Abstract
Simian immunodeficiency virus (SIV) infection leads to AIDS in experimentally infected Rhesus macaques similarly to HIV-infected humans. In contrast, SIV infection of natural hosts is characterized by a down-regulation of innate acute responses to the virus within a few weeks of infection and results in limited pathology. Chloroquine (CQ) has been used in the treatment or prevention of malaria and has recently been shown to cause a decrease of immune activation and CD4 cell loss in HIV-infected individuals treated with antiretroviral therapy. Here, we treated Rhesus macaques with CQ during the acute phase of SIVmac251 infection with the intent to decrease viral-induced immune activation and possibly limit disease progression. Contrary to what was expected, CQ treatment resulted in a temporary increased expression of interferon (IFN)-stimulating genes and it worsened the recovery of CD4+ T cells in the blood. Our findings confirm recent results observed in asymptomatic HIV-infected patients and suggest that CQ does not provide an obvious benefit in the absence of antiretroviral therapy.
Introduction
Chronic infection by the human immunodeficiency virus-1 (HIV) is associated with increased proinflammatory cytokines and chemokines1,2 and the maintenance of a chronic state of immune activation.3–6 HIV-induced immunopathogenesis is associated with increased apoptosis of CD4+ T cells7,8 and deregulation of CD4+ and CD8+ T cell functions.9 Many of these immunopathogenic mechanisms may be dependent on overexpression of interferon alpha (IFN-α),3,10,11 a pleiotropic cytokine that exerts potent antiviral activity against HIV.12
Chloroquine (CQ) is a synthetic quinoline that has been used worldwide for the treatment of malaria and autoimmune diseases.13–15 CQ inhibits HIV infection in vitro by blocking envelope glycosylation16 and inhibits HIV replication in T cells and monocytes.17 More recently, CQ was suggested as an inexpensive drug for the treatment of AIDS patients18 and for reducing HIV-induced chronic immune activation. In vitro studies demonstrated that CQ inhibits HIV-induced expression of several immune activation markers on plasmacytoid dendritic cells (pDC).3,19–21 While in vivo studies in macaques chronically infected with SIVmac239 confirmed a reduction in pDC activation following CQ administration, no effects were seen in the viral load or cellular composition in the treated animals.22 Because HIV activates pDC through TLR, including TLR7,19,21 CQ may interfere with endosomal maturation of TLR7 and with the HIV effect on TLR7 signaling.23
In the clinical setting, treatment with hydroxychloroquine (HCQ) or with chloroquine of antiretroviral-treated (ART) 24 as well as ART-naive patients25 resulted in the reduction of immune activation but had little or no effect on CD4+ T cell recovery and viral load reduction, possibly due to the limited number of subjects enrolled in these studies. Surprisingly, HCQ treatment in ART-naive nonprogressors resulted in a worsening of CD4+ T cell loss.26 A NIAID-sponsored Phase II clinical trial is currently ongoing to determine whether treatment of HIV-infected patients would reduce HIV-induced immune activation (NCT00819390).
The simian immunodeficiency virus (SIV) has been shown to infect both the pathogenesis-susceptible Rhesus macaque (RM) nonnatural host species and the pathogenesis-resistant African Green monkey (AGM) and sooty mangabey (SM) species.27,28 Evidence also supports a role for IFN-α in SIV-induced immunopathogenesis. Indeed, SIV exposure results in higher levels of IFN-α production by pDC from RM than SM.29 In addition, two recent studies compared the dynamics of IFN-stimulated genes (ISG) during acute SIV infection in RM with those seen in AGM and SM. Both reports indicated comparable up-regulation of ISG in AGM, SM, and RM. However, ISG expression returned to preinfection levels within 4 weeks of infection in AGM and SM, but not in RM.30,31 These findings are consistent with the hypothesis that chronic innate immune activation contributes to the immunopathogenesis induced by HIV.3
The recent findings reported above demonstrate that a major distinction between the pathogenesis-susceptible RM and pathogenesis-resistant AGM and SM primate species involves differences not only in immune activation, but also in the dynamics of the innate immune responses.30,31 Based on the hypothesis that a major contributor to the pathogenesis of HIV is the persistence of chronic innate immunity, we designed and performed an experiment in which we attempted to interrupt the innate immune response of RM early in SIV infection. We infected RM with SIVmac251 and initiated a daily CQ treatment for 16 weeks consecutively, starting 7 days postinfection. CQ treatment did not result in decreased viral replication and, surprisingly, it was associated with an increased expression of ISG genes in the rectal mucosa. CQ treatment also resulted in decreased recovery of the CD4+ T cell count in the blood when compared to untreated animals. Our results suggest that CQ treatment given early in infection neither decreases immune activation nor provides any long-term therapeutic benefit.
