Abstract
Mitochondria are no longer considered to be solely the static powerhouses of the cell. While they are undoubtedly essential to sustaining life and meeting the energy requirements of the cell through oxidative phosphorylation, they are now regarded as highly dynamic organelles with multiple funtions, playing key roles in cell survival and death. In this review, we discuss the emerging role of mitochondrial fusion and fission proteins, as novel therapeutic targets for treating a wide range of cardiovascular diseases.
Linked Articles
This article is part of a themed issue on Mitochondrial Pharmacology: Energy, Injury & Beyond. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2014.171.issue-8
Keywords: mitochondrial fusion, mitochondrial fission, MFN1, MFN2, Drp1, OPA1, mitochondrial morphology, mitochondrial dynamics
Introduction
Mitochondria are essential for eukaryotic life, meeting the cells’ energetic requirements through oxidative phosphorylation. This is especially true in the heart, where the mitochondria occupy around 30% of the total cell volume and produce a staggering 30 kg of ATP per day, in order to sustain normal contractile function of the heart. While their primary role is to generate ATP, mitochondria also play a number of important roles which affect cell survival and death (Pellegrini and Scorrano, 2007).
These important secondary roles mean that mitochondrial biology is a rejuvenated field, with mitochondrial dysfunction at the root of numerous diseases. In particular, the field of mitochondrial dynamics, in which mitochondria are dynamic organelles, and are able to change their morphology by undergoing fusion and fission, has recently been investigated and shown to be relevant to the cardiovascular system (Ong et al., 2013). This review investigates the role of the mitochondrial fusion and fission proteins as novel therapeutic targets for combating cardiovascular disease.
Changing mitochondrial morphology by fusion and fission
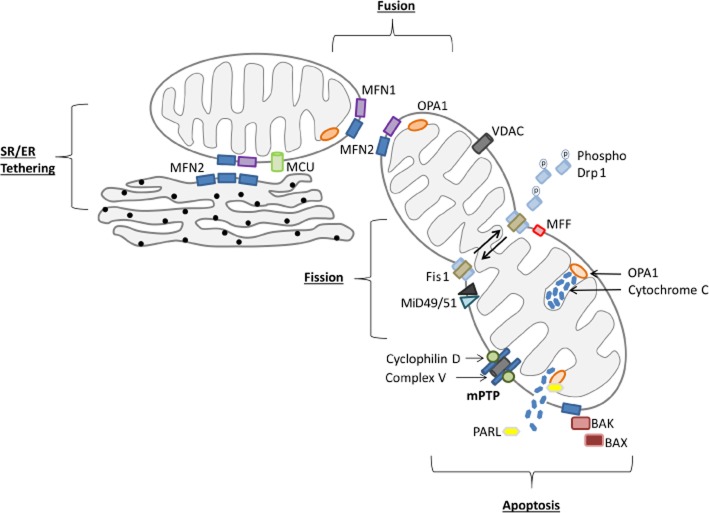
Proteins involved in shaping mitochondria are grouped as either mitochondrial fusion proteins (joining individual mitochondria together to become one), mitochondrial fission proteins (dividing one mitochondrion into two mitochondria) or mitochondrial distribution proteins (those which influence mitochondrial morphology through movement of mitochondria around the cell such as Miro and Milton) (see Figure 1 for summary).
Figure 1.
Mitochondrial fusion and fission proteins and their roles in changing mitochondrial morphology. MFN1, MFN2 and OPA1 are all key to mediating mitochondrial fusion, while Drp1 interacts with Fis1, MFF and MiD49/51 to mediate mitochondrial fission. Secondary to these mitochondrial-shaping roles, MFN2 tethers the SR/ER to the mitochondria allowing efficient Ca2+ transfer between the organelles. The mitochondria are also key to apoptosis with BAK and BAX binding to MFN2 stabilizing Drp1 binding. Drp1 binding leads to mitochondrial fragmentation and the release of cytochrome c. Cytochrome c is normally sequestered by OPA1 in the cristae, although the OPA1 oligomers maintaining cristae shape are disrupted by BAK and BAX binding.
Mitochondrial fusion
The fusion proteins are the outer mitochondrial membrane (OMM) proteins, mitofusin 1 (MFN1) and mitofusin 2 (MFN2), and the inner mitochondrial membrane (IMM) protein, optic atrophy factor 1 (OPA1) (as shown in Figure 1). These proteins hydrolyze GTP to fuse two neighbouring mitochondria together to allow sharing of mitochondrial DNA, proteins and metabolites. MFN1 and MFN2 mediate OMM fusion through the formation of either heterodimers [i.e. MFN1 binding to MFN2, and the most efficient method of fusion (Hoppins et al., 2011)] or homodimers (MFN2 binding to MFN2) (Chen et al., 2003; Koshiba et al., 2004). Fusion of IMM is mediated by OPA1 (Malka et al., 2005; Song et al., 2009), which itself is regulated by nuclease cleavage at the mRNA level, and proteolytic cleavage at the protein level (Song et al., 2007). In the absence of OPA1, MFN1 and MFN2 mediate only superficial mitochondrial fusion with the IMMs remaining unfused. This lack of IMM fusion and matrix content mixing result in loss of mitochondrial heterogeneity and the occurrence of metabolic disturbances, confirming that a key role of mitochondrial fusion is the mixing of matrix contents (mitochondrial DNA, metabolites and proteins) (Chen et al., 2005; Song et al., 2009). Whole body genetic ablation of MFN1 and MFN2 results in embryonic lethality due to a major placental defect (Chen et al., 2003). Mutations in human MFN2 are responsible for the autosomal dominant neurodegenerative disease Charcot-Marie-Tooth type 2A, a peripheral sensorimotor neuropathy (Zuchner et al., 2004). Mutations in the OPA1 gene were first identified in 2000 and found to be associated with the human neurogenerative condition, autosomal dominant optic atrophy (Delettre et al., 2000). How a deficiency in the mitochondrial fusion protein MFN2 or OPA1 results in such a specific neurological condition is currently unknown.
Mitochondrial fission
Mitochondrial fission describes the fragmentation or division of one mitochondrion into two or more mitochondria. Its main functions include: (i) increasing mitochondrial numbers to ensure mitochondria are passed on to daughter cells during mitosis (Taguchi et al., 2007); (ii) enabling shuttling of mitochondria to other regions of the cell; (iii) signalling to the cell that it is damaged and needs to be removed by mitophagy thereby maintaining a healthy mitochondrial network (Lee et al., 2011); and (iv) the initiation of apoptotic cell death (Lee et al., 2004).
The process of mitochondrial fission occurs through two coupled mechanisms. The first is the inhibition of the mitochondrial fusion proteins, and the second is the recruitment of the mitochondrial fission protein (see Figure 1), dynamin-related protein 1 (Drp1), from the cytosol (where it is primarily located) to the OMM where it mediates mitochondrial scission, through its interaction with other mitochondrial fission proteins: human fission factor-1 (Fis1), mitochondrial fission factor (MFF), and mitochondrial dynamics proteins of 49 and 51 kDa (MiD49 and MiD51, respectively) (Yoon et al., 2003; Gandre-Babbe and van der Bliek, 2008; Otera et al., 2010; Palmer et al., 2011; Loson et al., 2013). The pro-apoptotic protein PUMA (a BH3 member of the Bcl-2 protein family) has been reported to be critical for the recruitment of Drp1 to the mitochondria (Wang et al., 2009; Din et al., 2013). PUMA is reported to either displace BAX and Bad from their cytosolic sequestering proteins (Ren et al., 2010) which in turn causes Drp1 to translocate to the mitochondria (Din et al., 2013), or actually be present at the mitochondria where it aids Drp1 binding and assembly to mediate the scission events (Wang et al., 2009). Once Drp1 has bound to the OMM on one of its numerous receptors, it oligomerizes into a helical structure encircling the mitochondrion, and upon GTP hydrolysis, it constricts the mitochondrial membrane, until the lipid bilayers are sufficiently destabilized to break apart from each other (Mears et al., 2011). Drp1 then reverts back to its monomeric structure and moves back to the cytosol, leaving two mitochondria behind.
The dominance and specific roles of MFF, MiD49/51 and Fis1 in Drp1-mediated fragmentation are controversial (for recent reviews see Elgass et al., 2013; Otera et al., 2013). It is reported that MFF can recruit Drp1 independently of Fis1; while MiD49 and MiD51 can act independently of both MFF and Fis1 (Loson et al., 2013). The MiD proteins are reported to be Drp1 receptors on the mitochondrial membrane (Palmer et al., 2011), while MFF is reported to be a key protein in the recruitment of other proteins (potentially more Drp or other, as yet unidentified proteins) (Gandre-Babbe and van der Bliek, 2008) or a bone fide Drp1 receptor (Otera et al., 2010). With the oligermization of Drp1 less efficient without Fis1, it has a potential role as an assembly chaperone protein (Otera et al., 2010; Loson et al., 2013), although this role is distinct from MFF (Gandre-Babbe and van der Bliek, 2008). Clarification of the roles of MiD49/51, MFF and Fis1, the pathways (mitosis or apoptosis) in which Drp1 binds specifically to each protein could yield potential therapeutic targets.
Recently, it has been reported that the interaction of mitochondria with the endoplasmic reticulum (ER) (Friedman et al., 2011), inverted formin 2 (a formin that accelerates both actin polymerization and depolymerization) and the actin component of the cytoskeleton (De Vos et al., 2005; Korobova et al., 2013), may mark out sites for Drp1-mediated mitochondrial fission. The proposed paradigm suggests that the ER encircles mitochondria at sites of fission, and ER-associated inverted formin 2 then stimulates actin polymerization, providing the force required for partial constriction of the mitochondria, thereby facilitating the translocation of Drp1 to these pre-constriction contact sites in the OMM. The actual mechanism through which Drp1 localizes to these pre-constricted ER-contact sites on the OMM, and the roles MFF and MiD49/51 play in this process remains to be determined.
