, cytokines (IFNs, ILs, and chemokines), and cytokine-signaling components. Integration of the activities of multiple ARE-binding proteins likely determines the fate of ARE-containing transcripts (Mansfield and Keene 2009; Janga and Mittal 2011).
ARE-containing transcripts can interact with different ARE-binding proteins over the course of immune activation through competitive binding. For example, the ARE-binding proteins, ELAVL1 and ZFP36, compete with each other for certain ARE-containing transcripts in a dynamic process after T-cell activation. Cytoplasmic ELAVL1 levels increase shortly after T-cell activation, transiently stabilizing a network of ARE-containing transcripts to enable their increased expression. Later, in T-cell activation, ZFP36 is induced, displaces ELAVL1 on ARE-containing transcripts, and mediates their rapid decay (Raghavan and others 2001). This process enables transient expression and subsequent degradation of ARE-containing transcripts over the course of immune responses.
The functions of ARE-binding proteins can also change in response to cellular signals over the course of cellular activation during immune responses. For example, the ability of ZFP36 to bind to and recruit components of the cellular mRNA decay machinery to ARE-containing transcripts is regulated through phosphorylation of ZFP36 by p38 MAPK-activated protein kinase 2 after lipopolysaccharide (LPS) stimulation of macrophages. ZFP36 phosphorylation promotes ZFP36 association with 14-3-3 proteins and prevents ZFP36 from recruiting a deadenylase to the bound transcript (Sun and others 2007). Through LPS-mediated ZFP36 phosphorylation, ARE-containing transcripts encoding inflammatory mediators such as IL-1, IL-6, and COX-2 are induced as a part of an effective immune response (Ronkina and others 2010). As the immune response resolves, ZFP36 phosphorylation is reversed by the phosphatase PP2A, enabling ZFP36 to return to its baseline function and promote the degradation of transcripts encoding inflammatory mediators (Sun and others 2007; Frasca and others 2010). Thus, the coordinate regulation of networks of ARE-containing transcripts is a dynamic process after immune cellular activation that involves competition between ARE-binding proteins and altered activity of ARE-binding proteins over time through regulated phosphorylation.
Post-Transcriptional Regulation of Gene Expression by GREs
Studies regarding coordinate regulation by AREs and ARE-BPs have provided great insights into post-transcriptional regulatory mechanisms that have led to the identification of other cis-elements and proteins which regulate mRNA decay (Vlasova and Bohjanen 2008). Bioinformatic analysis of short-lived transcripts expressed in primary human T cells revealed that many of these transcripts were not regulated by AREs or other known mRNA regulatory elements. A search for novel conserved sequences in these short-lived transcripts led to the identification of the GU-rich sequence, UGUUUGUUUGU (known as a GRE), as a highly enriched sequence in the 3′UTRs of these transcripts (Vlasova and others 2008). Introduction of the GRE into the 3′UTR of a beta-globin reporter transcript conferred instability on the otherwise stable beta-globin transcript, demonstrating that the GRE is a functional mediator of mRNA decay. The RBP, CELF1, was shown to bind specifically to the GRE and to regulate the decay of GRE-containing transcripts (Vlasova-St. Louis and others 2013). Further verification of the role of CELF1 in GRE-mediated mRNA decay came from the observation that in HeLa cells, knockdown of CELF1 using siRNA led to stabilization of GRE-containing transcripts, implicating CELF1 as a mediator of GRE-dependent mRNA degradation (Vlasova and others 2008; Rattenbacher and others 2010). A database of GRE-containing transcripts (Halees and others 2011) similar to the ARE database was created in which GREs are placed into 5 clusters based on the number of overlapping GUUUG pentamers found in the 3′UTR of a transcript, with clusters 1–5 having 5 to 1 overlapping GUUUG pentamers, respectively. These GRE-containing transcripts encode a variety of proteins involved in cellular activation, growth, and apoptosis regulation.
In primary human T cells, GREs and CELF1 regulate rapid changes in gene expression after T-cell activation. T-cell receptor-mediated activation induces the phosphorylation of CELF1, which inhibits its ability to bind to GRE-containing transcripts (Beisang and others 2012b). The activation-induced inhibition of CELF1 binding to mRNA correlates with a transient up-regulation of GRE-containing transcripts. Many of these transiently up-regulated GRE-containing transcripts encode proteins involved in cellular activation and proliferation. It appears that CELF1 suppresses a network of GRE-containing mRNAs involved in proliferation in resting T cells. Subsequent activation-induced phosphorylation of CELF1 leads to de-repression and accumulation of these mRNA transcripts after T-cell activation.
