Abstract
Grape seed extract (GSE) is a by-product of the wine industry, with abundant polyphenolic compounds known for their anti-inflammatory and anti-oxidative effects. Using IL10-deficient mice (IL10KO), here we showed that GSE (1% of dry feed weight) ameliorated inflammatory bowel disease (IBD) indices, increased colonic goblet cell numbers and decreased myeloperoxidase levels in the large intestine. Concomitantly, GSE supplementation attenuated inflammation, decreased the expression of pore forming tight junction protein claudin2, and increased levels of Lactobacilli and Bacteroides in the gut microbiota of IL10KO mice. In summary, our study shows that GSE has protective roles on IBD through altering gut inflammation, tight junction protein expression, and gut microbiota composition.
Keywords: inflammatory bowel disease, grape seed extract, intestine, epithelium, IL10, microbiota
Inflammatory bowel disease (IBD) broadly refers to two major chronic intestinal disorders: Crohn's disease (CD) and ulcerative disease (UC), both of which incur huge health costs in U.S. and around the world. Though the etiology of IBD remain poorly defined, IBD pathogenesis involves the alternation of intestinal microbiota and defects in intestinal epithelial barrier function [1]. A “leaky” gut is the central predisposing factors to IBD, which transmits harmful bacteria and other antigens leading to chronic gut inflammation [2, 3]. Meanwhile, microbial community imbalances are correlated with the IBD pathogenesis [4, 5], and the modulation of gut microflora by probiotic [6] and prebiotic [7] treatments reduces inflammatory response in mouse models of colitis. Current pharmacological therapies for IBD long-term management rely on anti-inflammatory drugs, which can result in serious side effects, including secondary infections and immunosuppression [8]. Thus alternative approaches or adjuvant therapies are needed, which can provide long-term management of the disease with a low-risk profile.
Grape seed extract (GSE) is a by-product of the wine industry rich in polyphenolic compounds. Plant derived polyphenols are known for their anti-oxidative and anti-inflammatory effects, exerting a number of health beneficial effects including prevention of cancer, cardiovascular diseases and diabetes [9, 10]. Moreover, GSE and its constituents had protective effects on chemical induced ulcerative colitis in rats [11-14]. However, their efficacy in IL10-deficient mice colitis, a widely used animal model translatable to human chronic IBD, has not been tested. The study herein evaluated the protective role of dietary GSE on IBD symptoms of IL 10-deficient mouse and further explored the underlying mechanisms.
We supplemented wild-type (WT) and IL10-deficient (IL10KO) mice with 0% or 1% GSE (g GSE/g dry feed weight) for 16 weeks (See the Supporting Information for GSE specification and detailed methods), which resulted in four treatments; WT-CON (n=9), WT-GSE (n=9), IL10KO-CON (n=11) and IL10KO-GSE (n=11). There was no difference in feed intake or body weight gain among treatments (Supporting Information Fig. S1). At necropsy, IL10KO-CON showed splenomegaly, which was mitigated by GSE supplementation (Supporting Information Fig. S2). In both CON fed and GSE supplemented groups, WT mice showed no signs of IBD symptoms during 16 weeks feeding trial (Fig. 1A), while IL10KO-CON started to show colitis symptoms after 8 weeks (i.e. weight loss, rectal mucous secretion, diarrhea and rectal prolapses), which were delayed and attenuated by GSE supplementation (Fig. 1A), indicating the effectiveness of GSE in mitigating disease indices of IL10-deficient mice. The results was supported by previous studies on dextran sulfate sodium (DSS) or 2,4,6-trinitrobenzene sulfonic acid (TNBS) induced ulcerative colitis in rats[11-14], where GSE or its constituents supplementation for 7 to 10 days mitigated disease severity in the proximal colon [12] or exerted a protective effects in the recurrent phase of TNBS induced ulcerative colitis of rat [11, 13, 14].
Fig.1.