Regulation of the mitochondrial fission and fusion proteins
Recent studies have begun to describe the mechanisms that regulate the function of the mitochondrial fission and fusion proteins, providing insight into how mitochondrial dynamics may integrate into cell death and survival pathways.
Regulation of Drp1
Drp1 recruitment from the cytosol to the OMM is the major factor governing mitochondrial fission. As such, mitochondrial fission is regulated by the post-translational modification of Drp1, which alters both its localization and affinity for oligomerization. A number of post-translational modifications have been reported to occur on Drp1 (see Table 1) including sumoylation (Figueroa-Romero et al., 2009), phosphorylation (see below), ubiquitination (Nakamura et al., 2006), S-nitrosylation (Cho et al., 2009), O-GlcNAcylation (Gawlowski et al., 2012) and so on (reviewed in Otera et al., 2013). The most extensively reported Drp1 post-translational modification is phosphorylation, which occurs at Ser616 and Ser637 (in reference to the sequence of the human Drp1). Phosphorylation on Ser616 by cyclin-dependent kinase 1 (Cdk1)/cyclin B, a key mitotic kinase, induces Drp1 translocation to the mitochondria, where mitochondrial fragmentation ensures an equal division of the mitochondrial network between the daughter cells (Taguchi et al., 2007; Marsboom et al., 2011). Due to the slow mitotic turnover of adult cardiac myocytes, this form of Drp1 regulation may occur on an infrequent basis in the adult heart. Phosphorylation at the other site, Ser637, is mediated by PKA, Cam kinase and Pim1. Phosphorylation at Ser637 inhibits Drp1 oligomerization, suppresses mitochondrial fission and prevents cell death (Table 1). Indeed, phosphorylation by Pim1 is reported to play a key role in the sequestering of Drp1 in the cytosol (Din et al., 2013).
Table 1.
Post-translational modification of Drp1 by phosphorylation
| Reference | Cell-type | Regulator | Drp1 phosphorylation site | Effect |
|---|---|---|---|---|
| Taguchi et al., 2007 | HeLa | Cyclin-dependent kinase (Cdk1) | Ser585 with no effect of GTPase activity (Ser585 is the rat Drp1 splice variant 1 of Ser616) | Cdk1 phosphorylates Drp1 and activates it during cell division |
| Chang and Blackstone, 2007 | HeLa | PKA | Ser637 in the GTPase effector domain | PKA phosphorylates Drp1 and inhibits GTPase activity |
| Cribbs and Strack, 2007 | PC12 | PKA Calcineurin Calcium | Ser656 with no effect of GTPase activity (Ser656 is the rat Drp1 splice variant 1 of Ser637) | PKA phosphorylates Drp1 and inhibits GTPase activity preventing apoptosis Calcineurin and calcium dephosphorylates Drp1 and activates it enhancing apoptosis |
| Cereghetti et al., 2008 | Calcineurin Calcium | Ser637 | Calcineurin and calcium dephosphorylates Drp1 and activates it enhancing apoptosis | |
| Han et al., 2008 | HeLa neurones | Ca2+/calmodulin-dependent PKI 1 (CaMKIα) | Ser600 (Ser600 is the Drp1 splice variant of Ser637) | CaMKIα phosphorylates Drp1 and activates it |
| Marsboom et al., 2012 | Pulmonary arterial smooth muscle cells | Cdk1 | Ser616 | Cdk1 phosphorylates Drp1 and activates it during cell division |
| Din et al., 2013 | Neonatal cardiomyocytes | Pim-1 | Ser637 | Pim-1 phosphorylates Drp1 and inhibits fission |
Conversely, dephosphorylation by calcineurin induces mitochondrial fragmentation through Drp1 recruitment to the mitochondria (Cribbs and Strack, 2007; Chang and Blackstone, 2007). Dephosphorylation of this site is reported to initiate the transition towards apoptosis, with high Ca2+ concentrations, ATP depletion and the loss of mitochondrial membrane potential, all initiators of the calcineurin-dependent mitochondrial fission pathway (Cribbs and Strack, 2007; Estaquier and Arnoult, 2007; Cereghetti et al., 2008; Sandebring et al., 2009; Cho et al., 2010). In neonatal cardiomyocytes, it has recently been demonstrated that high glucose and the presence of diabetes induces mitochondrial fragmentation through the O-GlcNAcylation of OPA1 (Makino et al., 2011) and Drp1 (Gawlowski et al., 2012), resulting in the dephosphorylation of Ser637 and its translocation to the OMM.
Regulation of mitofusins
A prerequisite for mitochondrial fusion is a mitochondrial membrane potential (Legros et al., 2002), ensuring the mixing of healthy mitochondrial proteins and mtDNA. MFN1 has been reported to have higher GTPase activity than MFN2 (Ishihara et al., 2004), and this activity appears to be regulated by guanine nucleotide binding protein-β subunit 2 (Zhang et al., 2010). The binding of guanine nucleotide binding protein-β subunit 2 to MFN1 appears to decrease its mobility, allowing clustering of MFN1 to occur in specific foci and increasing mitochondrial fusion (Zhang et al., 2010).
MFN2 regulation has been reported to be mainly dependent on protein expression, rather than any protein modifications that alter GTPase activity or recruit MFN2 to the mitochondria. Under conditions of enhanced metabolic demand, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and PGC-1β have been shown to increase MFN2 expression (Liesa et al., 2008; Zorzano, 2009), linking mitochondrial fusion to changes in mitochondrial biogenesis.
Conversely, both MFN1 and MFN2 are reported to be regulated by ubiquitination, which immediately inhibits their activity, as well as providing the signal for these proteins to be degraded (Gegg et al., 2010). This modification inhibits mitochondrial fusion and is key to the process of removing damaged mitochondria by mitophagy (Gegg et al., 2010; Tanaka et al., 2010). The loss of the mitochondrial membrane potential, which indicates a loss of mitochondrial function, recruits the kinases PTEN-induced putative kinase 1 (PINK1) or JNK to the OMM (Leboucher et al., 2012). The phosphorylation of MFN2 on Thr111 and Ser442 by PINK1 then stabilizes the binding of Parkin, which ubiquitinates MFN2 (Chen and Dorn, 2013). The effect of the ubiquitination is twofold; a direct and immediate inhibition of MFN2 activity, thus preventing the damaged mitochondria fusing with the healthy mitochondrial population, and the recruitment of p62 (sequestosome 1) to mediate mitochondrial autophagy (Gegg et al., 2010; Tanaka et al., 2010; Glauser et al., 2011; Chen and Dorn, 2013). This modification and inhibition is not just limited to MFN2, as Parkin-mediated ubiquitination of MFN1 has also been reported (Gegg et al., 2010; Glauser et al., 2011). Thus the MFN proteins are regulated by their presence on the OMM, allowing mitochondrial fusion to occur when in contact with other mitochondria. As such, mitochondrial fusion must also be regulated through the proteins that manoeuvre the mitochondria around the cell, such as Miro and Milton (Koutsopoulos et al., 2010). Interestingly, the activity of both Miro and Milton has been reported to be regulated through their binding to MFN2 (Misko et al., 2010), demonstrating that mitochondrial fusion can also be controlled by the presence of these proteins in the OMM.
Regulation of OPA1
The regulation of OPA1 is complex and is dependent upon cleavage at both the mRNA transcript and protein levels (Delettre et al., 2001; Ishihara et al., 2006; Griparic et al., 2007; Ehses et al., 2009; Song et al., 2009). There are reported to be eight transcript variants of the OPA1 gene generated through the differential splicing of exon 4 (Delettre et al., 2001). Furthermore, each OPA1 isoform generated from these transcripts has two cleavage sites; S1 which is located on the N-terminus and cleaved by the mitochondrial processing peptidase to generate long form of optic atrophy factor 1 (L-OPA1), and S2 which is targeted by Yme1, presenilins-associated rhomboid-like protein (PARL), OMA1 and paraplegin to generate S-OPA1 (short OPA1) (Cipolat et al., 2006; Griparic et al., 2007; Song et al., 2007; Quiros et al., 2012). The sum of these processes results in five distinct OPA1 bands on electrophesis gels ranging from 100 to 85 kDa (Griparic et al., 2007; Song et al., 2007).
Yme1, PARL and paraplegin are all proteases reported to cleave OPA1 at S2 to create OPA1 short forms (Cipolat et al., 2006; Ishihara et al., 2006; Song et al., 2007). The cleavage of OPA1 is classified as either constitutive or inducible. Constitutive cleavage refers to OPA1 cleavage to form a range of fusion competent OPA1 isoforms, and is mediated by PARL and OMA1 (Cipolat et al., 2006; Frezza et al., 2006; Sanjuan Szklarz and Scorrano, 2012). PARL is reported to be sensitive to the phosphorylation status of OPA1, implicating another layer of regulation (Pellegrini and Scorrano, 2007), while Yme1 is only able to cleave certain splice variants of OPA1, with some being totally insensitive to Yme1 cleavage (Griparic et al., 2007). Inducible cleavage occurs in response to apoptotic stimuli to generate S-OPA1 isoforms, which have no fusion abilities, and is reported to also be mediated by OMA1 (Head et al., 2009). Genetic knockout of OMA1 results in mice that are unable to adapt their metabolism to utilize the appropriate substrates, revealing the importance of OMA1-mediated cleavage (Quiros et al., 2012). Formation of L-OPA1 is totally reliant upon de novo synthesis, with S-OPA1 unable to convert back to L-OPA1 (Griparic et al., 2007).