GRE-Containing Transcripts Encode Key Components of Cytokine Signaling Pathways
CELF1 binds numerous target transcripts that are components of intracellular signaling cascades, including components of cytokine signaling pathways (Vlasova-St. Louis and Bohjanen 2011). RNA-IP experiments that were carried out on a genome-wide level in HeLa cells (Rattenbacher and others 2010), primary human T cells (Beisang and others 2012b), and mouse myoblasts (Lee and others 2010) identified hundreds of CELF1 target transcripts. A comparison of the CELF1-bound transcripts in each of the different cell types revealed distinct sets of CELF1 targets, but for each cell type, an analysis of functional annotations of target transcripts identified the enrichment of mRNAs encoding regulators of transcription, post-transcriptional control, cell cycle, and apoptosis. Our recent analysis of CELF1 target revealed that targets contained not only the GRE sequence UGUUUGUUUGU, but also a GU-repeat sequence which was shown to bind to CELF1 and mediate mRNA decay. Based on these findings, the GRE was redefined as the sequence UGU[G/U]UGU[G/U]UGU (Rattenbacher and others 2010).
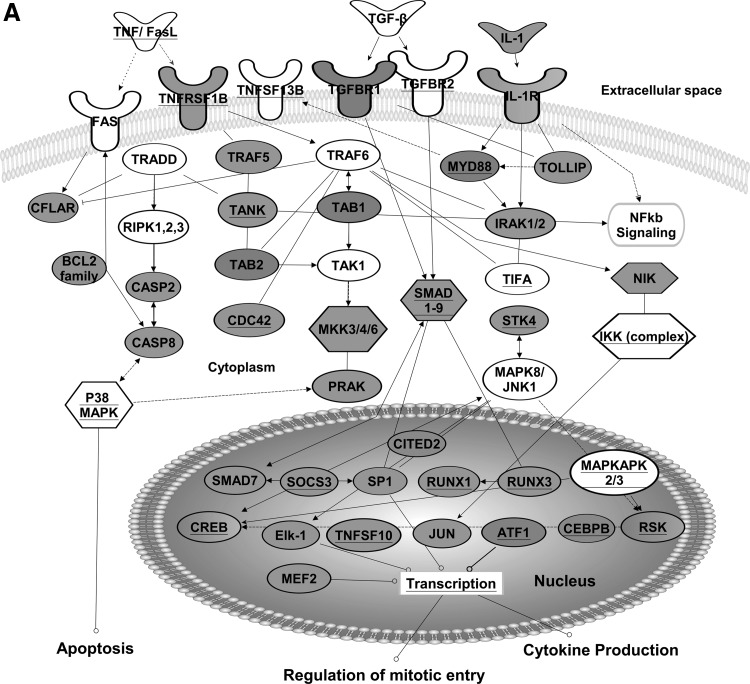
Cytokine signaling systems are pleotropic, and the functions of cytokines are diverse, controlling a variety of cellular activities such as immune responses, differentiation, chemotaxis, cell proliferation, and apoptosis (Vacchelli and others 2012). Post-transcriptional regulation impacts not only cytokine production but also the function of cytokine receptors and cytokine signaling pathways. For example, Fig. 2A shows transcripts encoding signaling components downstream of TNF-α, transforming growth factor (TGF)-β, and IL-1 cytokine receptors whose steady-state mRNA levels changed after T-cell receptor-mediated activation of primary human T cells. As can be seen, numerous components of signaling pathways downstream from these receptors are encoded by GRE-containing transcripts (gray transcripts). These receptors activate signaling pathways that transduce a broad range of intracellular signals which control cellular functions, including apoptosis, regulation of mitotic entry, and cytokine production.
FIG. 2.
The role of GREs and CELF1 in cytokine signaling. (A) Transcripts involved in tumor necrosis factor (TNF), transforming growth factor (TGF), or interleukin (IL)-1 receptor signaling. (B) Transcripts involved in interferon and IL receptor signaling. Transcripts shown exhibited changes in steady-state levels after T-cell receptor stimulation of primary human T cells. Transcripts in gray contain GREs. Transcripts with underlined text were identified as CELF1 target transcripts in human T cells by RNA immunoprecipitation (Beisang and others 2012b). This network diagram was built using Ingenuity Pathway Assistant Software.
