Abstract
PURPOSE
Monkey neurophysiology suggests that changes in neural drive rather than extraocular muscle structure underlie sensory-induced strabismus. If this is true, then extraocular muscle structure should be normal. We used magnetic resonance imaging to measure horizontal rectus muscle size and contractility to determine whether muscle structure is a factor in human concomitant esotropia.
METHODS
High-resolution, quasicoronal plane magnetic resonance imaging was performed in target-controlled central gaze, abduction, and adduction in 13 orthotropic controls (mean age, 38 ± 19 years) and 12 adults (mean age, 52 ± 16 years) who had concomitant esotropia averaging 28Δ ± 18Δ at distance. Thyroid ophthalmopathy was excluded. Horizontal rectus muscle cross sections were determined in 6 contiguous, 2-mm-thick midorbital image planes. Contractility was computed in each plane as the difference in cross section from contraction to relaxation.
RESULTS
Medial rectus muscle cross sections in multiple planes averaged up to 39% larger in esotropic patients than in controls (P <0.005), whereas lateral rectus muscle cross sections in esotropia were up to 28% larger but only significantly larger in one plane (P <0.02). Medial rectus contractility was increased by up to 60% in esotropic patients (P <0.005), whereas lateral rectus contractility in esotropia was slightly but not significantly supernormal.
CONCLUSIONS
Medial rectus muscle size is supernormal and lateral rectus muscle size is not subnormal in concomitant esotropia. This finding indicates that human concomitant esotropia is associated with peripheral muscular abnormality.
Concomitant esotropia frequently is encountered in clinical strabismus practice, yet there is little consensus on the cause or causes. Clearly, one or both eyes are excessively adducted in esotropia, implying that the medial rectus muscle is “overacting” in some sense or that the lateral rectus muscle is “underacting.” Yet such an assertion says nothing specific about the biological mechanisms of esotropia.1 It has been speculated that the medial rectus muscle eventually becomes mechanically shortened or “tight” in esotropia, yet this seems unlikely to be the initiating factor. A similar but etiologically implausible speculation has been made that the lateral rectus muscle eventually becomes mechanically lengthened or “loose,” yet this seems equally unlikely to be the primary cause of esotropia. Some have argued that the initiating factor in concomitant esotropia is excessive medial rectus innervation, perhaps associated with deficient lateral rectus innervation, representing a convergent “tonus.”2 Another possibility is that the medial rectus muscle becomes hyper-contractile, in the sense that it might become large and/or stronger, so that the normal innervational command evokes more adducting force. Conversely, the lateral rectus muscle might become smaller and/or weaker, so that the normal innervational command evokes less abducting force. It is even possible that abnormal innervation might induce secondary changes in the size and/or strength of the horizontal rectus muscles. Clinical observations cannot relate any of these putative mechanisms to concomitant esotropia.
Recent studies of strabismus in animals have helped narrow the range of possible causes of concomitant esotropia. Das3 and Joshi and Das4 made electrophysiologic recordings of the horizontal rectus muscle motor neurons in monkeys who began life with normal extraocular muscles and developed strabismus as a result of abnormal visual experience. They found that the relationship between motor neuron firing rate and eye position in these monkeys was the same as that found in normal monkeys. This finding implies that the extraocular muscles of these strabismic animals had normal lengths and strengths, ruling out the commonly assumed clinical explanations of “tight” or “overacting” extraocular muscles. This finding supports the idea that strabismus in these monkeys is attributable to abnormal central vergence commands to normal extra-ocular muscles.
Although strabismus in monkeys is similar in nearly every respect to strabismus in humans,5 extraocular muscle motor neuron electrophysiology studies cannot be performed in humans to validate the comparison. Magnetic resonance imaging (MRI), however, may be used to measure human extraocular muscle contractility, because, in part, a very close correlation exists between ocular duction angle and quantitative aspects of extraocular muscle morphology.6,7 Although functional MRI cannot directly indicate extraocular muscle force,8 the same criticism applies to recordings of extraocular muscle motor neurons, where a discrepancy between horizontal rectus muscle force and motor neuron firing during convergence has been reported.9,10 The purpose of the present study was to obtain functional MRI data on the size and contractility of human horizontal rectus muscles in concomitant esotropia.