The reason for such regulation of OPA1 splice lengths is that each OPA1 variant has a different solubility, which governs their localization within the inner mitochondrial membrane and their ability to mediate mitochondrial fusion of the IMMs. Controversy exists as to whether just the long forms of OPA1 are fusion competent (Ishihara et al., 2006), or whether a mixture of isoforms varying in length is required (Song et al., 2007). The long forms of OPA1 are reported to be stabilized by Higd1-a, with knockdown of Higd1-a resulting in excessive processing of OPA1 into the short form and collapse of cristae, fragmentation of the mitochondrial network, the loss of mitochondrial DNA stability and a retardation of cellular growth (An et al., 2013). In response to the loss of mitochondrial membrane potential, OPA1 is rapidly cleaved at the protein level into shorter forms (by a protease other than Yme1) and is classified as inducible OPA1 cleavage (Griparic et al., 2007). The loss of L-OPA1 reduces the ability of the mitochondrion to fuse, essentially signalling the mitochondria for autophagy (Duvezin-Caubet et al., 2006).
Such debate as to the processing of OPA1 arises because of the large number of splice variants (at both mRNA and protein level), cleavage being mediated by several different proteases, and cleavage occurring in response to both profusion and pro-apoptotic pathways. Indeed, the L-OPA1, are likely to be required to mediate IMM fusion; while OPA1 processing (by paraplegin or OMA1) to create shorter, less active forms of OPA also occurs to inhibit fusion (Duvezin-Caubet et al., 2006; Ishihara et al., 2006; Ehses et al., 2009). The inhibition of OPA1 immediately following the loss of a mitochondrial membrane potential, serves as a block of mitochondrial fusion, preventing the damage spreading to healthy mitochondria. As such, the regulation of OPA1 activity is the key to a mitochondrion's ability to fuse and remain part of the healthy network.
Mitochondrial fusion and fission proteins and cell death
The mitochondrial fusion and fission proteins have been shown to play critical roles in a number of different cell death pathways, with the mitochondria as a point of convergence for many of them (see Figure 1). However, their interplay with components of the cell death pathway can be quite complex and continues to be elucidated.
Apoptosis
Mitochondria play a major role in the initiation of the intrinsic pathway of apoptotic cell death and, in this respect, are controlled by a subclass of the Bcl-2 protein family. This subclass is further categorized into anti-apoptotic and pro-apoptotic proteins, such as BAX and BAK. In healthy cells, BAX is predominantly cytosolic, until pro-apoptotic stimuli cause BAX translocation to the mitochondria to mediate mitochondrial outer membrane permeabilization (MOMP), releasing cytochrome c along with other pro-apoptotic factors into the cytosol. Mitochondria are known to undergo fragmentation on induction of apoptosis, which has been suggested to facilitate MOMP. However, the interplay between the mitochondrial fusion and fission proteins, the members of the Bcl-2 protein family and MOMP is quite complex and is incompletely understood (Youle and Strasser, 2008).
Interestingly, both BAX and BAK are reported to colocalize with MFN2 at the outer mitochondrial membrane (Karbowski et al., 2002; Neuspiel et al., 2005). Indeed, BAX-mitochondrial binding, apoptosis and cytochrome c release are all inhibited by the expression of constitutively active MFN2 (Neuspiel et al., 2005). Therefore, during apoptosis, BAX could bind and inhibit MFN2, preventing any further mitochondrial fusion and prepare the mitochondria for fragmentation. If one proposes MFN2 as the BAX receptor, this may also explain why MFN2 knockouts are more resistant to apoptosis. With the BAX and BAK binding sites no longer present, binding of these pro-apoptotic proteins at the mitochondria is likely to be reduced.
MFN2 has also been proposed to decrease mitochondrial membrane stability, encouraging pores to form in the OMM and facilitate the opening of the mitochondrial permeability pore (MPTP) (Papanicolaou et al., 2012c). Potentially, the presence of MFN2 may reduce the stability of the mitochondrial membrane, making it easier for Drp1 to mediate mitochondrial fragmentation and stimulate cytochrome c release. Finally, the role of non-oligomerized or monomeric BAX and BAK in mitochondrial fusion has recently been evaluated in the context of MPTP opening and necrotic cell death (Whelan et al., 2012). This study confirmed that BAX/BAK double-knockout cells contain fragmented mitochondria and the double-knockout cells were resistant to MPTP opening and necrotic cell death. MFN2-deficient cells, tested in the same study (Whelan et al., 2012), also exhibited resistance against MPTP opening, supporting the observation that MFN2 promotes this process. Furthermore, monomeric BAX was demonstrated to be responsible for the observed sensitivity to MPTP. This may be in agreement with the notion that the localization of BAX to the OMM lowers the threshold for forming hemifusion-related holes, and if there is sufficient stress overload in the matrix/IMM, this hole can be employed in the precipitous exchange of ions during MPTP opening.
Drp1
Drp1 is also reported to colocalize with BAX at the OMM and mediate mitochondrial fragmentation in response to apoptotic stimuli. While Drp1 mutants failed to prevent BAX-mitochondrial binding, there were no subsequent changes in mitochondrial morphology, indicating that BAX recruits Drp1 rather than vice versa (Karbowski et al., 2002). The recruitment of Drp1 to the mitochondria has been reported to be independent of Fis1, with Drp1 binding to the mitochondria stimulated by BAX and stabilized by ubquitination by the small ubiquitin-like modifier (Wasiak et al., 2007). Once at the OMM, Drp1 mediates mitochondrial fragmentation, the loss of the membrane potential and facilitates the release of cytochrome c. These studies implicate Drp1 as a key player in apoptosis, with Drp1 inhibition reported to slow, rather than fully inhibit apoptosis (Frank et al., 2001; Estaquier and Arnoult, 2007). This may be due to the release of other pro-apoptotic proteins (other than cytochrome c) from the mitochondria when Drp1 is inhibited. However, there is growing evidence that apoptosis is able to proceed independently of Drp1 (Parone et al., 2006; Wakabayashi et al., 2009) despite the fact that mitochondrial fission mediated by Drp1 is considered to be a key hallmark of apoptosis (Martinou and Youle, 2006). As such, whether therapeutic inhibition of Drp1 would be effective in preventing apoptosis is still controversial.
OPA1
OPA1 is also regarded as an anti-apoptotic protein, with its overexpression reducing cell death and its knockdown increasing cell susceptibility to apoptosis (Olichon et al., 2003; Lee et al., 2004; Estaquier and Arnoult, 2007; Song et al., 2007). Localized in the IMM, OPA1 maintains IMM integrity and is able to sequester cytochrome c within the cristae (Frezza et al., 2006). While both long insoluble and short soluble forms of OPA1 are found at the cristae junctions, it is the short form that is reported to be anti-apoptotic. Cleavage by PARL increases the amount of shorter soluble OPA1, which is able to form oligomers and mediate cristae remodelling (Cipolat et al., 2006; Frezza et al., 2006; Sanjuan Szklarz and Scorrano, 2012). Such remodelling is reported to keep the cristae junctions tight, sequestering the cytochrome c within the cristae thereby inhibiting apoptosis. To mediate cytochrome c release, OPA1 oligomerization is disrupted by Bid, which opens up the cristae releasing cytochrome c into the intermembranous space (Frezza et al., 2006). In this regard, BAX-and BAK-mediated fission can also release cytochrome c into the cytoplasm leading to caspase activation (Scorrano et al., 2002). Furthermore, OPA1 cleavage is responsible for the protective response to heat shock, with PARL knockout mice being more susceptible to apoptosis. As such, this pathway is physiologically relevant (Cipolat et al., 2006).
Mitophagy
Mitophagy is required to remove damaged mitochondria and maintain a healthy mitochondrial network for normal cell function. The role of MFN2 in cellular autophagy has recently been explored in the adult murine heart by Zhao et al. (2012). These authors found that the cardiac-specific deletion of MFN2 in the adult heart resulted in the accumulation of autophagosomes, which was mostly shown to be due to impaired fusion of autophagosomes with lysosomes (Zhao et al., 2012). In this particular study, the role of MFN2 in mediating mitophagy was not clear, although starvation was found to increase its association with Ras-related protein, an autophagosome maturation-related protein (Zhao et al., 2012).
The current paradigm suggests that mitochondrial membrane depolarization of damaged mitochondria signals PINK1 translocation to the OMM where it mediates Parkin recruitment to the damaged mitochondria and induces their mitophagic removal by ubiquitination (Karbowski et al., 2002). Chen and Dorn (2013) have provided experimental data suggesting that in damaged mitochondria, PINK1 phosphorylates and activates MFN2 so the it can act as a receptor for Parkin on the OMM and mediate autophagy. These findings were shown to be consistent with the observation that hearts deficient in MFN2 displayed impaired mitochondrial respiration and went on to develop a dilated cardiomyopathy (Chen and Dorn, 2013).
In summary, these two experimental studies suggest another non-fusion pleiotropic role for MFN2 as a critical mediator of cellular autophagy and mitophagy. Given the importance of these processes in the healthy and diseased cardiovascular system, the therapeutic potential for manipulating these processes may be mediated via MFN2.