Materials and Methods
Animals and study design
All of the animals used in this study were colony-bred rhesus macaques (Macaca mulatta) obtained from Covance Research Products (Alice, TX). The animals were housed and maintained in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Advanced BioScience Laboratories, Inc., Institutional Animal Care and Use Committee approved the protocol. All surgery was performed under general anesthesia, and all efforts were made to minimize suffering. All macaques were negative for simian retrovirus, simian T cell leukemia virus type 1, and herpesvirus B.
All the nine animals enrolled in the study were challenged intrarectally with a single high dose of 105 TCID50 of SIVmac251. Chloroquine was administered by gavage starting at day 7 for 112 consecutive days in seven animals while three animals were used as controls and they were left untreated. Each group was composed of one MamuA*01 animal.
Chloroquine and desethylchloroquine measurement
Plasma concentrations of CQ and its main metabolite monodesethylchloroquine (CQm) were quantified using solid-phase extraction and high-performance liquid chromatography with UV detection.32 Triplicates of quality control samples at three levels (i.e., 15 ng/ml, 972 ng/ml, and 2,916 ng/ml) were analyzed within each batch to ensure accurate and precise drug measurements. Relative standard deviations (RSD) of quality control samples were below 10% at all tested levels. The limit of detection was set to 5 ng/ml for both parent drug and metabolite.
Detection of viral replication in plasma and tissues
Plasma SIV-RNA was quantified by nucleic acid sequence-based amplification (NASBA), as previously described.33 SIV-DNA was quantified in the blood and tissue of macaques by quantitative polymerase chain reaction (PCR), as previously described.34
Enumeration of CD4+ T cells in blood and tissues
CD4+ T cell counts were periodically determined from whole blood by flow cytometry, as previously described.35 CD4+ T cell number, in tissue, was determined in rectal pinch biopsy specimens by immunohistochemistry. All slides were stained using the Dako autostainer (Dako Inc., Carpinteria, CA). Slides were visualized with epifluorescence illumination using a Zeiss Axioplan 2 microscope (Carl Zeiss) and appropriate filters. Digital images were captured and analyzed by using the Zeiss Axiocam System and Openlab software (Inprovision), as previously described.34 Briefly, the primary antibodies used included monoclonal anti-CD4+ T cell mouse serum antibody (clone IF6; Vector, Burlingame, CA). Binding of CD4+ T cells was detected using Alexa Fluor 488-labeled polyclonal goat antirabbit IgG (Molecular Probes, Eugene, OR). Slides were visualized with epifluorescence illumination using a Zeiss Axioplan 2 microscope (Carl Zeiss Inc., Thornwood, NY) and appropriate filters. Digital images were captured and analyzed by using a Zeiss Axiocam System and Openlab software (Inprovision Inc., Waltham, MA). Only cells with perfect membrane staining, strong fluorescence, and correct cellular structure were considered positive. The number of positive cells is presented as the number of cells per square millimeter.
Preparation of lymphocytes from lymph nodes
Mononuclear cells from lymph nodes were isolated by density-gradient centrifugation on Ficoll and resuspended in RPMI 1640 medium (GIBCO BRL, Gaithersburg, MD) containing 10% fetal bovine serum (FBS) (R-10). Whole blood was used for the detection of dendritic cells.
Flow cytometry
Cells were stained for surface marker expression by standard methods, and permeabilized and stained intracellularly using Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA). Acquisition was performed on a BD LSRII (BD Biosciences, San Jose, CA) and analyzed using FlowJo software, version 9 (Treestar, Portland, OR). The following antibodies were used to discriminate phenotypic markers on T cells: Ki67-FITC, CD4-PerCP-Cy5.5, CD3-Alexa700, and CCR5 PE from BD Biosciences and CD8-APC-Cy7 from Biolegend (San Diego, CA). One hundred microliters of peripheral blood mononuclear cells (PBMCs) was incubated with CD4-PerCP-Cy5.5, CD3-Alexa700, CD14-PerCP-Cy5.5, and CD20-PerCP-Cy5.5, HLA-DR− APC-Cy7) (Becton-Dickinson); CD11c-APC (Pharmingen, San Jose, CA); and PE-conjugated antibody to CD123. The cells were incubated at 4°C for 30 min and were then washed twice with phosphate-buffered saline (PBS) solution. The percentage of mDC [lin−/HLA-DR+/CD123−/CD11c+] and the percentage of pDC [lin−/HLA-DR+/CD123bright] were determined.