Signals downstream of TNF-α receptors interact with signals downstream of FAS to regulate apoptosis. In cancer cell lines, CELF1 regulates a number of apoptotic transcripts downstream of FAS (also TNFRSF6) receptor through destabilization of pro-apoptotic mRNAs, which prevents cell death and promotes proliferation. CELF1 has been shown to co-immunoprecipitate with transcripts encoding TNF receptor super family members and other regulators of cytokine signaling (represented by a grey color and/or underlined text nodes in Fig. 2A) (Lee and others 2010; Rattenbacher and others 2010; Beisang and others 2012b). The outcomes of CELF1 binding to most of these transcripts on mRNA stability have not been studied and should be the focus of future experiments. Interestingly, both CELF1 and the ARE-binding protein ELAVL1 bind to and affect the stability of TNF mRNA (Dean and others 2001; Zhang and others 2008) and other transcripts involved in signaling that regulate apoptosis. For example, both CELF1 and ELAVL1 proteins regulate the stability of transcripts encoding members of the BCL2 superfamily such as BCL2, BNIP3, BCL2L2, BAD, and BAX, which modulate programmed cell death (Abdelmohsen and others 2007; Rattenbacher and others 2010; Talwar and others 2013). CELF1 also binds to transcripts that encode other regulators of apoptosis, including effector caspases and cytochrome c mRNA which are activated downstream of TNF receptors. Thus, proper signaling mediated by IL-1 cytokine requires the coordination of ARE and GRE networks.
TGF-β signals are transduced by trans-membrane type I and type II serine/threonine kinase receptors (TGFR1/2), which are encoded by GRE-containing mRNAs (Fig. 2A). TGF-β maintains tissue homeostasis by regulating cellular proliferation rate, differentiation, and survival. The activated TGF receptor complex induces oligomerization of several downstream SMAD protein family members. Activated SMADs regulate the transcription of a number of GRE-containing mRNAs (eg, CITED2, CXCL2, NKX, CDKN1A, VEGF, IL3RA, TGFBR1, etc.), which, in turn, become a subject of post-transcriptional regulation by CELF1, once they leave the nucleus.
As shown in Fig. 2A, multiple GRE-containing transcripts encode signaling components downstream of the IL-1 receptor. Thus, proper signaling mediated by IL-1 cytokine requires the coordination of ARE and GRE networks downstream of IL-1 receptor. Several of these signaling components, including STK4, CDC42, MYD88, TIFA, CREB, MEF2, RSK, RUNX1, and JUN, have been shown to be ELAVL1 targets in RNA-IP experiments (Mukherjee and others 2009). Many of target transcripts, shared by CELF1 and ELAVL1, are transcription factors or early response genes such as JUN that are transiently up-regulated after cellular activation and then down-regulated through rapid mRNA decay. Imbalance of these receptor signaling cascades affect mRNA turnover rates, and it could lead to diseases such as autoimmunity or cancer through aberrant immune cell activation, abnormal immune cell differentiation, excess inflammation, or inappropriate cell death [reviewed in Vlasova and others (2005); Moudgil and Choubey (2011); Fuxe and Karlsson (2012); Candido and Hagemann 2013)].
Many other IL and IFN receptors also utilize signaling proteins that are encoded by GRE-containing transcripts (Fig. 2B). Cytokines that bind to receptors containing the common gamma chain, such as IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, are pivotal for immune responses (Liao and others 2011). These receptors often activate Janus kinases (JAK1 and JAK2) to phosphorylate members of the STAT (Signal transducers and activators of transcription) family of transcription factors (Fig. 2B). STATs form hetero- or homodimers, and upon translocation into the nucleus, they induce the transcription of genes, including cytokine, chemokine, and adhesion molecules. One of the induced gene products, SOCS3, plays a role in inhibiting JAK kinase activity and, consequently, down-modulates immune activation (Linossi and others 2013). Interestingly, SOCS3 mRNA is also a CELF1 and ELAVL1 target, perhaps to enable its expression only at the appropriate time during the resolution phase of the immune response. Other GRE-containing transcripts, such as CCL1–5, IRF, ETS family, and NFAT family of mRNA molecules, encode proteins that play important roles in mediating the biological effects of cytokines. Overall, these cytokine signaling pathway analyses indicate that the GRE/CELF1 network plays important roles in regulating the expression of cytokine signaling components through mRNA degradation, thereby modulating the strength and duration of immune responses.