MPTP
The opening of the MPTP is a critical determinant of cell death. Therefore, preventing or delaying its opening increases cell survival. This is of particular importance in the setting of acute ischaemic reperfusion injury (IRI), where MPTP opening at the point of reperfusion mediates cardiomyocyte death, and inhibiting MPTP opening at this time prevents cell death (Hausenloy et al., 2003; Hausenloy and Yellon, 2003). The mitochondrial fusion and fission proteins have been reported to influence MPTP opening susceptibility and, therefore, affect cell viability. In the HL-1 cardiac cell line, Ong et al. (2010) found that inhibiting mitochondrial fission during acute IRI could prevent MPTP opening. Similarly, MFN2 ablation in neonatal cardiomyocytes was also found to increase MPTP opening susceptibility (Papanicolaou et al., 2011). However, the results of manipulating the mitochondrial fusion proteins in the adult heart have had opposing effects on MPTP opening susceptibility. Genetic ablation of MFN1, MFN2 or OPA1 has been reported in the adult heart to reduce MPTP opening susceptibility, the mechanism for which is unclear. It has been suggested that the mitofusins may act as indirect mediators of MPTP formation (Papanicolaou et al., 2012c). During the process of mitochondrial fusion, membrane destabilization occurs. As well as leading to a leak of solutes from the mitochondria, the destabilization of the membrane may make it easier for MPTP formation to occur. Conversely, in the MFN1 and MFN2 knockout mice, the lack of mitochondrial fusion stabilizes the mitochondrial membranes, thus reducing the ability of the pore proteins to break the membrane, and form the MPTP (Papanicolaou et al., 2012c). OPA1 down-regulation may inhibit pore formation through a similar stabilization of the inner mitochondrial membrane, or the disturbances in cristae formation may alter the way Ca2+ is handled within the mitochondria (Piquereau et al., 2012). Whether the mitofusin proteins or OPA1 play a direct role in MPTP formation remains to be determined, although how these proteins would interact with the ATP synthase, a recently proposed component of the MPTP is unclear (Giorgio et al., 2013).
Programmed cell necrosis
The current paradigm has suggested that cell death by necrosis is an unregulated process. However, a pathway of programmed cell necrosis has recently been described, in which the cytokine TNF-α activates the receptor-interacting serine-threonine kinases (RIP), RIP1 and RIP3, and their interaction with the mixed lineage kinase domain like protein resulting in ROS generation, calcium overload and the opening of the MPTP (Vanlangenakker et al., 2008). RIP3 forms a complex mitochondrial protein phosphatase PGAM5, which recruits Drp1 to the OMM by dephosphorylating its Ser637 site causing mitochondrial fragmentation (Wang et al., 2012). These findings implicate a role for Drp1 in mediating programmed cell death necrosis. Importantly, and relevant to the heart, pharmacological inhibition of this pathway, using Necrostatin-1 to target RIP1, has been reported to limit myocardial infarction (MI) size and prevent post-left ventricular (LV) remodelling in animal models of acute IRI (Lim et al., 2007; Oerlemans et al., 2012).
Tethering to ER or sarcoplasmic reticulum
Tethering between the ER and mitochondria was initially observed in the 1970s using electron microscopy (Morre et al., 1971). Subsequent studies have gone on to show that this interaction is physiologically important, and they have identified some of the key proteins which mediate this interaction including the voltage-dependant anion channel (Szabadkai et al., 2006), Tespa1 (Matsuzaki et al., 2013), Trichoplein/mitostatin (Cerqua et al., 2010) and, most recently MFN2 (de Brito and Scorrano, 2008), although the latter has not been universally reported (Cosson et al., 2012). The contact region between the ER and mitochondria is known as the mitochondrial associated membrane (MAM) and contains proteins and phospholipids which facilitate inter-organelle signalling, energy regulation and apoptosis all of which occur at the ER-mitochondria interface (Vance, 1990).
Scorrano's group (de Brito and Scorrano, 2008) first proposed the key role for MFN2 in tethering these organelles together. Present at both the mitochondrial and ER membranes, MFN2 is enriched within the MAM region (see Figure 1). As such, MFN2 present on the ER membrane is able to interact with either MFN1 or MFN2 on the mitochondrial membrane, and tether the organelles together. Loss of MFN2 diminishes this interaction between the mitochondria and ER, increasing the distance between the two organelles. The interaction mediated by MFN2 is physiologically important for IP3 signalling, as well as preventing ER stress. Indeed, Ngoh et al. (2012) showed that MFN2 expression increases under ER stress, and without this adaptive tethering, apoptosis follows.
MFN2-mediated ER-mitochondrial tethering is regulated by MITOL (ubiquitin ligase) (Sugiura et al., 2013). Interestingly, MITOL only ubiquitinates mitochondrial MFN2 on lysine residues 63 and 192, while ER MFN2 remains unmodified. Rather than signalling MFN2 for degradation, ubquitination increases the affinity of MFN2 for GTP, stimulating MFN2 activity. Through this mechanism, MFN2 undergoes GTP-dependent oligomerization and facilitates the formation of mitochondrial ER tethering domains (Sugiura et al., 2013).
The dynamic nature of cardiac contractility is mirrored by alterations in energy demand, which must be met by mitochondrial respiration. Key to this supply-demand coupling is the interaction and close proximity of the sarcoplasmic reticulum (SR) and mitochondria. With Ca2+ stimulating both contraction and mitochondrial respiration (specifically the Kreb's cycle dehydrogenases), the transfer of Ca2+ from the SR to the mitochondria enables a tight coupling of energy demand and supply (Chen et al., 2012b). Central to this pathway is the uptake of Ca2+ into the mitochondria through the mitochondrial Ca2+ uniporter, whose molecular identity was recently discovered (de Brito and Scorrano, 2008; Baughman et al., 2011; De et al., 2011). Despite the mitochondrial Ca2+ uniporter possessing a low affinity for Ca2+ (10 μM) (Kirichok et al., 2004), mitochondrial Ca2+ uptake is rapid, suggesting that high concentrations of Ca2+ exist (around 25 μM) (Giacomello et al., 2010) within a very localized area to the SR (Rizzuto et al., 1998). Mitochondrial tethering to the SR through MFN2 enables the Ca2+ present in these subcellular microdomains to stimulate mitochondrial respiration on a beat-to-beat basis (Csordas et al., 2010; Giacomello et al., 2010; Chen et al., 2012b).
The effect of disturbing the interaction between the SR and mitochondria by ablating MFN2 or by a pharmacological strategy is not clear, but it may in part contribute to the development of a cardiomyopathy through its effect on Ca2+ signalling.
Mitochondrial fusion and fission proteins and the adult heart
The majority of experimental studies investigating the phenomenon of mitochondrial dynamics have been restricted to immortal cell lines because their mitochondrial networks are highly mobile, unconstrained by sarcomeres, easy to image and the cells themselves are compliant to genetic manipulation. More recently, mitochondrial fusion and fission proteins have been investigated in the adult heart, in which mitochondria have a distinct arrangement and in which mitochondrial movement is constrained by the cellular architecture.
The mitofusins and the adult heart
In terms of the cardiovascular system, a number of experimental studies have investigated the mitofusins in cardiac and vascular cell lines and neonatal cardiomyocytes in which mitochondrial movement is unrestricted. In the adult heart, however, the role of the mitofusins in the adult heart have been somewhat varied and in some cases unexpected (see Table 2). This difference may be due in part to the pleiotropic non-fusion effects of mitofusins, in particular MFN2 (de Brito and Scorrano, 2008). Of particular interest and importance is the recent observation suggesting that mitochondrial dynamics may be relevant to the adult heart, with the description of ‘nanotunneling’ and ‘kiss-and-tell’ events in isolated adult cardiomyocytes (Huang et al., 2013; Kasahara et al., 2013). ‘Kiss-and-tell’ events were observed between adjacent mitochondria and are hypothesized to mediate local mixing of mitochondrial material. ‘Nanotunneling’ was also observed as a means of communication between mitochondria that are more remote. The highest rate of fusion events was observed in the perinuclear region where the mitochondria are not restricted by the sarcomeres, as with the interfibrillar mitochondria. Interestingly, these fusion events occurred relatively infrequently (compared to mitochondrial mobility in HeLa and MEF cell lines) with mitochondrial material taking up to a day to transverse the cell (Huang et al., 2013). These observations add substantial evidence to the idea that adult cardiac mitochondria are dynamic, and form a large interconnected network as observed in other tissues. An early study by Chen et al. (2003) was the first to report that mice deficient in both MFN1 and MFN2 die in utero during the mid-gestation period, highlighting the critical role the mitofusins play in embryonic development. Cardiac-specific ablation of both MFN1 and MFN2 in the embryo was shown to be lethal at day 9.5–10.5, demonstrating that these mitochondrial fusion proteins also play a critical role in cardiac development (Chen et al., 2011).
Table 2.