RT-PCR
For real-time PCR, total RNA was extracted from rectal biopsy samples with RNeasy Plus (Qiagen) and was retrotranscribed with a Quantitect Reverse Transcription kit (Qiagen) following the manufacturer's instruction. The cDNA quantification for 18S, myxovirus resistance GTPase protein A (MxA), apolipoprotein B mRNA-editing, 2′,5′-oligoadenylate synthetase (OAS) was performed by real-time PCR (ABI 7000). Reactions were performed using a SYBRGreenEr PCR mix (Invitrogen), following the manufacturer's instructions. Primer sequences were designed to specifically target 18S, 5′-GCCCGAAGCGTTTACTTTGA-3′ [forward] and 5′-TCCATTATTCCTAGCTGCGGTATC-3′ [reverse]); for OAS, 5′-GGAAA GGGCTCCAGTGTTATC-3′ [forward] and 5′-GGATCAAGAGTCCCACCTG AAAA-3′ [reverse]; and for MxA, 5′-AGGAGTTGCCCTTCCCAGA-3′ [forward] and 5′-TCGTTCACAAGTTTCTTCAGTTTCA-3′ [reverse].36,37 Results were expressed as ΔΔCt (where Ct is the cycle threshold) and presented as ratios between the target gene and the 18S housekeeping mRNA.
Data and statistical analyses
Statistical analyses were performed with the Mann–Whitney U test to compare the values of two different groups, or with the paired Student's t-test for comparison of values in two samples from the same group. The p-values less than 0.05 were considered to indicate statistical significance. The correlation between variables was analyzed by linear regression analysis.
Results
Study design and CQ kinetic in plasma
Nine adult Rhesus macaques were challenged intrarectally with 105 TCID50 of SIVmac251. At 1 week from the challenge (day 7), six macaques (P265, P267, P268, P269, P270, and P272) were started on a daily dose of 100 mg of CQ given by gavage for 16 consecutive weeks (112 days) with the intent to decrease immune activation in the gut and possibly viral replication during the set point, as observed in AGM and SM30,31 (Fig. 1A). Three macaques were left untreated (P266, P273, and P274). The dose was approximately 18.7 mg/kg corresponding to almost double the amount typically given to arthritis patients38 and was carefully chosen to limit drug-related toxicity and side effects.39 We measured the levels of chloroquine and CQm, its pharmacologically active metabolite, in the plasma of all nine animals (Fig. 1B and C). Both CQ and CQm levels increased in the plasma of the six treated macaques and reached a peak at week 11/16 and began to decrease, as expected, when CQ administration was ceased at week 16. Of note, animal P267 presented a different kinetic for plasma CQ accumulation with levels around 500 ng/ml of plasma at week 5 followed by a rapid contraction at week 11 (Fig. 1B and C).
FIG. 1.
(A) Study design. (B) Chloroquine (CQ) and (C) monodesethylchloroquine (CQm) concentration equivalents in plasma samples collected at baseline (week −3) and after infection.
Chloroquine treatment during the acute phase of infection did not affect the level of cell activation in the lymph nodes and the gut
Treatment with chloroquine has resulted in decreased immune activation both in preclinical and clinical settings. To test the hypothesis that CQ treatment may decrease immune activation, we measured the frequency of the proliferation marker Ki67+ and the activation marker CD69+ on CD8+ T and CD4+ T cells from blood mononuclear cells collected at weeks 2 and 16, the latter time point corresponding to the peak of CQ levels in the plasma. No significant differences were observed in immune activation in this site between four CQ-treated and three untreated animals at any time point considered (Fig. 2A–D). Interestingly, the frequency of CCR5 and of Ki67+ CD4+ and CD8+ T cells measured in the jejunum at week 2 postinfection showed some trends for decrease in treated animals when compared to untreated animals (Supplementary Fig. S1A and B; Supplementary Data are available online at www.liebertpub.com/aid), but the overall differences in activation and proliferation in the tissue did not reach statistical significance possibly because of the small number of animals. No differences were observed in the frequency of CCR5 and of Ki67+ CD4+ and CD8+ T in lymph nodes analyzed at the same time point (Supplementary Fig. S1C and D).
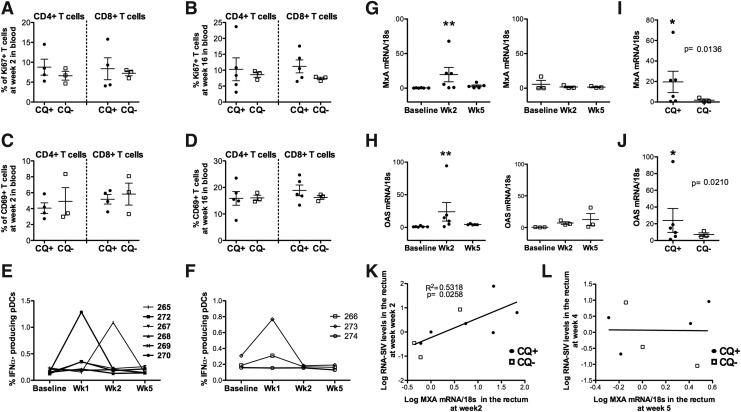
FIG. 2.