Subjects and Methods
Subjects prospectively provided written informed consent according to an institutional review board–approved protocol compliant with the requirements of the Health Insurance Portability and Accountability Act. Paid control subjects were recruited by advertising and underwent complete examinations to verify normal corrected vision, normal ocular versions, orthotropia in all gaze positions, and normal stereopsis of 40 arcsec by Titmus testing. Subjects with esotropia were recruited from an academic strabismus practice into a long-term, prospective study of strabismus and underwent complete sensorimotor evaluation and MRI. The ongoing study includes a large number of potentially eligible subjects. Power analysis indicated that a sample size of 12 subjects would provide at least 80% power to confirm at the 5% level a difference in rectus muscle contractility. We therefore selected from the study database the first alphabetically consecutive 13 controls and a similar number of esotropia cases that had adequate imaging quality for analysis. Cases of paralytic or restrictive esotropia, such as abducens palsy or thyroid ophthalmopathy, were excluded. Deviations were measured at distance and near by cover testing with prisms.
Control data were thus obtained from 13 orthotropic adult volunteers (mean age, 38 ± 19; range, 18–74; 5 females) and 12 adult subjects (mean age, 52 ± 16; range 31–71 years; 5 females) with esotropia. Strabismic subjects had concomitant esotropia averaging 28Δ ± 18Δ at distance. The duration of esotropia ranged from <3 months to 49 years. Of the 12 subjects with esotropia, 4 had undergone previous surgery (1, orbital fracture repair not causative of the strabismus; 3, previous strabismus surgery). Subjects did not have restrictive strabismus. Best-corrected visual acuities ranged from 20/16 to 20/50 and were ≥20/20 one eye of each patient. Of the 12 esotropic patients, 9 were myopic (range, −8.00 to −0.50 D), 1 was hyperopic (+1.50 D), and 2 were emmetropic.
A 1.5-T MRI scanner (Signa; General Electric, Milwaukee, WI) was used to obtain imaging using T111,12 or T2 fast spin-echo pulse sequences.13 The 2 scanning protocols provide equivalent measurements. Crucial aspects of this technique, described in detail elsewhere, include use of the dual-phased surface coil array (Medical Advances, Milwaukee, WI) and fixation targets.14–16 High-resolution (312-μm), axial and quasi-coronal images of 2-mm thickness and matrix of 256 × 256 perpendicular to the long axis of the orbit were obtained in target controlled central gaze, abduction, and adduction for each eye (Figure 1). Because the scanned eye was centered on a monocularly viewed target, this procedure avoided any confounding caused by an angle of strabismus. Ancillary experiments have verified that this method of target presentation does not evoke vergence to the target, which is a fine, afocal, fiberoptic light. Interocular differences of individual patients were not analyzed.
FIG 1.
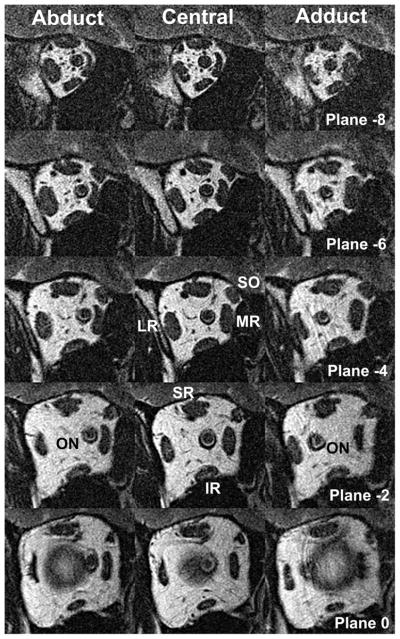
Quasicoronal MRI of right orbit of esotropic subject in abduction (left column), central gaze (middle column) and adduction (right column), in 2-mm-thick image planes numbered positively in the anterior direction from the globe-optic nerve junction at plane 0. IR, inferior rectus muscle; LR, lateral rectus muscle; MR, medial rectus muscle; ON, optic nerve; SR, superior rectus muscle.