Effect of ablating the mitochondrial fusion proteins in the adult heart
| Study | Fusion protein | Murine model | Mitochondrial morphology (TEM) | MPTP susceptibility | IRI sensitivity | Other effect |
|---|---|---|---|---|---|---|
| Papanicolaou et al., 2012a | MFN1 | Cardiac knockout (KO) | Smaller | Reduced | Reduced | No phenotype |
| Papanicolaou et al., 2011 | MFN2 | Cardiac KO | Pleomorphic, larger | Reduced | Reduced | Modest LV hypertrophy, mild LV impairment |
| Wang et al., 2012 | MFN2 | Cardiac KO | Pleomorphic, larger | Not tested | Increased | Impaired mito function and autophagy |
| Chen et al., 2011 | MFN1/MFN2 | Cardiac KO | Smaller | No change | Not known | Impaired mito function |
| Piquereau et al., 2012 | OPA1 | Whole body OPA1 +/− | Pleomorphic larger | Reduced | Not known |
Papanicolaou et al. (Papanicolaou et al., 2011; Chen et al., 2012b) first investigated the effects of conditional ablation of cardiomyocyte-specific MFN2 (α-MHC-Cre) in the adult murine heart. Interestingly, they found that subsarcolemmal mitochondria in this heart were pleomorphic and slightly enlarged, and that the hearts themselves displayed modest LV hypertrophy and a mild deterioration in LV systolic function. MFN2 ablation was found to be associated with a decreased sensitivity to MPTP opening and reduced susceptibility to acute IRI, despite there being no major effect on mitochondrial function other than a mild mitochondrial membrane depolarization (Papanicolaou et al., 2011; Chen et al., 2012b). In contrast, the effect of genetic ablation of MFN2 using small interfering RNA in neonatal cardiomyocytes had opposing effects with mitochondrial fragmentation, increased susceptibility to MPTP opening and less cell death following oxidative stress. These studies demonstrate the cell-specific effects of MFN2 ablation. Zhao et al. (2012) have also investigated the effects of cardiac-specific MFN2 ablation (Mlc2v-Cre) in the adult murine heart. At 4 months of age, there was no obvious cardiac phenotype in MFN2 depleted hearts (Zhao et al., 2012). Although they were able to confirm the appearance of pleomorphic large mitochondria in these hearts, the MFN2 knockout hearts also displayed increased sensitivity to acute IRI and developed late-onset LV dysfunction at 17 months of age (Zhao et al., 2012). These findings were associated with a major metabolic disturbance with impaired autophagy, defective lipid metabolism and decreased mitochondrial respiration (primarily at complex III) (Zhao et al., 2012). The explanation for the observed differences in effects of MFN2 ablation in the adult heart is not clear. Whether the difference in response to MFN2 ablation can be attributed to the use of different cardiac-specific promoters is not known.
Papanicolaou et al. (2012b) were also the first to investigate the effects of deleting cardiomyocyte-specific MFN1 (Myh6-Cre) in the adult murine heart from embryonic day 9.5. They found evidence of mitochondrial fragmentation (smaller spherical mitochondria), preserved mitochondrial function, resistance to oxidative stress in terms of mitochondrial membrane depolarization and cell viability, and reduced sensitivity to MPTP opening. The explanation for the differing effects on MFN1 versus MFN2 ablation on mitochondrial morphology is not known but may be attributed to the high profusion affinity of MFN1.
The effect of genetic ablation of both MFN1 and MFN2 in the adult murine heart (8 weeks of age) has also been investigated. It resulted in mitochondrial fragmentation, impairment in mitochondrial respiration, and a severe lethal cardiomyopathy after 7–8 weeks, suggesting that the mitofusins are essential for normal cardiac mitochondrial morphology and respiratory function (Chen et al., 2011; Papanicolaou et al., 2012a).
OPA1 and the adult heart
Mice completely deficient in OPA1 die in utero, confirming the importance of this protein in embryonic development and maintenance of mitochondrial integrity (Davies et al., 2007). As an alternative approach, mice partially deficient in OPA1 (heterozygous OPA1 +/−) have been used to investigate the role of OPA1 in the adult heart (Piquereau et al., 2012; Chen et al., 2012a). In this transgenic mouse, there was a 50% reduction in myocardial OPA expression, enlarged mitochondria with disturbed cristae and altered mitochondrial organization, with a mild cardiac phenotype at 3 and 6 months, but severe deterioration and heart failure occurring at 12 months. Indeed, at 12 months, the mutation of OPA1 led to cardiac dysfunction with a reduced cardiac output, blunted inotropic reserve and reduced pressure-volume loops (Chen et al., 2012a). This reduction in cardiac reserve was associated with mitochondrial dysfunction with a general reduction in complex activity and a blunted respiratory response when stimulated with either ADP or FCCP. In addition, the 10-week-old mouse was more susceptible to pressure-load LV hypertrophy when challenged with the transverse aortic constriction model (Piquereau et al., 2012). Coupled with a reduction in mitochondrial DNA, these results suggest that maintenance of mitochondrial integrity, at either the DNA or protein level, is a key physiological requirement for mitochondrial fusion in the adult heart. The requirement for OPA1 in the adult heart is also the same for skeletal muscle, in which OPA1 plays a pivotal role (Chen et al., 2010).
Drp1 and the adult heart
The Drp1-knockout mouse is embryonically lethal at embryonic day 12.5 and surprisingly, there are no phenotypical differences between the wild type and heterozygote Drp1-knockout mouse (Manczak et al., 2012). These studies demonstrate a vital role for Drp1 during development (Ishihara et al., 2009; Wakabayashi et al., 2009), and that Drp1 localization, rather than its quantity may regulate its action. Despite the lack of a Drp1-knockout mouse, many of its roles in mitochondrial fission (apoptosis, mitophagy and increasing the mitochondrial network) are likely to hold true in the heart, since these functions of Drp1 have been reported in numerous other tissues and appear fundamental to mammalian life. The only effect of Drp1 that is unlikely to occur in the heart, or occur on a very infrequent basis, is its effect in mitosis as cardiomyocytes rarely undergoing replication.
While there are few studies directly investigating the role and molecular mechanisms of Drp1 in cardiac tissue (due to the aforementioned difficulties of manipulation and imaging in the heart), those studies conducted on cardiac tissue, have focused mainly on the involvement of Drp1 in apoptosis. Indeed, several studies have all reported that inhibition of Drp1 reduces cardiac cell death post-IRI (Ong et al., 2010; Din et al., 2013; Gao et al., 2013) or prevents cardiac dysfunction following aortic banding (Givvimani et al., 2012). These studies are discussed in greater detail later on, but they all demonstrate that Drp1 and mitochondrial fission play a key role in the regulation of apoptosis in cardiac tissue. As mentioned previously, not all mitochondrial fission should be considered detrimental. Drp1 recruitment to the mitochondria mediated by Bnip3 serves to mediate mitophagy in cardiomyocytes (Lee et al., 2011), and, because of the high amount of oxidative phosphorylation that occurs in the heart, this pathway is especially important to remove damaged mitochondria and maintain the quality of mitochondria.
Mitochondrial fusion/fission proteins as therapeutic targets
The postnatal period
In the first few days of postnatal cardiac growth there are drastic changes in cardiac metabolism and intracellular architecture as the heart switches from the placental circulation and anaerobic metabolism to the pulmonary circulation and oxidative phosphorylation. The role of the mitochondrial fusion and fission proteins in mediating this adaptive response has recently been investigated (Papanicolaou et al., 2012a). It was found that over the first 7 days of postnatal cardiac growth, mitochondrial morphology changes from fragmented and randomly orientated to more elongated mitochondria aligned to myofibrils; this change is associated with a significant increase in the expression of MFN1 and MFN2 (Papanicolaou et al., 2012a). Crucially, transgenic mice (loxP/Myh6-Cre) deficient in cardiac-specific MFN1 and MFN2 from the late embryonic period display severe mitochondrial dysfunction at 7 days (abnormal mitochondrial structure, down-regulated mitochondrial biogenesis genes, reduced mitochondrial DNA), develop cardiomyopathy, and all die before 14 days old, implicating an important role for the mitofusins in cardiac growth over the initial postnatal period (Papanicolaou et al., 2012a).
Another critical change, which occurs at the time of birth, is closure of the ductus arteriosus (DA) in response to the increase in oxygen thereby diverting blood from the right ventricle into the pulmonary circulation. Failure of the DA to close can result in pulmonary congestion and failure to thrive. Hong et al. (2013) have investigated the role of Drp1-mediated mitochondrial fission in oxygen-induced DA closure. They provided evidence that oxygen-induced phosphorylation of Drp1 at Ser616 induces mitochondrial fission in DA smooth muscle cells, which then generates mitochondrial-signalling ROS required to activate DA closure through a complex mechanism involving inhibition of oxygen-sensitive potassium channels, membrane depolarization of DA smooth muscle cells, L-type channel activation, increased intracellular calcium and subsequent vasoconstriction of the DA (Hong et al., 2013). These findings implicate Drp1 as a potential therapeutic target for mediating DA closure (see Figure 2).
Figure 2.
This scheme depicts the mitochondrial fission and fusion proteins as novel therapeutic targets for treating cardiovascular disease. It is important to bear in mind that chronic therapeutic targeting of these shaping proteins may have detrimental effects and that there may be off-target effects as these mitochondrial-shaping proteins may have non-fusion or non-fission pleiotropic actions within the cell.
Vascular smooth muscle cell (VSMC) proliferation
VSMC proliferation and hyperplasia is a key feature of a variety of cardiovascular diseases including atherosclerosis, hypertension, pulmonary arterial hypertension (PAH), and its existence contributes to the failure of coronary artery bypass vein grafts and to the restenosis following percutaneous coronary intervention. As such, novel therapeutic targets are required to inhibit VSMC proliferation in these settings. In this regard, the mitochondrial fusion and fission proteins may provide novel targets for preventing this pathological process.
MFN2 and proliferation of VSMCs
Chen et al. were the first to implicate MFN2, as a novel hyperplasia suppressor gene (HSG), capable of inhibiting VSMC proliferation in a variety of vasculo-proliferative conditions (Chen et al., 2004). These authors found that the expression of HSG (MFN2) in VSMCs was reduced in several experimental models of vasculo-proliferation, and HSG (MFN2) overexpression inhibited VSMC proliferation in angioplasty balloon-induced neointimal injury, oxidized LDL and subsequent atheroma formation and restenosis in rat carotid arteries. These vasculo-proliferative effects appeared to be mediated by inducing apoptotic death of VSMCs through the suppression of the Ras-Raf-MAPK-Erk1/2 and PI3K-Akt signalling pathways (Chen et al., 2004; Guo et al., 2007). The same researchers found that PKA-induced phosphorylation of MFN2 at Ser442 is central to the anti-proliferative effect of MFN2 on VSMCs (see Figure 2) (Zhou et al., 2010).