(A, B) Frequency of Ki67+ and (C, D) CD69+ CD4+ and CD8+ T cells in blood collected in four CQ macaques and three controls at weeks 2 and 16 after infection. (E) Frequency of circulating plasmacytoid dendritic cells (pDC) defined as lineage negative (CD3−, CD20−, CD14−, CD16−)/HLA-DR+/CD123+ that produce interferon (IFN)-α after in vitro stimulation with CpG in CQ-treated and (F) untreated animals. (G) Levels of myxovirus resistance-A (MxA)-RNA and (H) 2′,5′-oligoadenylate synthetase (OAS)-RNA levels measured in total rectal mucosa tissue at baseline (week −3) and 2 and 5 weeks after infection in CQ-treated (left panels) and untreated animals (right panels). (I) Levels of MxA-RNA and (J) OAS-RNA measured in total rectal mucosa tissue 2 weeks after infection. Significant p-values are indicated by an asterisk (*p<0.05, **p<0.005). (K) Correlation between the log transformed levels of MxA-RNA in the rectal mucosa and the RNA-SIV levels measured at the same site at week 2 and (L) at week 5.
Type I interferons (IFN-α/β) play an important role as a first line defense mechanism during innate immune responses against viral infection.40 In vivo CQ reduces HIV-induced production of IFN-α/β by pDC, which in turn could mitigate the CD4+ T cell loss during the first weeks of infection. In our study we did not observe differences in the frequency of monocytes and pDCs, nor in the percentage of pDC producing IFN-α in the blood of CQ-treated versus untreated macaques, within the first weeks of infection over the course of the infection (Fig. 2E and F).
In sooty mangabeys a decrease in immune activation is observed at the set point after SIV infection, corresponding to weeks 4 to 6 in this model.30,31 Although we could not measure the accumulation of the drugs in the mucosal tissues, studies have shown that CQ accumulates in tissues before being released in the blood.13–15 Therefore, we measured the levels of interferon-inducible genes 2′,5′-oligoadenylate synthetase 1 (OAS-1) and myxovirus resistance-A (MxA) by RT-PCR in rectal mucosa sampled from all the animals at weeks 2 and 5 postinfection (Fig. 2G–J). Surprisingly, the level of expression of both OAS-1 and MxA significantly increased at week 2 after infection (p=0.008 and p=0.003, respectively) (Fig. 2G and H) only in CQ-treated animals. Although the significant difference was probably driven by one animal (P270) for both measurements, overall this difference was observed only in the CQ-treated group. Also, at week 2 CQ-treated animals had significantly higher levels of both OAS-1 and MxA when compared to nontreated animals (p=0.02, p=0.013, respectively) (Fig. 2I and J). Interestingly, when all treated and untreated animals were considered, the levels of MxA but not of OAS at week 2 directly correlated with the RNA-SIV levels at the mucosal sites at the same time point (Fig. 2K and data not shown). No correlation was found with the levels of both interferon-inducible genes at week 5 and the RNA-SIV levels measured at week 4 (Fig. 2L).
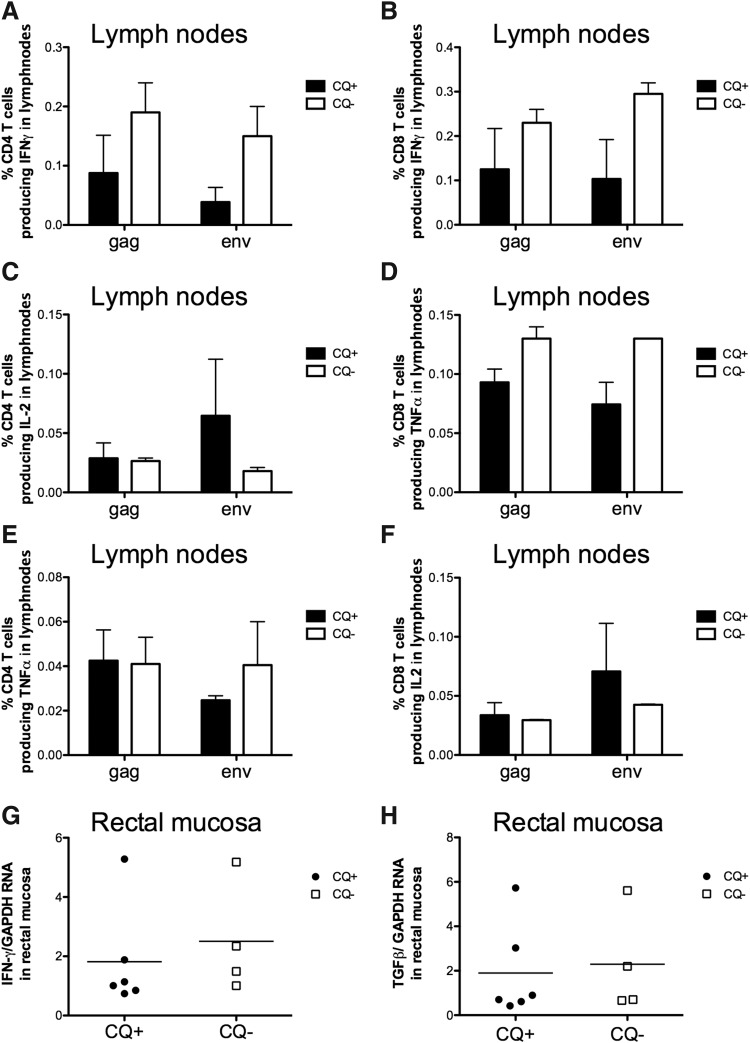