Cytokine Signaling Involves Cross-Talk Between ARE and GRE Networks
Numerous GRE-containing CELF1 target transcripts are also targets of the ARE-binding protein ELAVL1. RNA recognition sequences for CELF1 require precise GU repeats or GUUU repeats, whereas the recognition sequence for ELAVL1 is less precise, and ELAVL1 binds to a variety of U-rich sequences, including GU-rich sequences or a poly U sequence. Thus, CELF1 and ELAVL1 may compete for binding to the same target transcripts. In contrast to ELAVL1, other ARE-binding proteins that recognize precise AUUU repeats, such as ZFP36, do not bind to GRE sequences and do not compete for binding sites. To better understand the relationship between CELF1 and ELAVL1 target transcripts in cytokine signaling pathways, we compared CELF1 targets from normal T cells (Beisang and others 2012b) with ELAVL1 targets from HeLa (Lopez de Silanes and others 2004) and Jurkat cells (Mukherjee and others 2009), and show a subset of transcripts involved in cytokine signaling that are targets of both proteins (Table 1). The consequences of binding by these proteins to target transcripts often have opposite biological effects, with CELF1 mediating degradation and ELAVL1 mediating stabilization. We hypothesize that in resting cells, these transcripts are bound to CELF1 and targeted for degradation, but in activated immune cells, CELF1 becomes phosphorylated and is rendered unable to bind to GRE sequences. ELAVL1 then bind to the GRE, displacing CELF1, stabilizing the transcripts, and enabling them to be translated. Later in the immune response, CELF1 becomes dephosphorylated and then displaces ELAVL1 to mediate the transcripts' degradation. Further investigation is necessary to better characterize the interaction between CELF1 and ELAVL1.
Table 1.
Examples of Transcripts Involved in Cytokine Signaling That Have Been Shown to Be Targets of Both CELF1 and ELAVL1 in RNA-Immunoprecipitation Experiments
| Gene symbol | Gene title | Entrez gene | Function in cytokine signaling | Upstream cytokine |
|---|---|---|---|---|
| AEBP2 | AE-binding protein 2 | 121536 | Transcription regulator | TNF |
| ARCN1 | Archain 1 | 372 | Viral entry, replication | TNF |
| ATF4 | Activating transcription factor 4 (tax-responsive enhancer element B67) | 468 | Cell cycle progression | IL-2,5,15 |
| CDC42 | Cell division cycle 42 | 998 | Cell cycle progression | TNF |
| CLDND1 | Claudin domain containing 1 | 56650 | Adhesion, growth | TGF |
| DAZAP2 | DAZ-associated protein 2 | 9802 | Transcription | IL-17 |
| EIF4EBP2 | Eukaryotic translation initiation factor 4E binding protein 2 | 1979 | Translation | IFN-α/β |
| HNRNPC | Heterogeneous nuclear ribonucleoprotein C (C1/C2) | 3183 | mRNA processing | TGF |
| HNRNPDL | Heterogeneous nuclear ribonucleoprotein D-like | 9987 | mRNA processing | TGF |
| HNRNPH1 | Heterogeneous nuclear ribonucleoprotein H1 | 3187 | mRNA processing | TGF |
| HSPD1 | Heat shock 60 kDa protein 1 (chaperonin) | 3329 | Transport | IFN-γ |
| LCP2 | Lymphocyte cytosolic protein 2 (SH2 domain containing leukocyte protein) | 3937 | Cell cycle progression | IL-15 |
| OPTN | Optineurin | 10133 | Cell cycle progression | TNF |
| PAK2 | p21 protein (Cdc42)-activated kinase 2 | 5062 | Signaling | TNF |
| POLR2K | Polymerase (RNA) II polypeptide K | 5440 | Signaling, survival | IL-15 |
| RAB8B | RAB8B, member RAS oncogene family | 51762 | Cell cycle progression | IL-15 |
| RBPJ | Recombination signal-binding protein for immunoglobulin kappa J region | 3516 | Signaling | IL-5,15 |
| RPS6KA3 | Ribosomal protein S6 kinase, 90 kDa, 3 | 6197 | Signaling, survival | IL-1 |
| SFPQ | Splicing factor proline/glutamine-rich | 6421 | mRNA processing | IL-5 |
| SMAD2 | SMAD family member 2 | 4087 | Cell cycle progression | TGF |
| SUB1 | SUB1 homolog (S. cerevisiae) | 10923 | Transcription, survival | IL-4 |
| SUMO2 | SMT3 suppressor of mif 2 homolog 2 | 6613 | Protein expression | IFN-γ |
Gene symbol, the identifier of the mRNA in common targets dataset.