Drp1 and proliferation of VSMCs
Chalmers et al. (2012) have investigated the role of the mitochondrial fission protein, Drp1, in VSMCs in relation to their proliferative response. They found that in native non-proliferative quiescent VSMCs mitochondria were fairly static, mainly ovoid in shape whereas during proliferation, the mitochondria became more mobile and displayed various shapes. Interestingly, treatment of VSMCs with the Drp1 inhibitor, Mdivi-1, was able to inhibit the proliferative response, suggesting that mitochondrial fission may be required for the proliferation of VSMCs. The requirement for mitochondrial fission in VSMC proliferation has been recently explored in the setting of PAH (see Figure 2).
PAH
PAH, in which the pulmonary arteries become obstructed, results in right ventricular hypertrophy and heart failure. Despite new vasodilator therapy, the morbidity and mortality remain high (15% death at 1 year) (Thenappan et al., 2010). Therefore, novel therapeutic targets for preventing the progression of PAH are desirable. In this regard, recent experimental data have implicated the mitochondrial fusion and fission proteins as potential novel therapeutic targets for treating PAH.
The ability of pulmonary arterial smooth muscle cells (PASMC) to hyperproliferate is an essential part of the pathophysiology underlying PAH. It has been demonstrated that this pathological process is dependent on the ability of the mitochondria to undergo division as this allows the equal redistribution of mitochondria during cell proliferation. Marsboom et al. (2011) noted that in PAH, the mitochondria in PASMCs are fragmented, findings which were associated with the up-regulation of Drp1 and the down-regulation of MFN2 (see Figure 2). They demonstrated that mitochondrial fission, mediated by cyclin B1/Cdk1 phosphorylation of Drp1 at Ser616, is required for the hyperproliferation of PASMCs. Interestingly, treatment with the small molecule Drp1 inhibitor, Mdivi-1, was shown to prevent the progression of PAH in three different experimental models of PAH, suggesting Drp1-mediated mitochondrial fission as a novel therapeutic target for PAH (Marsboom et al., 2011). However, preventing PAH by using this therapeutic approach would require prolonged and chronic inhibition of mitochondrial fission, the result of which may be detrimental over the long term, given the essential role mitochondrial fission plays in maintaining a healthy mitochondrial network. The same research group have gone on to implicate the mitochondrial fusion protein, MFN2, as a potential therapeutic target for treating PAH. They found that in two different experimental models of PAH, and in patients with PAH, both MFN2 and PGC-1α were down-regulated in PASMCs. Importantly, they demonstrated that genetic ablation of MFN2 and PGC-1α induced mitochondrial fragmentation and worsened PAH, whereas in contrast, overexpressing MFN2 was able to prevent the progression of PAH.
Heart failure
A critical role for the mitochondrial fusion and fission proteins in normal development and function of the heart has been indicated by the finding that deficiencies in either the mitochondrial fusion or fission proteins result in the development of a cardiomyopathy.
OPA1 and heart failure
The changes in mitochondrial morphology that occur with ischaemia-induced heart failure have been investigated by Chen et al. (2009), using a post-MI rat heart failure model and human dilated and ischaemic cardiomyopathy tissue samples. These authors observed mitochondrial fragmentation and decreased myocardial levels of OPA1 (Chen et al., 2009). A subsequent experimental study by the same authors has recently demonstrated that heterozygous mice deficient in OPA1 had reduced mitochondrial DNA copy number and decreased expression of nuclear antioxidant genes at 3 to 4 months of age (Chen et al., 2012a). However, baseline cardiac function was normal in these OPA1-deficient mice, although at 12 months of age, the mice developed a cardiomyopathy associated with mitochondrial fragmentation and impaired mitochondrial function (Chen et al., 2012a). The reason for the decline in OPA1 levels in heart failure requires further investigation.
Mitofusins and heart failure
Silencing of mitochondrial assembly regulatory factor orthologue of mammalian mitofusin and OPA1 in the Drosophila fly heart tube resulted in a dilated cardiomyopathy, which could be rescued by overexpressing either of the human mitofusins (MFN1 or MFN2) or superoxide dismutase 1, implicating impaired mitochondrial fusion and oxidative stress in the pathogenesis of heart failure (Dorn et al., 2011).
Conditional cardiac-specific ablation of MFN1 and MFN2 in the adult murine heart has been reported to result in a severe lethal dilated cardiomyopathy after 6–8 weeks, a finding which was associated with mitochondrial fragmentation and impaired mitochondrial respiration, implicating a role for the mitofusins in maintaining normal mitochondrial function in the adult heart (Chen et al., 2011).
Drp1 and heart failure
Ashrafian et al. (2010) have described a novel mutation in the Drp1 gene (C452F) which gives rise to an autosomal dominant form of dilated cardiomyopathy in the python mouse. Although the homozygous mutation is embryonically lethal, the heterozygous form survives until adulthood and develops a severe dilated cardiomyopathy after 11 weeks, a finding which was associated with reduced content of mitochondrial respiratory enzymes and ATP (Ashrafian et al., 2010).
Acute IRI
Ischaemic heart disease is the leading cause of death and disability worldwide. Its clinical manifestations are due to the detrimental effects of acute IRI on the myocardium. The susceptibility of the heart to acute IRI and its recovery is critically dependant on the function of its mitochondria. Mitochondrial dysfunction in response to acute IRI and subsequent opening of the MPTP at reperfusion are critical determinants of cell death. Therefore, the preservation of mitochondrial viability and the prevention of MPTP opening during acute IRI are important therapeutic strategies for cardioprotection. Recent experimental data suggest that manipulating the mitochondrial fission and fusion proteins in the heart may affect the susceptibility to acute IRI, providing novel therapeutic targets for cardioprotection.
Mitochondrial fission proteins as targets for cardioprotection
In response to acute IRI, the mitochondrial fission protein, Drp1, has been demonstrated to translocate to the OMM and induce mitochondrial fission (Ong et al., 2010; Din et al., 2013). In the HL-1 cardiac cell line, Ong et al. (2010) showed that genetic or pharmacological (using Mdivi-1) inhibition of Drp1 during simulated IRI prevented the opening of the MPTP and reduced cell death. In the murine heart, Mdivi-1-induced inhibition of Drp1 reduced cell death in isolated cardiomyocytes subjected to simulated IRI and reduced MI size in the adult murine heart subjected to in vivo acute IRI. A number of experimental studies have subsequently confirmed Drp1 to be a therapeutic target for cardioprotection in the adult heart. Din et al. (2013) found that using Mdivi-1 to inhibit Drp1 translocation to the OMM during acute IRI protected neonatal murine cardiomyocytes and the adult murine heart. Interestingly, the same authors were able to reduce Drp1 translocation to the mitochondria in simulated IRI through the overexpression of Pim1, implicating this kinase as another mechanism through which cardioprotection through the modulation of Drp1 may be achieved. Using the non-specific dynamin inhibitor, Dynasore, to inhibit Drp1 during acute IRI, Gao et al. (2013) were able to protect the isolated adult murine heart. Inhibiting mitochondrial fission has also been reported to protect the kidney and the brain against acute IRI, suggesting that therapeutic targeting of mitochondrial fission may be beneficial in other organs (Zhang et al., 2013). Most recently, a specific peptide inhibitor of Drp1, named P110, has been used to demonstrate that inhibiting mitochondrial fission at reperfusion can reduce myocardial infarct size and prevent adverse LV remodelling post-MI in the adult rat heart (Disatnik et al., 2013). The discovery of other components of the mitochondrial fission machinery, such as MFF and MiD49/51, raises the possibility of inhibiting these other proteins to mediate cardioprotection. However, this therapeutic strategy will only be useful in protecting the heart against acute episodes of IRI, as this can be achieved by transient pharmacological inhibition of mitochondrial fission. Chronic inhibition of mitochondrial fission would be detrimental to the heart and other organs as this process is critical to maintaining a health mitochondrial network.
Mitochondrial fusion proteins as targets for cardioprotection
The role of the mitochondrial fusion proteins (MFN1, MFN2 and OPA1) as targets for cardioprotection has produced some unexpected findings. This may relate to their well-established non-fusion effects, which may interfere with the myocardial response to acute IRI. In the HL-1 cardiac cell line, Ong et al. (2010) found that overexpressing MFN1 or MFN2 prevented the opening of the MPTP and reduced cell death following simulated IRI. These findings were consistent with those of Papanicalou et al. (Papanicolaou et al., 2011; Chen et al., 2012b), who demonstrated that small interfering RNA knockdown of MFN2 delayed MPTP opening and rendered neonatal cardiomyocytes more susceptible to oxidative stress.
The genetic manipulation of the mitochondrial fusion proteins in the adult heart has had contrasting effects in terms of susceptibility to MPTP opening and sensitivity to acute IRI (see Table 2). The deletion of MFN1, MFN2 or OPA1 appears to render the heart less resistant to MPTP opening, and in the case of MFN1 and MFN2, the hearts have been reported to be more resistant to acute IRI. The explanation for this is not clear, but could possibly be related to the non-fusion effects of the mitofusins described earlier. Although partial genetic ablation of OPA1 has been shown to delay MPTP opening, its effect on acute IRI susceptibility has not been investigated (Piquereau et al., 2012). Similar to the mitofusins, OPA1 has non-fusion pleiotropic effects such as inhibiting apoptosis by preventing cytochrome c release, and therefore, the effect of manipulating OPA1 on the susceptibility to acute IRI may be less straightforward to predict.