Changes in immune activation could impact specific T cell responses against the virus. Moreover, CQ treatment has been shown to reduce both innate and adaptive responses. Thus, we measured the frequency of CD4+ and CD8+ T cells isolated from the lymph nodes and producing interleukin (IL)-2, IFN-γ, or tumor necrosis factor (TNF)-α after stimulation with gag or envelope overlapping peptides (Fig. 3). Although, we observed a trend for a decrease, no significant differences were observed between CQ-treated and nontreated animals (Fig. 3A–F) in either the frequency of CD4+ T cells or of CD8+ T cells producing cytokines at the peak of viral load (week 2). Also no differences were found in the quality of the immune responses in CQ-treated versus nontreated animals (data not shown). Lastly, the RNA levels for IFN-γ and TGF-β were also analyzed at the same time point in the rectal mucosa by RT-PCR and no changes were observed within the two groups (Fig. 3G and H).
FIG. 3.
(A) Frequency of memory (CD95+) CD4+ and (B) CD8+ T cells producing interferon (IFN)-γ, (C, D) interleukin (IL)-2, and (E, F) tumor necrosis factor (TNF)-α after in vitro stimulation with SIV-gag and env overlapping peptides of cells isolated from peripheral lymph nodes collected at 2 weeks after infection. Values obtained from unstimulated cells are subtracted. (G) Levels of IFN-γ RNA and (H) tumor growth factor (TGF)-β RNA in rectal mucosa intact tissue collected 2 weeks after the challenge time.
Effects of chloroquine treatment during the acute phase of infection on viral replication and CD4+ T cell loss
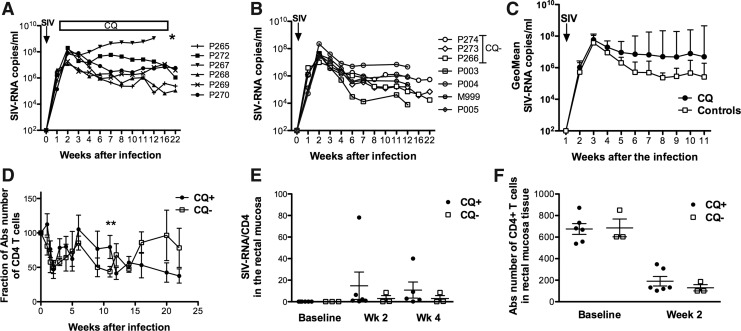
CQ has been shown to inhibit HIV-1 replication in vitro and in vivo through its ability to alter the glycosylation pattern of the gp120 envelope and to reduce CD4+ T cell infectivity mediated by the interaction with DC-SIGN.41 The effects of CQ treatment during the acute phase of infection are unknown. Thus, we measured the SIV-RNA levels in the plasma and in the rectal mucosal (Fig. 4). All the animals were infected by a single high dose of SIVmac251. Treatment with chloroquine did not decrease viral replication compared to the three untreated animals (P266, P273, and P274) and to four additional historical controls challenged with the same viral stock (P003, P004, P005, and M999) (Fig. 4A–C). Conversely, we observed a trend for increased viral load in CQ-treated animals at set point. Moreover, one of the six treated animals, P267, rapidly progressed to disease and was euthanized at week 12. Interestingly, CQ plasma levels had peaked earlier in P267 compared to the other animals in the same group (Fig. 1B). Despite this observation, no correlation was found between the CQ levels and the SIV-RNA levels in the plasma (data not shown). The CD4+ T cell number in the blood did differ in treated animals only at week 12 from infection (p=0.003), but controls underwent better regeneration of CD4+ T cells during the chronic phase of infection. (Fig. 4D).
FIG. 4.
(A) SIV-RNA measured in plasma of six animals treated with chloroquine and (B) three concurrent (CQ−) and four historical controls. (C) Geometric mean of SIV-RNA levels in the plasma in six treated animals (CQ) and seven nontreated macaques. (D) Changes of CD4+ T cell count in blood with respect to the baseline levels in six treated and three untreated macaques (CQ−). The average±standard error is shown for each group. (E) SIV-RNA measured in rectal mucosal biopsies collected at baseline and at weeks 2 and 4 after infection by PCR. (F) Absolute number of CD4+ T cells measured in rectal mucosa.