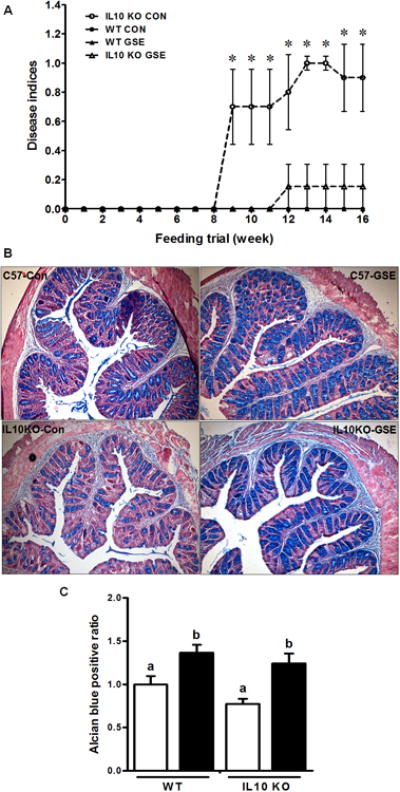
Disease indices of inflammatory bowel disease *: P < 0.05 (A); Goblet cell density Alcian blue staining representative pictures (B) and statistical results (C) of the large intestine of IL10KO or WT mice fed a control (□) or GSE supplemented (■) diet. Original magnification at 100 ×. Histogram bars with the same letter do not differ significantly at P < 0.05 (Mean ± SEM, n = 9-11) (for detailed methods, see Supporting Information).
To further explore mechanisms responsible for the observed beneficial effects of GSE on disease indices of IL 10-deficient mice, we analyzed gut epithelial barrier function, a primary pre-disposing factor for the incidence of IBD, type 1 diabetes and other autoimmune diseases [2, 3, 15]. The intestinal mucosal layer provides the first line of defense against harmful agents and is crucial in maintaining gut integrity [1]. Severe active UC patients have reduced mucus layer thickness and goblet cell density [16]. In line with published data [16], we found that the colonic goblet cell density of IL10KO mice were numerically lower than that of WT mice, while GSE supplementation increased goblet cell density in both WT and IL10KO mice (Fig. 1B & C). The elevated goblet cell density in IL10KO-GSE mice is expected to increase mucin biosynthesis and secretion, improving barrier function. In addition to the mucus layer, the formation of tight junctions among epithelial cells is critical in the regulation of epithelial permeability [3, 17]. Increased expression of the barrier weakening claudin 2, and the decreased and redistribution of barrier forming occludin, claudin 5 and 8 were associated with gut inflammation and active Crohn's disease [18]. Previously, dietary GSE or GSE procyanidins supplementation was shown to modulate tight junction protein expression in in vitro cultured Caco-2 cells [19] as well as rat intestinal tissues [20, 21]. Consistently, claudin 2 mRNA expression was higher in IL10KO mice compared to the WT mice (Supporting Information Fig. S3), while GSE supplementation reduced the mRNA and protein levels of claudin 2 in both WT and IL10KO mice (Supporting Information Fig. S3). This is consistent with improved IBD disease indices in GSE fed IL10KO mice, and also a previous publication showing that active Crohn's disease is associated with high expression of claudin 2 [18]. On the other hand, mRNA expression of claudin 3, which enhances barrier function, was significantly lower in IL10-deficient mice regardless of dietary treatment (Supporting Information Fig. 3A).