Experimental studies thus far have investigated the effects of genetically ablating the mitochondrial fusion proteins. However, the effect of overexpressing the mitochondrial fusion proteins in the adult heart on sensitivity to acute IRI is currently unknown. Whether this would worsen the response to acute IRI is unclear.
In summary, the role of the mitochondrial fusion proteins in the adult heart in terms of susceptibility to acute IRI is quite complex. However, the development of small molecule inhibitors of MFN1 and MFN2 may provide a novel therapeutic strategy for cardioprotection.
LV hypertrophy (LVH)
Hypertrophy of the left ventricle can be both physiological (in response to exercise) and pathological (congenital or acquired – most often a detrimental response to an increase load) and can lead to an increased risk of arrhythmias, regions of ischaemia and heart failure (Frey and Olson, 2003; Frey et al., 2004). As such, novel therapeutic agents are required to prevent the progression of LVH and reduce the onset of heart failure.
Mitochondrial fusion proteins and LVH
A number of experimental studies have implicated the mitochondrial fusion proteins MFN2 and OPA1 as potential therapeutic targets for treating LVH. Previous work by Fang et al. demonstrated that MFN2 (formerly known as hyperplasia suppressor gene or HSG) can inhibit VSMC proliferation through the suppression of MEK1/2-Erk1/2 (Fang et al., 2007), a signalling pathway that is up-regulated in LVH. As such, they investigated whether MFN2 can also suppress pathological LVH. They found that MFN2 expression was down-regulated and Erk1/2 up-regulated in four different experimental models of LVH (phenylephrine induced LVH in neonatal rat cardiomyocytes, spontaneously hypertensive rats, β2-adrenoceptor transgenic mice and pressure overload LVH by transverse aortic constriction) (Fang et al., 2007). Yu et al. (2011) went on to demonstrate that MFN2 is down-regulated and Akt up-regulated in neonatal rat cardiomyocytes treated with angiotensin-II, and that genetic overexpression of MFN2 could prevent angiotensin-II-induced LVH in both neonatal cardiomyocytes and the intact rat heart. While these studies all show a reduction in total MFN2 protein, there is also the possibility that these observations are merely due to the reduction in mitochondrial mass per se. It is, therefore, important to quantify the amount of mitochondrial dynamic protein to total mitochondrial mass in order to obtain the correct conclusion, especially in pathologies that are associated with dynamic changes in mitochondrial number. Consistent with a role for MFN2 in hypertrophy are the observations of cardiac hypertrophy in mice with cardiac-specific MFN2 knockout (Papanicolaou et al., 2011; Chen and Dorn, 2013). These implicated MFN2 as a potential cause, and therefore target for treating LVH. MFN2 and the mitochondria are presumably implicated in such a condition through the generation of ROS, and an energy supply–demand mismatch. One can hypothesize that by increasing the activity of MFN2 in the early stages of hypertrophy, the mitochondrial network is maintained, ensuring healthy mitochondria, which produce less ROS. Furthermore, given the ability of MFN2 to tether mitochondria to the SR, this pathway is not only important in the maintenance of Ca2+ homeostasis, but also in energy supply–demand coupling. Through these mechanisms, MFN2 could reduce the need for the heart to hypertrophy pathologically.
Another mitochondrial fusion protein, OPA1, may also be a potential therapeutic target for preventing LVH. Mice that are partially deficient in OPA1 are more susceptible to LVH and cardiac dysfunction induced by total aortic constriction (Piquereau et al., 2012). While the anti-hypertrophic effects of OPA1 overexpression in the adult heart are yet been investigated, it is supposed that beneficial effects would be observed. The benefits could operate on two levels, the maintenance of a healthy mitochondrial network (less ROS, better ATP coupling etc.) and a reduction in apoptosis. With OPA1 a strongly anti-apoptotic protein (as previously discussed), OPA1 manipulation may reduce the apoptosis of cardiomyocytes, reducing subsequent collagen deposition and the associated decline in cardiac function. If the next lines of investigation prove MFN2 and OPA1 to be beneficial in the treatment of hypertrophy, the next challenges are to discover drugs that will specifically activate these fusion proteins, and pinpoint the exact time-point of disease progression where activation of these proteins would prove most beneficial.
Mitochondrial fission proteins and LVH
In a recent study it was reported that Drp1 is up-regulated, and MFN2 and OPA1 are down-regulated in a cell model of phenylephrine-induced cardiomyocyte hypertrophy, suggesting a change in the balance of mitochondrial morphology to fragmentation may be associated with the development of LVH (Javadov et al., 2011). Drp1 has recently been investigated as a potential mediator of LVH given its role in mediating mitophagy, a process that may contribute to the pathogenesis of LVH and heart failure (Givvimani et al., 2012). Treatment with the Drp1 inhibitor Mdivi-1 prevented the progression of LVH and development to heart failure induced by pressure overload transaortic constriction. Treatment with Mdivi-1 was associated with the maintenance of the mitochondrial population, a release of pro-angiogenic factors (CD31 and VEGF) and a reduced collagen deposition. Assuming that Mdivi-1 is acting upon the mitochondria through Drp1 inhibition, this study implicates changes in mitochondrial dynamics as a key stage in the development of pathological hypertrophy (Givvimani et al., 2012).
Differentiation of stem cells into cardiomyocytes
In the cardiomyocyte differentiation of stem cells, changes in mitochondrial function and architecture are required to cater for the increased metabolic demands of the differentiated beating cardiomyocyte (reviewed in Rehman, 2010). For the embryonic stem cell (ESC) to differentiate into a cardiomyocyte, there needs to be a metabolic switch from anaerobic glycolysis to oxidative phosphorylation (Chung et al., 2007). Crucially, this change in mitochondrial metabolism has been reported to require a change in mitochondrial morphology from fragmented rounded mitochondria (lacking cristae) present in the ESC, to an elongated interconnected mitochondria (with well-developed cristae) closely aligned with the myofibrils of the differentiated contractile cardiomyocyte (Chung et al., 2007). This change in mitochondrial morphology was associated with alterations in the expression of Drp1 and MFN2 as well as expected changes in the metabolic transcriptome (Chung et al., 2007). A recent experimental study has shown that the presence of OPA1 and MFN2 are required in the development of the heart, with developmental arrest occurring at e13.5 (Kasahara et al., 2013). Similarly, differentiation from ESC to cardiac myocytes was associated with an increased expression of MFN2 and OPA1. While knockout of these genes failed to affect mitochondrial biogenesis, the mitochondrial network failed to elongate and the cells were no longer able to differentiate into beating cardiomyocytes. Through a mechanism that was unrelated to ATP production, the interruption of mitochondrial fusion reduced signalling calcium entry, which suppressed calcineurin activity and Notch signalling (Kasahara et al., 2013). These findings suggest that therapeutic targeting of mitochondrial fusion and fission protein may allow one to facilitate the cardiac differentiation of stem cells in future regenerative therapy.
Limitations and future therapeutic potential
Despite the therapeutic potential of pharmacologically targeting mitochondrial fusion and fission proteins to treat cardiovascular disease, several hurdles still need to be overcome to make this a clinical reality. These include the ability to target the therapy in terms of treatment duration and its organ-specificity. Given the important physiological roles the mitochondrial fusion and fission proteins play in normal cellular physiology, therapeutic activation or inhibition of either the mitochondrial fission or fusion proteins is likely to have detrimental effects as both processes are required to maintain a healthy mitochondrial network for normal cell function. As such, the application of this therapeutic approach may be limited to temporary modulation in acute rather than chronic cardiovascular conditions. Another important issue to consider will be the ability to target the drug to a specific organ and avoid off-target side effects. One potential approach could be the intracoronary delivery of the drug or coating coronary stents with the drug, thereby enabling the local delivery of the drug to the myocardium or the coronary endothelium.
Summary
Mitochondria are now regarded as highly dynamic organelles with multiple functions. Emerging data suggest that the mitochondrial fusion and fission proteins may provide novel therapeutic targets for treating a variety of cardiovascular diseases including acute IRI, heart failure, LVH, pulmonary arterial hypertension and diabetes mellitus (see Figure 2). There is also the potential to target mitochondrial fusion and fission proteins to promote DA closure and facilitate cardiomyocyte differentiation from stem cells for regenerative therapy.
Funding
This work was supported by the British Heart Foundation (FS/10/039/28270), the RoseTrees Trust, and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. A. R. H. was supported by the Medical Research Council (MR/J003530/1).
Conflict of interest
None.