There was no difference in the SIV-RNA levels (Fig. 4E) or in the CD4+ T cell count at the rectal mucosa level between the two groups (Fig. 4F). These data go hand to hand with a trend for a possible early decrease of immune activation in the CQ-treated macaques, but overall the data were not significantly different between the two groups.
Discussion
Chronic immune activation is a hallmark of HIV infection in humans and pathogenic SIV infection in nonnatural nonhuman primate (NHP) hosts. Conversely, SIV infection of natural NHP hosts such as sooty mangabeys or African green monkeys does not induce signs of chronic immune activation.42 It has been proposed that the continuous stimulation of IFN-α-producing pDCs may lead to CD4+ loss and lack of CD4+ T cell regeneration as well as the onset of chronic immune activation.29–31 Moreover, the defective CD4+ T cell recovery observed in HIV-infected immunological nonresponders may be associated with TLR-mediated immune activation that is driven by microbial translocation.43–45
CQ is a diprotic weak base traditionally used for treating malaria and some autoimmune diseases.46,47 CQ down-regulates toll-like receptors TLR4 and TLR9-mediated immune activation, resulting in decreased cytokine release even in the presence of potent activators of the immune system such as bacterial DNA or lipopolysaccharide (LPS).48–51 Moreover, CQ has been shown to block HIV-induced IFN-α production and PDL-1 expression by pDC in cultures of PBMCs from healthy donors.52 Because of its immune-modulatory and antiretroviral properties, CQ has been proposed to be an anti-HIV drug. However, two small clinical trials reported only modest reduction in plasma virus of 0.4–0.6 log copies/ml. More recently, two studies have shown that prolonged CQ treatment during chronic HIV infection has resulted in decreased immune activation and a reduction of viral replication in ART-treated patients. In contrast, CQ treatment of nonprogressors resulted in a greater decline of CD4+ T cells in CQ-treated rather then nontreated subjects, and no effects on immune activation were observed.26
Here, we reason that therapies to decrease immune activation soon after the peak of viral replication, thus mimicking the drop in inflammatory responses observed in the nonpathogenic animal model of HIV infection, might be of benefit in slowing HIV/SIV disease progression.
We treated six animals with CQ given by gavage to target the Gut-associated lymphoid tissue (GALT), where most of the inflammation occurs during the HIV/SIV acute phase. Surprisingly, the treatment changed the immune activation status of the mucosal site and increased the expression of ISG (OAS and MxA) in the rectal mucosa. Although no significant differences were found between the levels of RNA-SIV measured from the all rectal mucosa tissue collected from treated and untreated animals (Fig. 1H), we observed a significant correlation between the levels of MxA and the levels of RNA-SIV at the mucosal site at week 2. Nonetheless, the increase in interferon-inducible genes was temporary and occurred only at the mucosal site, while in the periphery, the frequency of the marker of immune activation showed only a trend for reduction in the CQ-treated animals that was not significant, nor was reflected by the RNA levels in the two groups in the SIV-RNA levels in the blood.
Type I IFN provides a potent innate immune mechanism against a wide range of viruses, including HIV, but it may also promote pathogenic immune activation, thus resulting in greater loss of activated CD4+ T cells. Both untreated and CQ-treated animals suffered a deep loss of CD4+ T cells during the acute phase of infection, but the ability to regenerate peripheral CD4+ T cells was apparently improved early on but hampered by CQ treatment in the long term. Interestingly, CQ treatment during chronic SIV infection demonstrated a reduction of immune activation and a better recovery of CD4+ T cells but this did not affect virus levels.22 Although our study was limited by the small number of control animals, the data suggest that treatment with CQ during the acute onset of infection may not sufficiently reduce chronic immune activation to have a beneficial effect in the long term.
We did observe an overall decrease in the frequency of SIV-specific T cell responses in CQ-treated animals during acute infection, but it is possible that the down-modulatory effect on immunoglobulin levels or on natural killer activity (not demonstrated here) may also have participated to the apparent worst outcome in this group in the long term.
When considered together with the recent results of human clinical trials, our study suggests that immunotherapeutic approaches aimed at dampening antiviral innate immunity similar to CQ may not be beneficial when SIV/HIV replication occurs at a high rate, such as in acute infection. Further studies are necessary to determine whether CQ or similar approaches can be applied later on during the chronic phase or under HAART regimen or in specific subsets of patients depending on disease phenotype such as in immunologic nonresponders.24
Supplementary Material
Acknowledgments
We would like to thank J. Treece, D. Weiss, and P. Markham for animal husbandry and care and Teresa Habina for manuscript editing. This work was supported by federal funds from the National Cancer Institute, National Institutes of Health. The Mahidol-Oxford Tropical Medicine Research Unit is supported by the Welcome Trust of Great Britain.