In alignment with known anti-inflammatory effects of GSE and associated polyphenolic compounds [13, 22-24], here, we demonstrated dietary GSE supplementation attenuated inflammatory responses in the large intestine of IL10KO mice. The mRNA levels of key inflammatory mediators, TNF-α, IL1β, IL6 and IFN-γ, were dramatically elevated in IL10KO-CON mice compared to those of WT-CON mice, which were mitigated by GSE supplementation (Fig. 2A). T-bet is a transcriptional factor promoting Th1-mediated colitis in vivo [25]. Compared to WT mice, T-bet mRNA expression was enhanced in the large intestine of IL10KO mice regardless of the dietary treatment (Fig. 2B), which might contribute to strong Th1 inflammatory responses in the colon of IL10KO mice [26]. However, GSE had no significant effects on T-bet mRNA expression in both WT and IL10KO mice (Fig. 2B), suggesting GSE exerted anti-inflammatory effects independent of T-bet. IL10KO-CON had similar GATA3 mRNA expression to that of WT-CON mice, but GSE supplementation decreased GATA3 mRNA level in WT mice but not in IL10KO mice (Fig. 2B). The NF-κB signaling pathway is a major inflammatory signaling pathway. In line with elevated pro-inflammatory cytokine expression, phosphorylation of p65, the key mediator of NF-κB inflammatory signaling was enhanced in IL10KO-CON compared to that of WT, which was again alleviated by dietary GSE supplementation (Fig. 2C). These data indicated that the protective role of GSE in IL10KO mice was at least partially due to its anti-inflammatory effects.
Fig.2.
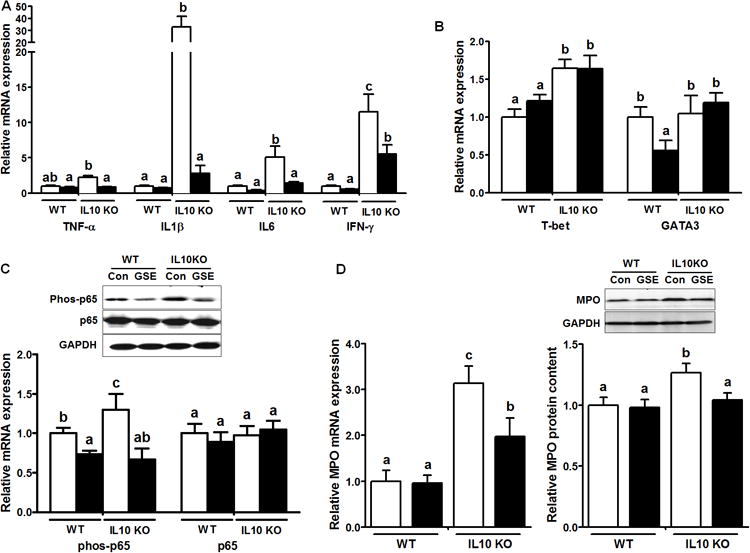
Inflammatory cytokines (A), T-bet and GATA3 (B), inflammatory NF-kB p65 signaling (C), and myeloperoxidase mRNA and protein levels (D) in IL10KO or WT mice fed a CON (□) or GSE supplemented (■) diet; Histogram bars with the same letter do not differ significantly at P < 0.05 (Mean ± SEM, n = 9-11) (for detailed methods, see Supporting Information).
Infiltration of neutrophils to the inflammatory site is a prominent feature for active IBD [27]. The severity of colitis is commonly assessed by analyzing colonic or fecal myeloperoxidase (MPO) activity [27] or MPO content [6], due to the fact that MPO abundantly presents in neutrophils and its content is correlated with the neutrophil number. In consistent with observed inflammatory response, both mRNA and protein levels of MPO were augmented in IL10KO mice compared to these in WT mice, which were mitigated by GSE supplementation (Fig. 2D), eliciting that GSE supplementation reduced neutrophil infiltration into the large intestine of IL10KO mice. These data were supported by a recent study in healthy Wistar Furth rats, where GSE supplementation reduces the level of fecal neutrophil protein calprotectin, another maker of gut neutrophil infiltration [21].