Glossary
- Cdk1
cyclin-dependent kinase 1
- Drp1
dynamin-related peptide
- ER
endoplasmic reticulum
- ESC
embryonic stem cell
- Fis1
human fission protein 1
- IMM
inner mitochondrial membrane
- IRI
ischaemic reperfusion injury
- L-OPA1
long form of optic atrophy factor 1
- LV
left ventricular
- LVH
left ventricular hypertrophy
- MAM
mitochondria-associated membranes
- MFF
mitochondrial fission factor
- MFN1
mitofusin 1
- MFN2
mitofusin 2
- MI
myocardial infarction
- MiD49/MiD51
mitochondrial dynamics proteins of 49 kDa and 51 kDa
- MOMP
mitochondrial outer membrane permeabilization
- MPTP
mitochondrial permeability pore
- OMM
outer mitochondrial membrane
- OPA1
optic atrophy factor 1
- PAH
pulmonary arterial hypertension
- PARL
presenilins-associated rhomboid-like protein
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1-α
- PINK1
PTEN-induced putative kinase 1
- RIP
receptor-interacting serine-threonine kinases
- S-OPA1
short/cleaved form of optic atrophy factor 1
- VSMC
vascular smooth muscle cells
References
- An HJ, Cho G, Lee JO, Paik SG, Kim YS, Lee H. Higd-1a interacts with Opa1 and is required for the morphological and functional integrity of mitochondria. Proc Natl Acad Sci U S A. 2013;110:13014–13019. doi: 10.1073/pnas.1307170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqua C, Anesti V, Pyakurel A, Liu D, Naon D, Wiche G, et al. Trichoplein/mitostatin regulates endoplasmic reticulum-mitochondria juxtaposition. EMBO Rep. 2010;11:854–860. doi: 10.1038/embor.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers S, Saunter C, Wilson C, Coats P, Girkin JM, McCarron JG. Mitochondrial motility and vascular smooth muscle proliferation. Arterioscler Thromb Vasc Biol. 2012;32:3000–3011. doi: 10.1161/ATVBAHA.112.255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins MFN1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu T, Tran A, Lu X, Tomilov AA, Davies V, et al. OPA1 mutation and late-onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc. 2012a;1:3012–3024. doi: 10.1161/JAHA.112.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dorn GW. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu Y, Dorn GW. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, et al. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res. 2012b;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SG, Du Q, Huang S, Dong Z. Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol. 2010;299:F199–F206. doi: 10.1152/ajprenal.00716.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4:S60–S67. doi: 10.1038/ncpcardio0766. (Suppl 1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cosson P, Marchetti A, Ravazzola M, Orci L. Mitofusin-2 independent juxtaposition of endoplasmic reticulum and mitochondria: an ultrastructural study. PLoS ONE. 2012;7:e46293. doi: 10.1371/journal.pone.0046293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, et al. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet. 2007;16:1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- De SD, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, et al. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109:584–591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci U S A. 2013;110:5969–5974. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, Clark CF, Eschenbacher WH, Kang MY, Engelhard JT, Warner SJ, et al. MARF and Opa1 control mitochondrial and cardiac function in Drosophila. Circ Res. 2011;108:12–17. doi: 10.1161/CIRCRESAHA.110.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, et al. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgass K, Pakay J, Ryan MT, Palmer CS. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta. 2013;1833:150–161. doi: 10.1016/j.bbamcr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- Fang L, Moore XL, Gao XM, Dart AM, Lim YL, Du XJ. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci. 2007;80:2154–2160. doi: 10.1016/j.lfs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Figueroa-Romero C, Iniguez-Lluhi JA, Stadler J, Chang CR, Arnoult D, Keller PJ, et al. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J. 2009;23:3917–3927. doi: 10.1096/fj.09-136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins BO, Micaroni M, Beznoussenko GV, Rudka T, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Zhang L, Dhillon R, Hong TT, Shaw RM, Zhu J. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS ONE. 2013;8:e60967. doi: 10.1371/journal.pone.0060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287:30024–30034. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS ONE. 2012;7:e32388. doi: 10.1371/journal.pone.0032388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser L, Sonnay S, Stafa K, Moore DJ. Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1. J Neurochem. 2011;118:636–645. doi: 10.1111/j.1471-4159.2011.07318.x. [DOI] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Chen KH, Guo Y, Liao H, Tang J, Xiao RP. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res. 2007;101:1113–1122. doi: 10.1161/CIRCRESAHA.107.157644. [DOI] [PubMed] [Google Scholar]
- Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol. 2003;35:339–341. doi: 10.1016/s0022-2828(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Kutty S, Toth PT, Marsboom G, Hammel JM, Chamberlain C, et al. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res. 2013;112:802–815. doi: 10.1161/CIRCRESAHA.111.300285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, et al. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell. 2011;41:150–160. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Sun L, Ji S, Zhao T, Zhang W, Xu J, et al. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc Natl Acad Sci U S A. 2013;110:2846–2851. doi: 10.1073/pnas.1300741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117(Pt 26):6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Javadov S, Rajapurohitam V, Kilic A, Hunter JC, Zeidan A, Said FN, et al. Expression of mitochondrial fusion-fission proteins during post-infarction remodeling: the effect of NHE-1 inhibition. Basic Res Cardiol. 2011;106:99–109. doi: 10.1007/s00395-010-0122-3. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Cipolat S, Chen Y, Dorn GW, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and notch signaling. Science. 2013;342:734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- Koutsopoulos OS, Laine D, Osellame L, Chudakov DM, Parton RG, Frazier AE, et al. Human Miltons associate with mitochondria and induce microtubule-dependent remodeling of mitochondrial networks. Biochim Biophys Acta. 2010;1803:564–574. doi: 10.1016/j.bbamcr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TD, et al. Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell. 2012;47:547–557. doi: 10.1016/j.molcel.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1924–H1931. doi: 10.1152/ajpheart.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M, Borda-d'Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, et al. Mitochondrial fusion is increased by the nuclear coactivator PGC-1beta. PLoS ONE. 2008;3:e3613. doi: 10.1371/journal.pone.0003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther. 2007;21:467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, et al. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1296–R1302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, et al. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6:853–859. doi: 10.1038/sj.embor.7400488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Sesaki H, Kageyama Y, Reddy PH. Dynamin-related protein 1 heterozygote knockout mice do not have synaptic and mitochondrial deficiencies. Biochim Biophys Acta. 2012;1822:862–874. doi: 10.1016/j.bbadis.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ. 2006;13:1291–1295. doi: 10.1038/sj.cdd.4401985. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Fujimoto T, Tanaka M, Shirasawa S. Tespa1 is a novel component of mitochondria-associated endoplasmic reticulum membranes and affects mitochondrial calcium flux. Biochem Biophys Res Commun. 2013;433:322–326. doi: 10.1016/j.bbrc.2013.02.099. [DOI] [PubMed] [Google Scholar]
- Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18:20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morre DJ, Merritt WD, Lembi CA. Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma. 1971;73:43–49. doi: 10.1007/BF01286410. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2-and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- Ngoh GA, Papanicolaou KN, Walsh K. Loss of mitofusin 2 promotes endoplasmic reticulum stress. J Biol Chem. 2012;287:20321–20332. doi: 10.1074/jbc.M112.359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270–283. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833:1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12:565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, et al. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012a;111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Ngoh GA, Dabkowski ER, O'Connell KA, Ribeiro RF, Jr, Stanley WC, et al. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol. 2012b;302:H167–H179. doi: 10.1152/ajpheart.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Phillippo MM, Walsh K. Mitofusins and the mitochondrial permeability transition: the potential downside of mitochondrial fusion. Am J Physiol Heart Circ Physiol. 2012c;303:H243–H255. doi: 10.1152/ajpheart.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, James DI, Da CS, Mattenberger Y, Donze O, Barja F, et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L, Scorrano L. A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ. 2007;14:1275–1284. doi: 10.1038/sj.cdd.4402145. [DOI] [PubMed] [Google Scholar]
- Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, et al. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res. 2012;94:408–417. doi: 10.1093/cvr/cvs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodriguez F, Peinado JR, et al. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31:2117–2133. doi: 10.1038/emboj.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med. 2010;88:981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX-and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Sandebring A, Thomas KJ, Beilina A, van der Brug M, Cleland MM, Ahmad R, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE. 2009;4:5701–5719. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan Szklarz LK, Scorrano L. The antiapoptotic OPA1/Parl couple participates in mitochondrial adaptation to heat shock. Biochim Biophys Acta. 2012;1817:1886–1893. doi: 10.1016/j.bbabio.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Nagashima S, Tokuyama T, Amo T, Matsuki Y, Ishido S, et al. MITOL regulates endoplasmic reticulum-mitochondria contacts via mitofusin2. Mol Cell. 2013;51:20–34. doi: 10.1016/j.molcel.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De SD, Wieckowski MR, Cavagna D, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Li Q, Li PF. Apoptosis repressor with caspase recruitment domain contributes to chemotherapy resistance by abolishing mitochondrial fission mediated by dynamin-related protein-1. Cancer Res. 2009;69:492–500. doi: 10.1158/0008-5472.CAN-08-2962. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yu H, Guo Y, Mi L, Wang X, Li L, Gao W. Mitofusin 2 inhibits angiotensin II-induced myocardial hypertrophy. J Cardiovasc Pharmacol Ther. 2011;16:205–211. doi: 10.1177/1074248410385683. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu W, Liu J, Xiao W, Liu L, Jiang C, et al. G-protein beta2 subunit interacts with mitofusin 1 to regulate mitochondrial fusion. Nat Commun. 2010;1:101–111. doi: 10.1038/ncomms1099. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wang S, Li Y, Che L, Zhao Q. A selective inhibitor of Drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. Neurosci Lett. 2013;535:104–109. doi: 10.1016/j.neulet.2012.12.049. [DOI] [PubMed] [Google Scholar]
- Zhao T, Huang X, Han L, Wang X, Cheng H, Zhao Y, et al. Central role of mitofusin 2 in autophagosome-lysosome fusion in cardiomyocytes. J Biol Chem. 2012;287:23615–23625. doi: 10.1074/jbc.M112.379164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chen KH, Cao W, Zeng J, Liao H, Zhao L, et al. Mutation of the protein kinase A phosphorylation site influences the anti-proliferative activity of mitofusin 2. Atherosclerosis. 2010;211:216–223. doi: 10.1016/j.atherosclerosis.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Zorzano A. Regulation of mitofusin-2 expression in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:433–439. doi: 10.1139/H09-049. [DOI] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]