Author Disclosure Statement
There are no conflicts of interest.
References
- 1.Valdez H. and Lederman MM: Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev 1997;1997–1998:187–228 [PubMed] [Google Scholar]
- 2.Connolly NC, Riddler SA, and Rinaldo CR: Proinflammatory cytokines in HIV disease–a review and rationale for new therapeutic approaches. AIDS Rev 2005;7(3):168–180 [PubMed] [Google Scholar]
- 3.Boasso A. and Shearer GM: Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol 2008;126(3):235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascher MS, Sheppard HW, Krowka JF, and Bremermann HJ: AIDS as immune system activation. Key questions that remain. Adv Exp Med Biol 1995;374:203–210 [DOI] [PubMed] [Google Scholar]
- 5.Sodora DL. and Silvestri G: Immune activation and AIDS pathogenesis. AIDS 2008;22(4):439–446 [DOI] [PubMed] [Google Scholar]
- 6.Douek D: HIV disease progression: Immune activation, microbes, and a leaky gut. Top HIV Med 2007;15(4):114–117 [PubMed] [Google Scholar]
- 7.Herbeuval JP, Nilsson J, Boasso A, et al.: Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci USA 2006;103(18):7000–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Accornero P, Radrizzani M, Delia D, et al.: Differential susceptibility to HIV-GP120-sensitized apoptosis in CD4+ T-cell clones with different T-helper phenotypes: Role of CD95/CD95L interactions. Blood 1997;89(2):558–569 [PubMed] [Google Scholar]
- 9.Boasso A, Hardy AW, Anderson SA, Dolan MJ, and Shearer GM: HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One 2008;3(8):e2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbeuval JP. and Shearer GM: HIV-1 immunopathogenesis: How good interferon turns bad. Clin Immunol 2007;123(2):121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boasso A, Vaccari M, Fuchs D, et al.: Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J Immunol 2009;182(7):4313–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitha PM: Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antiviral Res 1994;24(2–3):205–219 [DOI] [PubMed] [Google Scholar]
- 13.Fox NW. and McArthur CP: Acquired immunodeficiency syndrome—a review and update History, characteristics of human immunodeficiency virus infection, its replication, biologic factors, and pathogenicity. Arch Fam Med 1993;2(10):1068–1077 [DOI] [PubMed] [Google Scholar]
- 14.Wallace DJ: The use of chloroquine and hydroxychloroquine for non-infectious conditions other than rheumatoid arthritis or lupus: A critical review. Lupus 1996;5(Suppl 1):S59–S64 [PubMed] [Google Scholar]
- 15.Klinger G, Morad Y, Westall CA, et al.: Ocular toxicity and antenatal exposure to chloroquine or hydroxychloroquine for rheumatic diseases. Lancet 2001;358(9284):813–814 [DOI] [PubMed] [Google Scholar]
- 16.Tsai WP, Nara PL, Kung HF, and Oroszlan S: Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res Hum Retroviruses 1990;6(4):481–489 [DOI] [PubMed] [Google Scholar]
- 17.Sperber K, Kalb TH, Stecher VJ, Banerjee R, and Mayer L: Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res Hum Retroviruses 1993;9(1):91–98 [DOI] [PubMed] [Google Scholar]
- 18.Savarino A, Lucia MB, Rastrelli E, et al.: Anti-HIV effects of chloroquine: Inhibition of viral particle glycosylation and synergism with protease inhibitors. J Acquir Immune Defic Syndr 2004;35(3):223–232 [DOI] [PubMed] [Google Scholar]
- 19.Beignon AS, McKenna K, Skoberne M, et al.: Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest 2005;115(11):3265–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt B, Ashlock BM, Foster H, Fujimura SH, and Levy JA: HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology 2005;343(2):256–266 [DOI] [PubMed] [Google Scholar]
- 21.Hardy AW, Graham DR, Shearer GM, and Herbeuval JP: HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci USA 2007;104(44):17453–17458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma M, Wang L, Yang J, et al.: Age-related impaired Th1 responses to PRV vaccine in vivo in aged pigs. Mol Immunol 2012;52(3–4):217–223 [DOI] [PubMed] [Google Scholar]
- 23.Rutz M, Metzger J, Gellert T, et al.: Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur J Immunol 2004;34(9):2541–2550 [DOI] [PubMed] [Google Scholar]
- 24.Piconi S, Parisotto S, Rizzardini G, et al.: Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 2011;118(12):3263–3272 [DOI] [PubMed] [Google Scholar]
- 25.Murray SM, Down CM, Boulware DR, et al.: Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol 2010;84(22):12082–12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton NI, Goodall RL, Dunn DT, et al.: Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. JAMA 2012;308(4):353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liovat AS, Jacquelin B, Ploquin MJ, Barre-Sinoussi F, and Muller-Trutwin MC: African non human primates infected by SIV—why don't they get sick? Lessons from studies on the early phase of non-pathogenic SIV infection. Curr HIV Res 2009;7(1):39–50 [DOI] [PubMed] [Google Scholar]