Gut microbiota are increasingly recognized an important player in intestinal permeability and associated metabolic diseases. A balance between beneficial and pathogenic bacteria plays a central role in the mucosal immune response in IBD [4, 28]. In the present study, GSE supplementation resulted in increased Bacteroides abundance in both WT and IL10KO mice (Fig. 3A). Compared to WT-CON mice, Lactobacilli were diminished in quantity in IL10KO-CON fecal samples, which was compensatory increased by GSE supplementation (Fig. 3B). In line with these observations, the previous reports found that the abundance of Bacteroides were reduced in the mucosal samples of IBD patients [4], while probiotic [2, 6] and prebiotic [7] supplements attenuate IBD symptoms. As in the current study, regular intake of a high-cocoa flavonol drink results in increased Lactobacillus spp. and Bifidobacterium in fecal samples of human volunteers [29], and the red wine polyphenols ingestion augments Bacteroides and Bifidobacterium in the healthy male participants' gut microbial ecosystem [30]. In addition, we observed that GSE supplementation decreased F. prausnitzii in both WT and IL10KO mice (Fig. 3C). The mechanisms responsible for the reduction of this bacterium and its relationship with increased Bacteroides and Lactobacilli abundance warrant further studies. These data suggested GSE supplementation could have a note-worthy effect on the abundance of select gut microbiota, which might contribute to improved gut immune and barrier function as well as IBD symptoms. In this study, commercial GSE product (Gravinol-S) was used, which contains a minimal 80% of proanthocyanidins. Considering the protective roles of proanthocyanidins on chemical induced ulcerative colitis in rats [11-14]; we speculate that proanthocyanidins in GSE were likely to be the key component for the observed beneficial effects of GSE supplementation.
Fig.3.
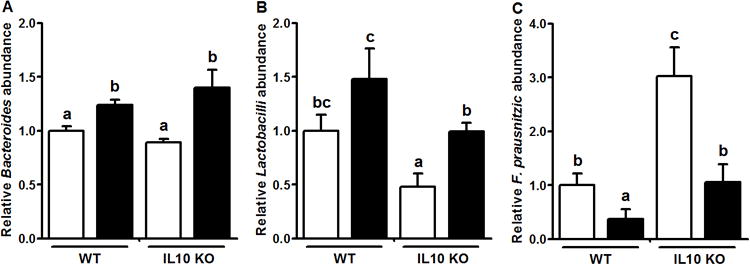
Fecal microflora composition in IL10KO or WT mice fed a CON (□) or GSE supplemented (■) diet. A: Bacteroides; B: lactobacilli; C: Faecalibacterium prausnitzii Histogram bars with the same letter do not differ significantly at P = 0.05 (Mean ± SEM, n=9-11).
In summary, the current study demonstrated that dietary GSE supplementation exerted protective effects in IBD indices of IL10KO mice through several mechanisms including modulating gut microflora, blocking gut inflammatory response and decreasing pore forming tight junction protein claudin 2. Therefore, dietary GSE supplementation might be an alternative approach for preventive or therapeutic treatments of IBD and related gut diseases.
Supplementary Material
Acknowledgments
This work was financially supported by USDA-AFRI 2009-65203-05716, NIHR15HD073864 and Washington State University seed grant.
Abbreviations
- CD
Crohn's disease
- DSS
dextran sulfate sodium
- GSE
grape seed extract
- IBD
inflammatory bowel disease
- IFN-γ
interferon γ
- IL
interleukin
- KO
knockout
- MPO
myeloperoxidase
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- Th-1
T helper 1
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- TNF-α
tumor necrosis factor α
- UC
ulcerative disease
- WT
wild-type
References
- 1.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 2.Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L, Gasbarrini A. The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol. 2012;(46 Suppl):S12–17. doi: 10.1097/MCG.0b013e31826ae849. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank DN, St Amand AL, Feldman RA, Boedeker EC, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America; 2007; pp. 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira M, Bosco N, Perruisseau G, Nicolas J, et al. Lactobacillus paracasei reduces intestinal inflammation in adoptive transfer mouse model of experimental colitis. Clin Dev Immunol. 2011;2011:807483. doi: 10.1155/2011/807483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Videla S, Vilaseca J, Antolin M, Garcia-Lafuente A, et al. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96:1486–1493. doi: 10.1111/j.1572-0241.2001.03802.x. [DOI] [PubMed] [Google Scholar]
- 8.Melmed GY, Targan SR. Future biologic targets for IBD: potentials and pitfalls. Nat Rev Gastroenterol Hepatol. 2010;7:110–117. doi: 10.1038/nrgastro.2009.218. [DOI] [PubMed] [Google Scholar]