Entrez gene, according to http://ncbi.nlm.nih.gov/gene.
Upstream cytokine, canonical pathway based on Ingenuity Pathway Assistant Database Knowledge.
TNF, tumor necrosis factor; IL, interleukin; TGF, transforming growth factor; IFN, interferon.
Numerous studies describe an important role of ELAVL1 in the pathogenesis of inflammatory diseases that involve over-production of inflammatory cytokines or diseases characterized by cytokine deficiency. Most studies support a role for ELAVL1 in the promotion of inflammation and proliferation in human diseases such as autoimmune diseases and cancer (Khabar 2010; Srikantan and Gorospe 2012). In contrast, CELF1 functions as an inhibitor of a network of transcripts that promote cellular activation and proliferation (Beisang and Bohjanen 2012). In this way, CELF1 and ELAVL1 seem to have opposite effects with regard to sustaining inflammation and cellular proliferation. The opposing effects of CELF1 and ELAVL1 may have important implications for new therapies for proliferative diseases such as cancer or autoimmunity. Many studies show that ELAVL1 is over-expressed in a variety of malignancies and proliferative diseases (Srikantan and Gorospe 2012). The development of drugs that block binding by ELAVL1 to its mRNA targets could render these target transcripts vulnerable to degradation by CELF1, thereby promoting an anti-proliferative state. Thus, understanding the molecular mechanisms of CELF1 and ELAVL1 cross-talk may uncover novel therapeutic strategies for the blockade of proliferative or proinflammatory pathways involved in human diseases.
Concluding Remarks
Post-transcriptional networks defined by AREs and GREs regulate cytokine production and cytokine signaling. The ARE and GRE networks represent distinct subsets of transcripts that work in concert to coordinate the function of cytokines over the course of an immune response. AREs are critical regulators of cytokine production and play important roles in cytokine signaling, but a distinct subset of cytokine signaling components that are regulated by GREs is needed for the precise regulation of cytokine function. Thus, an effective immune response requires cross-talk between ARE and GRE pathways to appropriately regulate cytokine responses over time. A better understanding of the roles of the GRE, CELF1, and ELAVL1 as modulators of coordinate gene expression in immune cellular activation, cellular proliferation, and apoptosis may establish GRE/CELF1/ELAVL1 interactions as a therapeutic target in autoimmune diseases and cancer.
Acknowledgments
This work was supported by the National Institutes of Health grants AIO57484 and AIO72068 to P.R.B. The authors would like to thank the University of Minnesota Supercomputing Institute for providing the access to Ingenuity Pathway Assistant; Mai Lee Moua, for her critical reading of the article; and Robert St. Louis for his assistance with the preparation of the figures.
Author Disclosure Statement
No competing financial interests exist.