- 28.Paiardini M, Pandrea I, Apetrei C, and Silvestri G: Lessons learned from the natural hosts of HIV-related viruses. Annu Rev Med 2009;60:485–495 [DOI] [PubMed] [Google Scholar]
- 29.Mandl JN, Barry AP, Vanderford TH, et al.: Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med 2008;14(10):1077–1087 [DOI] [PubMed] [Google Scholar]
- 30.Jacquelin B, Mayau V, Targat B, et al.: Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest 2009;119(12):3544–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosinger SE, Li Q, Gordon SN, et al.: Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest 2009;119(12):3556–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blessborn D, Neamin G, Bergqvist Y, and Lindegardh N: A new approach to evaluate stability of amodiaquine and its metabolite in blood and plasma. J Pharm Biomed Anal 2006;41(1):207–212 [DOI] [PubMed] [Google Scholar]
- 33.Romano JW, Shurtliff RN, Dobratz E, et al.: Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods 2000;86(1):61–70 [DOI] [PubMed] [Google Scholar]
- 34.Vaccari M, Boasso A, Ma ZM, et al.: CD4+ T-cell loss and delayed expression of modulators of immune responses at mucosal sites of vaccinated macaques following SIV(mac251) infection. Mucosal Immunol 2008;1(6):497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaccari M, Trindade CJ, Venzon D, Zanetti M, and Franchini G: Vaccine-Induced CD8+ central memory T cells in protection from simian AIDS. J Immunol 2005;175(6):3502–3507 [DOI] [PubMed] [Google Scholar]
- 36.Boasso A, Herbeuval JP, Hardy AW, Winkler C, and Shearer GM: Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood 2005;105(4):1574–1581 [DOI] [PubMed] [Google Scholar]
- 37.Hughes R, Towers G, and Noursadeghi M: Innate immune interferon responses to human immunodeficiency virus-1 infection. Rev Med Virol 2012;22(4):257–266 [DOI] [PubMed] [Google Scholar]
- 38.Miller DR, Khalil SK, and Nygard GA: Steady-state pharmacokinetics of hydroxychloroquine in rheumatoid arthritis patients. DICP 1991;25(12):1302–1305 [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, and Hopkins JL: Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci 1978;17(12):1158–1175 [PubMed] [Google Scholar]
- 40.Samuel CE: Antiviral actions of interferons. Clin Microbiol Rev 2001;14(4):778–809, table [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naarding MA, Baan E, Pollakis G, and Paxton WA: Effect of chloroquine on reducing HIV-1 replication in vitro and the DC-SIGN mediated transfer of virus to CD4+ T-lymphocytes. Retrovirology 2007;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestri G, Sodora DL, Koup RA, et al.: Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 2003;18(3):441–452 [DOI] [PubMed] [Google Scholar]
- 43.Brenchley JM, Price DA, Schacker TW, et al.: Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12(12):1365–1371 [DOI] [PubMed] [Google Scholar]
- 44.Funderburg N, Luciano AA, Jiang W, et al.: Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One 2008;3(4):e1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benveniste O, Flahault A, Rollot F, et al.: Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. J Infect Dis 2005;191(10):1670–1679 [DOI] [PubMed] [Google Scholar]
- 46.Fox RI: Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum 1993;23(2 Suppl 1):82–91 [DOI] [PubMed] [Google Scholar]
- 47.Wallace DJ: Antimalarial agents and lupus. Rheum Dis Clin North Am 1994;20(1):243–263 [PubMed] [Google Scholar]
- 48.Macfarlane DE. and Manzel L: Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol 1998;160(3):1122–1131 [PubMed] [Google Scholar]
- 49.Manzel L, Strekowski L, Ismail FM, Smith JC, and Macfarlane DE: Antagonism of immunostimulatory CpG-oligodeoxynucleotides by 4-aminoquinolines and other weak bases: Mechanistic studies. J Pharmacol Exp Ther 1999;291(3):1337–1347 [PubMed] [Google Scholar]
- 50.Weber F, Kochs G, and Haller O: Inverse interference: How viruses fight the interferon system. Viral Immunol 2004;17(4):498–515 [DOI] [PubMed] [Google Scholar]
- 51.Hong Z, Jiang Z, Liangxi W, et al.: Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int Immunopharmacol 2004;4(2):223–234 [DOI] [PubMed] [Google Scholar]
- 52.Martinson JA, Montoya CJ, Usuga X, et al.: Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: Implication for T-cell activation. Antimicrob Agents Chemother 2010;54(2):871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.