Abstract
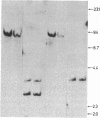
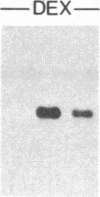
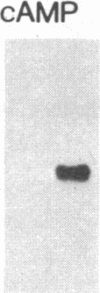
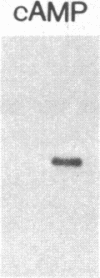
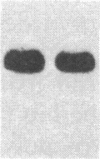
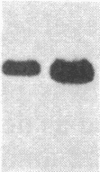
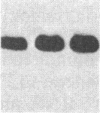
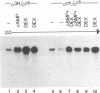
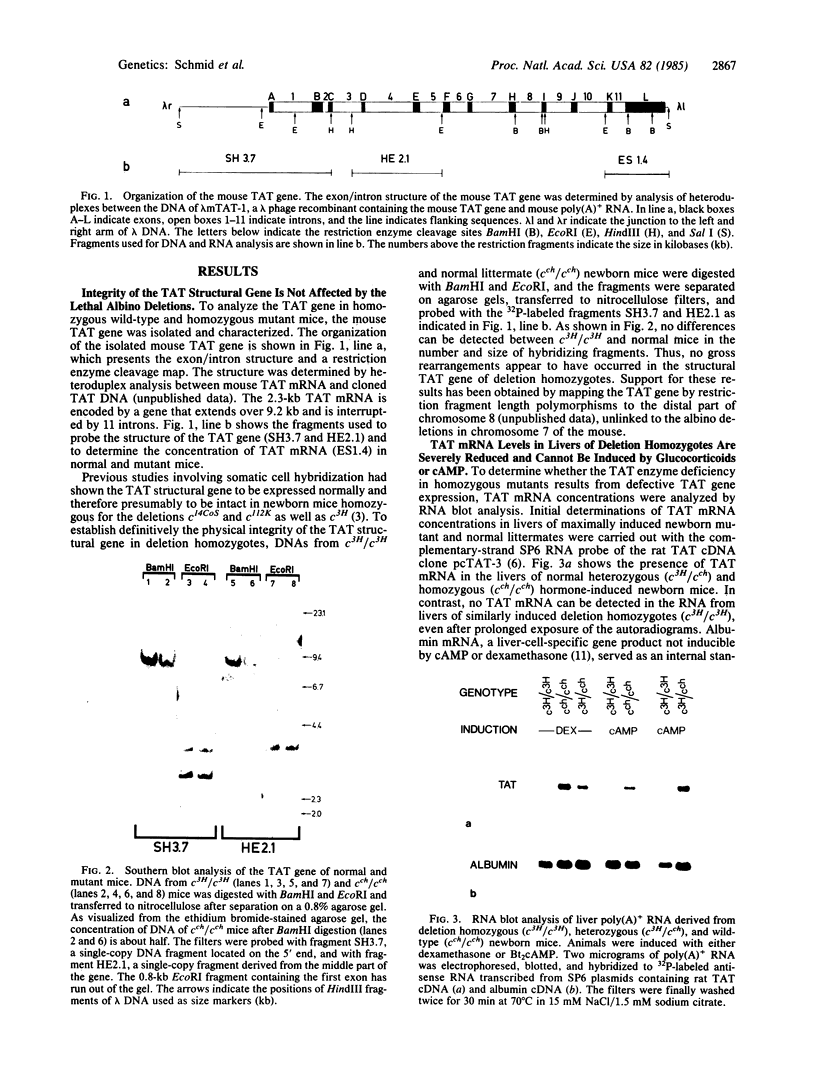
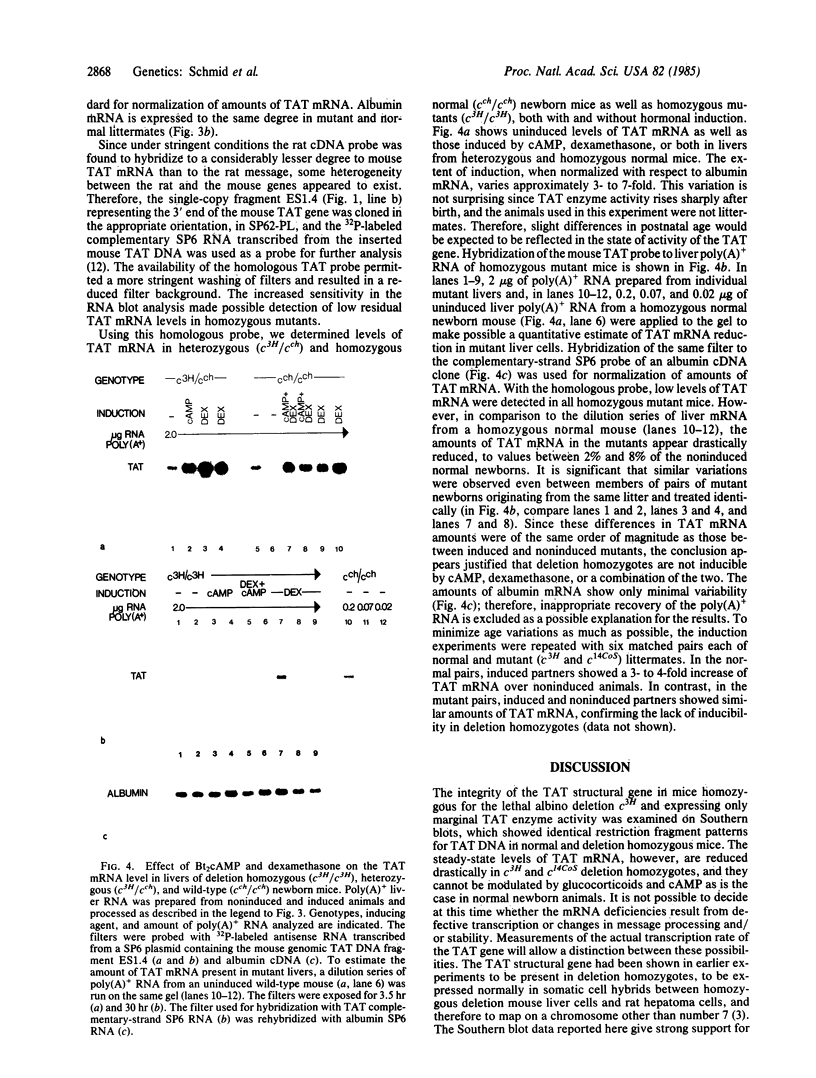
Overlapping chromosomal deletions at the albino locus on chromosome 7 of the mouse affect the expression of several liver enzymes, including tyrosine aminotransferase (TAT; L-tyrosine:2-oxoglutarate aminotransferase, EC 2.6.1.5). With cloned TAT DNA the integrity of the TAT structural gene and its expression and inducibility by glucocorticoids and cAMP were examined in deletion homozygous mice. No difference in the structure of the gene between normal and mutant mice was detected by Southern blotting. Severely reduced amounts of TAT mRNA were detected in homozygous mutants. The residual mRNA levels could not be modulated by glucocorticoids or cAMP. We conclude that a trans-acting control function required for expression and inducibility of mouse TAT can be assigned to the chromosomal region near the albino locus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P., Renkawitz R., Schütz G. Tissue-specific DNaseI hypersensitive sites in the 5'-flanking sequences of the tryptophan oxygenase and the tyrosine aminotransferase genes. EMBO J. 1984 Sep;3(9):2015–2020. doi: 10.1002/j.1460-2075.1984.tb02084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori C. F., Gluecksohn-Waelsch S., Klinger H. P., Pick L., Schlagman S. L., Teicher L. S., Wang-Chang H. F. Complementation of gene deletions by cell hybridization. Proc Natl Acad Sci U S A. 1981 Jan;78(1):479–483. doi: 10.1073/pnas.78.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori C. F., Gluecksohn-Waelsch S., Shaw P. A., Robinson C. Correction of a genetically caused enzyme defect by somatic cell hybridization. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6611–6614. doi: 10.1073/pnas.80.21.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesch U., Hashimoto S., Renkawitz R., Schütz G. Transcriptional regulation of the tryptophan oxygenase gene in rat liver by glucocorticoids. J Biol Chem. 1983 Apr 25;258(8):4750–4753. [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Granner D. K., Hargrove J. L. Regulation of the synthesis of tyrosine aminotransferase: the relationship to mRNATAT. Mol Cell Biochem. 1983;53-54(1-2):113–128. doi: 10.1007/BF00225249. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Schmid W., Schütz G. Transcriptional activation of the rat liver tyrosine aminotransferase gene by cAMP. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6637–6641. doi: 10.1073/pnas.81.21.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Schmid W., Strange C. M., Röwekamp W., Schütz G. Isolation of cDNA clones coding for rat tyrosine aminotransferase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7205–7208. doi: 10.1073/pnas.79.23.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Scherer G., Schmid W., Zentgraf H., Schütz G. Isolation and characterization of the rat tyrosine aminotransferase gene. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1346–1350. doi: 10.1073/pnas.81.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]