- 9.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(1):S139–151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 10.Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr. 2012;52:936–948. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- 11.Li XL, Cai YQ, Qin H, Wu YJ. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Canadian journal of physiology and pharmacology. 2008;86:841–849. doi: 10.1139/Y08-089. [DOI] [PubMed] [Google Scholar]
- 12.Cheah KY, Bastian SE, Acott TM, Abimosleh SM, et al. Grape seed extract reduces the severity of selected disease markers in the proximal colon of dextran sulphate sodium-induced colitis in rats. Digestive diseases and sciences. 2013;58:970–977. doi: 10.1007/s10620-012-2464-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang YH, Ge B, Yang XL, Zhai J, et al. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. International immunopharmacology. 2011;11:1620–1627. doi: 10.1016/j.intimp.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Yang XL, Wang L, Cui MX, et al. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Canadian journal of physiology and pharmacology. 2010;88:888–898. doi: 10.1139/y10-071. [DOI] [PubMed] [Google Scholar]
- 15.Yu LC. The epithelial gatekeeper against food allergy. Pediatr Neonatol. 2009;50:247–254. doi: 10.1016/S1875-9572(09)60072-3. [DOI] [PubMed] [Google Scholar]
- 16.Strugala V, Dettmar PW, Pearson JP. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn's disease. International journal of clinical practice. 2008;62:762–769. doi: 10.1111/j.1742-1241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cellular and molecular life sciences : CMLS. 2012 doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeissig S, Burgel N, Gunzel D, Richter J, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T, Tanabe S, Hara H. Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. The Journal of nutrition. 2011;141:87–94. doi: 10.3945/jn.110.125633. [DOI] [PubMed] [Google Scholar]
- 20.Song P, Zhang R, Wang X, He P, et al. Dietary grape-seed procyanidins decreased postweaning diarrhea by modulating intestinal permeability and suppressing oxidative stress in rats. Journal of agricultural and food chemistry. 2011;59:6227–6232. doi: 10.1021/jf200120y. [DOI] [PubMed] [Google Scholar]
- 21.Goodrich KM, Fundaro G, Griffin LE, Grant A, et al. Chronic administration of dietary grape seed extract increases colonic expression of gut tight junction protein occludin and reduces fecal calprotectin: a secondary analysis of healthy Wistar Furth rats. Nutr Res. 2012;32:787–794. doi: 10.1016/j.nutres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Hogan S, Canning C, Sun S, Sun X, et al. Dietary supplementation of grape skin extract improves glycemia and inflammation in diet-induced obese mice fed a Western high fat diet. Journal of agricultural and food chemistry. 2011;59:3035–3041. doi: 10.1021/jf1042773. [DOI] [PubMed] [Google Scholar]
- 23.Gessner DK, Ringseis R, Siebers M, Keller J, et al. Inhibition of the pro-inflammatory NF-kappaB pathway by a grape seed and grape marc meal extract in intestinal epithelial cells. J Anim Physiol Anim Nutr (Berl) 2012;96:1074–1083. doi: 10.1111/j.1439-0396.2011.01222.x. [DOI] [PubMed] [Google Scholar]
- 24.Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol Carcinog. 2010;49:641–652. doi: 10.1002/mc.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neurath MF, Weigmann B, Finotto S, Glickman J, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. The Journal of experimental medicine. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg DJ, Davidson N, Kuhn R, Muller W, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. The Journal of clinical investigation. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masoodi I, Tijjani BM, Wani H, Hassan NS, et al. Biomarkers in the management of ulcerative colitis: a brief review. German medical science : GMS e-journal. 2011;9:Doc03. doi: 10.3205/000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki M, Klapproth JM. The role of bacteria in the pathogenesis of ulcerative colitis. J Signal Transduction. 2012;2012:704953. doi: 10.1155/2012/704953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, et al. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 30.Queipo-Ortuno MI, Boto-Ordonez M, Murri M, Gomez-Zumaquero JM, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. The American journal of clinical nutrition. 2012;95:1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