Image analysis was similar to published methods.6,17,18 Investigators were not masked to subject diagnosis, but because they did not have a strong previous hypothesis regarding the expected effect of strabismus on extraocular muscles, this was not expected to be a source of bias. Digital MRIs were quantified using ImageJ (Rasband WS. ImageJ, U.S. National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/, 1997–2009, accessed February 2009). To summarize, each rectus muscle’s cross-sectional area was determined automatically after manually outlining it with a cursor. Horizontal rectus muscle cross sections were determined in 6 contiguous, 2-mm-thick midorbital image planes along the length of the extraocular muscles. Contractility was computed in each plane as the difference in cross section from contraction to relaxation, as a function of the change in cross section of the extraocular muscles. The plane of the globe–optic nerve junction served as the reference point for determining image plane location, with image planes more posterior assigned a negative designation, and positive designation anteriorly. Statistical analyses were performed using the t test.
Results
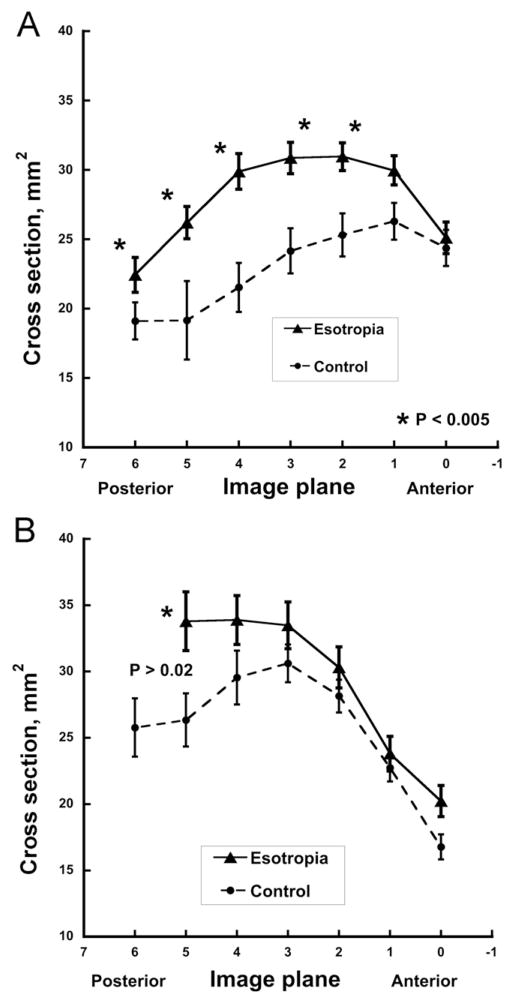
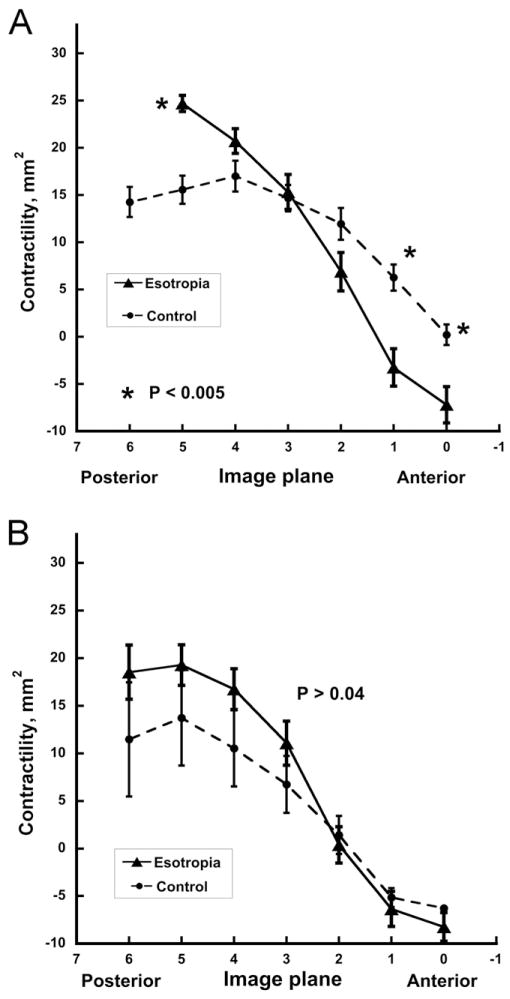
As previously described, the horizontal rectus muscles had maximum cross sections in midorbit (Figure 2) and thinned toward their origins in the annulus of Zinn and anteriorly as the extraocular muscle bellies transitioned to their insertional tendons. Medial rectus muscle cross sections in multiple planes averaged up to 39% larger in esotropic patients than controls (P<0.005, Figure 2A), whereas lateral rectus muscle cross sections in esotropia were up to 28% greater but not significantly so (Figure 2B, P > 0.02). Differences were greatest in the deep orbit. Contractility of the horizontal rectus muscles was greatest in the mid- to deep orbit (Figure 3). Medial rectus muscle contractility was significantly increased by up to 60% in esotropic subjects (P <0.005, Figure 3A), whereas lateral rectus muscle contractility in esotropia was slightly but not significantly supernormal (Figure 3B).