References
- Abdelmohsen K, Lal A, Kim HH, Gorospe M. 2007. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle 6(11):1288–1292 [DOI] [PubMed] [Google Scholar]
- Al-Haj L, Khabar KS. 2012. Cloning of cytokine 3′ untranslated regions and posttranscriptional assessment using cell-based GFP assay. Methods Mol Biol 820:91–104 [DOI] [PubMed] [Google Scholar]
- Anderson P. 2008. Post-transcriptional control of cytokine production. Nat Immunol 9(4):353–359 [DOI] [PubMed] [Google Scholar]
- Anderson P. 2010. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev Immunol 10(1):24–35 [DOI] [PubMed] [Google Scholar]
- Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS. 2001. ARED: Human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res 29(1):246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakheet T, Williams BR, Khabar KS. 2006. ARED 3.0: The large and diverse AU-rich transcriptome. Nucleic Acids Res 34(Database issue):D111–D114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisang D, Bohjanen P, Vlasova-St. Louis I. 2012a. CELF1, a multifunctional regulator of posttranscriptional networks. In: Abdelmohsen K, ed. Binding protein, Chapter 8. InTech(Open Access). DOI: 10.5772/48780, pp 181–206 [DOI] [Google Scholar]
- Beisang D, Bohjanen PR. 2012. Perspectives on the ARE as it turns 25 years old. Wiley Interdiscip Rev RNA 3(5):719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisang D, Rattenbacher B, Vlasova-St. Louis IA, Bohjanen PR. 2012b. Regulation of CUG-binding protein 1 (CUGBP1) binding to target transcripts upon T cell activation. J Biol Chem 287(2):950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronicki LM, Jasmin BJ. 2013. Emerging complexity of the HuD/ELAVl4 gene; implications for neuronal development, function, and dysfunction. RNA 19(8):1019–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SA, Blackshear PJ. 2013. Tristetraprolin (TTP): Interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta 1829(6–7):666–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido J, Hagemann T. 2013. Cancer-related inflammation. J Clin Immunol 33(Suppl 1):S79–S84 [DOI] [PubMed] [Google Scholar]
- Chen CY, Shyu AB. 1995. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem Sci 20(11):465–470 [DOI] [PubMed] [Google Scholar]
- Dean JL, Wait R, Mahtani KR, Sully G, Clark AR, Saklatvala J. 2001. The 3′ untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol Cell Biol 21(3):721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Shi J, Ge X, Dolken L, Moy W, He D, Shi S, Sanders AR, Ross J, Gejman PV. 2013. Genome-wide survey of interindividual differences of RNA stability in human lymphoblastoid cell lines. Sci Rep 3:1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons J, Townley-Tilson WH, Deleault KM, Skinner SJ, Gross RH, Whitfield ML, Brooks SA. 2008. Identification of TTP mRNA targets in human dendritic cells reveals TTP as a critical regulator of dendritic cell maturation. RNA 14(5):888–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Ishmael FT, Fang X, Myers A, Cheadle C, Huang SK, Atasoy U, Gorospe M, Stellato C. 2011. Chemokine transcripts as targets of the RNA-binding protein HuR in human airway epithelium. J Immunol 186(4):2482–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Romero M, Landin AM, Diaz A, Riley RL, Blomberg BB. 2010. Protein phosphatase 2A (PP2A) is increased in old murine B cells and mediates p38 MAPK/tristetraprolin dephosphorylation and E47 mRNA instability. Mech Ageing Dev 131(5):306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel CC, Dolken L, Ruzsics Z, Koszinowski UH, Zimmer R. 2009. Conserved principles of mammalian transcriptional regulation revealed by RNA half-life. Nucleic Acids Res 37(17):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe J, Karlsson MC. 2012. TGF-beta-induced epithelial-mesenchymal transition: A link between cancer and inflammation. Semin Cancer Biol 22(5–6):455–461 [DOI] [PubMed] [Google Scholar]
- Gratacos FM, Brewer G. 2010. The role of AUF1 in regulated mRNA decay. Wiley Interdiscip Rev RNA 1(3):457–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, Bartel DP. 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466(7308):835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halees AS, El-Badrawi R, Khabar KS. 2008. ARED organism: Expansion of ARED reveals AU-rich element cluster variations between human and mouse. Nucleic Acids Res 36(Database issue):D137–D140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halees AS, Hitti E, Al-Saif M, Mahmoud L, Vlasova-St. Louis IA, Beisang DJ, Bohjanen PR, Khabar K. 2011. Global assessment of GU-rich regulatory content and function in the human transcriptome. RNA Biol 8(4):681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Baltimore D. 2009. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol 10(3):281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Anderson P. 2013. Post-transcriptional regulatory networks in immunity. Immunol Rev 253(1):253–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janga SC, Mittal N. 2011. Construction, structure and dynamics of post-transcriptional regulatory network directed by RNA-binding proteins. Adv Exp Med Biol 722:103–117 [DOI] [PubMed] [Google Scholar]
- Keene JD. 2007. RNA regulons: Coordination of post-transcriptional events. Nat Rev Genet 8(7):533–543 [DOI] [PubMed] [Google Scholar]
- Khabar KS. 2007. Rapid transit in the immune cells: The role of mRNA turnover regulation. J Leukoc Biol 81(6):1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabar KS. 2010. Post-transcriptional control during chronic inflammation and cancer: A focus on AU-rich elements. Cell Mol Life Sci 67(17):2937–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabar KS, Young HA. 2007. Post-transcriptional control of the interferon system. Biochimie 89(6–7):761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Lee JY, Wilusz J, Tian B, Wilusz CJ. 2010. Systematic analysis of cis-elements in unstable mRNAs demonstrates that CUGBP1 is a key regulator of mRNA decay in muscle cells. PLoS One 5(6):e11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lin WJ, Chen CY, Si Y, Zhang X, Lu L, Suswam E, Zheng L, King PH. 2012. KSRP: A checkpoint for inflammatory cytokine production in astrocytes. Glia 60(11):1773–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. 2011. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol 23(5):598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linossi EM, Babon JJ, Hilton DJ, Nicholson SE. 2013. Suppression of cytokine signaling: The SOCS perspective. Cytokine Growth Factor Rev 24(3):241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. 2005. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol Cell Biol 25(21):9520–9531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. 2004. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci U S A 101(9):2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KD, Keene JD. 2009. The ribonome: A dominant force in co-ordinating gene expression. Biol Cell 101(3):169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AE, Young LE, Dixon DA. 2011. MicroRNA and AU-rich element regulation of prostaglandin synthesis. Cancer Metastasis Rev 30(3–4):419–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil KD, Choubey D. 2011. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J Interferon Cytokine Res 31(10):695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N, Lager PJ, Friedersdorf MB, Thompson MA, Keene JD. 2009. Coordinated posttranscriptional mRNA population dynamics during T-cell activation. Mol Syst Biol 5:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy V, Jakymiw A, Van Tubergen EA, D'Silva NJ, Kirkwood KL. 2012. Control of cytokine mRNA expression by RNA-binding proteins and microRNAs. J Dent Res 91(7):651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan A, Dhalla M, Bakheet T, Ogilvie RL, Vlasova IA, Khabar KS, Williams BR, Bohjanen PR. 2004. Patterns of coordinate down-regulation of ARE-containing transcripts following immune cell activation. Genomics 84(6):1002–1013 [DOI] [PubMed] [Google Scholar]
- Raghavan A, Robison RL, McNabb J, Miller CR, Williams DA, Bohjanen PR. 2001. HuA and tristetraprolin are induced following T cell activation and display distinct but overlapping RNA binding specificities. J Biol Chem 276(51):47958–47965 [DOI] [PubMed] [Google Scholar]
- Rattenbacher B, Beisang D, Wiesner DL, Jeschke JC, von Hohenberg M, St. Louis-Vlasova IA, Bohjanen PR. 2010. Analysis of CUGBP1 targets identifies GU-repeat sequences that mediate rapid mRNA decay. Mol Cell Biol 30(16):3970–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronkina N, Menon MB, Schwermann J, Tiedje C, Hitti E, Kotlyarov A, Gaestel M. 2010. MAPKAP kinases MK2 and MK3 in inflammation: Complex regulation of TNF biosynthesis via expression and phosphorylation of tristetraprolin. Biochem Pharmacol 80(12):1915–1920 [DOI] [PubMed] [Google Scholar]
- Schott J, Stoecklin G. 2010. Networks controlling mRNA decay in the immune system. Wiley Interdiscip Rev RNA 1(3):432–456 [DOI] [PubMed] [Google Scholar]
- Seko Y, Cole S, Kasprzak W, Shapiro BA, Ragheb JA. 2006. The role of cytokine mRNA stability in the pathogenesis of autoimmune disease. Autoimmun Rev 5(5):299–305 [DOI] [PubMed] [Google Scholar]
- Srikantan S, Gorospe M. 2012. HuR function in disease. Front Biosci 17:189–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklin G, Tenenbaum SA, Mayo T, Chittur SV, George AD, Baroni TE, Blackshear PJ, Anderson P. 2008. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J Biol Chem 283(17):11689–11699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo DJ, Broxmeyer HE, Ward T, Cooper S, Hangoc G, Chung YJ, Shelley WC, Richfield EK, Ray MK, Yoder MC, Aplan PD, Blackshear PJ. 2009. Targeted disruption of Zfp36l2, encoding a CCCH tandem zinc finger RNA-binding protein, results in defective hematopoiesis. Blood 114(12):2401–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo DJ, Lai WS, Blackshear PJ. 2010. Inflammation: Cytokines and RNA-based regulation. Wiley Interdiscip Rev RNA 1(1):60–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Stoecklin G, Van Way S, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP. 2007. Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-alpha mRNA. J Biol Chem 282(6):3766–3777 [DOI] [PubMed] [Google Scholar]
- Talwar S, Balasubramanian S, Sundaramurthy S, House R, Wilusz CJ, Kuppuswamy D, D'Silva N, Gillespie MB, Hill EG, Palanisamy V. 2013. Overexpression of RNA-binding protein CELF1 prevents apoptosis and destabilizes pro-apoptotic mRNAs in oral cancer cells. RNA Biol 10(2):277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Hodson D. 2012a. Regulation of lymphocyte development and function by RNA-binding proteins. Curr Opin Immunol 24(2):160–165 [DOI] [PubMed] [Google Scholar]
- Turner M, Hodson DJ. 2012b. An emerging role of RNA-binding proteins as multifunctional regulators of lymphocyte development and function. Adv Immunol 115:161–185 [DOI] [PubMed] [Google Scholar]
- Vacchelli E, Galluzzi L, Eggermont A, Galon J, Tartour E, Zitvogel L, Kroemer G. 2012. Trial Watch: Immunostimulatory cytokines. Oncoimmunology 1(4):493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kouwenhove M, Kedde M, Agami R. 2011. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer 11(9):644–656 [DOI] [PubMed] [Google Scholar]
- Vlasova IA, Bohjanen PR. 2008. Posttranscriptional regulation of gene networks by GU-rich elements and CELF proteins. RNA Biol 5(4):201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova IA, McNabb J, Raghavan A, Reilly C, Williams DA, Bohjanen KA, Bohjanen PR. 2005. Coordinate stabilization of growth-regulatory transcripts in T cell malignancies. Genomics 86(2):159–171 [DOI] [PubMed] [Google Scholar]
- Vlasova IA, Tahoe NM, Fan D, Larsson O, Rattenbacher B, Sternjohn JR, Vasdewani J, Karypis G, Reilly CS, Bitterman PB, Bohjanen PR. 2008. Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Mol Cell 29(2):263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova-St. Louis I, Bohjanen PR. 2011. Coordinate regulation of mRNA decay networks by GU-rich elements and CELF1. Curr Opin Genet Dev 21(4):444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova-St. Louis I, Dickson AM, Bohjanen PR, Wilusz CJ. 2013. CELFish ways to modulate mRNA decay. Biochim Biophys Acta 1829(6–7):695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Roretz C, Di Marco S, Mazroui R, Gallouzi IE. 2011. Turnover of AU-rich-containing mRNAs during stress: A matter of survival. Wiley Interdiscip Rev RNA 2(3):336–347 [DOI] [PubMed] [Google Scholar]
- Whelan JT, Hollis SE, Cha DS, Asch AS, Lee MH. 2012. Post-transcriptional regulation of the Ras-ERK/MAPK signaling pathway. J Cell Physiol 227(3):1235–1241 [DOI] [PubMed] [Google Scholar]
- Winzen R, Thakur BK, Dittrich-Breiholz O, Shah M, Redich N, Dhamija S, Kracht M, Holtmann H. 2007. Functional analysis of KSRP interaction with the AU-rich element of interleukin-8 and identification of inflammatory mRNA targets. Mol Cell Biol 27(23):8388–8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chesoni S, Rondeau G, Tempesta C, Patel R, Charles S, Daginawala N, Zucconi BE, Kishor A, Xu G, Shi Y, Li ML, Irizarry-Barreto P, Welsh J, Wilson GM, Brewer G. 2013. Combinatorial mRNA binding by AUF1 and Argonaute 2 controls decay of selected target mRNAs. Nucleic Acids Res 41(4):2644–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lee JE, Wilusz J, Wilusz CJ. 2008. The RNA-binding protein CUGBP1 regulates stability of tumor necrosis factor mRNA in muscle cells: Implications for myotonic dystrophy. J Biol Chem 283:22457–22463 [DOI] [PMC free article] [PubMed] [Google Scholar]