FIG 2.
Horizontal rectus extraocular muscle size, ± standard error of the mean, represented by cross-sectional area along extraocular muscle length in contiguous 2 mm thick image planes referenced to zero at the globe–optic nerve junction. A. Medial rectus muscle. B. Lateral rectus muscle. Note significantly larger than normal medial rectus, but not lateral rectus, cross sections in subjects with esotropia. Data from 24 orbits with esotropia, and 26 control orbits.
FIG 3.
Horizontal rectus muscle contractility, ± standard error of the mean, represented by change in cross-sectional area from contraction to relaxation along the length of the muscle in contiguous 2 mm thick image planes referenced to zero at the globe–optic nerve junction. A. Medial rectus muscle. B. Lateral rectus muscle. Note for esotropic subjects significantly larger than normal medial rectus but not lateral rectus contractility at image planes in the deep orbit and lesser contractility in the anterior orbit. Data from 24 orbits with esotropia, and 26 control orbits.
Discussion
In the present study, subjects with concomitant esotropia had larger medial rectus muscles and lateral rectus muscles than controls. This effect could not have been attributable to confounding by position of the scanned eye or by strabismus angle because each orbit was imaged during monocular fixation of a central target. Although outlining of extraocular muscle cross sections was not masked to alignment status, all analysis subsequent to that step was digital, and there was no a priori hypothesis that would have predicted the current results. The horizontal rectus muscles were not only larger but also demonstrated hypercontractile properties, the medial rectus more so than the lateral rectus muscle, implying an element of central gaze co-contraction. These effects would tend to balance out the tensile forces, allowing for binocular alignment to remain determined by the balance of neural commands. The neural commands in humans with concomitant esotropia therefore might predicted to show larger-than-normal changes with eye position. Because at least the medial rectus muscle is larger than normal and the lateral rectus muscle is not smaller, total tensions of medial rectus plus lateral rectus muscles in esotropia are therefore predicted to be greater than normal.
Both the medial rectus and lateral rectus muscles are significantly larger than those in normal in subjects with concomitant esotropia, as measured in cross sections throughout the orbit. This finding appears specific to the presence of concomitant esotropia, rather than potential confounding factors such as myopathy or eye position during MRI scanning. The esotropic subjects had no evidence of thyroid ophthalmopathy or any other condition known to enlarge extraocular muscles. All subjects were imaged in the same central gaze position; thus results were not influenced by instantaneous gaze angle. Moreover, the total volume of any given extraocular muscle is the same regardless of eye position.
Some of the subjects with esotropia had undergone previous strabismus surgery, presumably for esotropia, but surgical details were unavailable. In such instances, if a horizontal rectus muscle resection had been performed, reduced extraocular muscle volume would have been the expected MRI finding. Nevertheless, in the subjects with esotropia, increased volume of the horizontal rectus muscles was observed. Recession of an extraocular muscle would have a negligible effect on volume. The present approach of simultaneously evaluating extraocular muscle cross sections throughout the orbit amounts to considering whole extraocular muscle volumes. It has been demonstrated that strabismus surgery does not significantly alter extraocular muscle volume or cross sections.18 Whatever effect previous strabismus surgery might have had on the horizontal rectus muscles studied here, it could not have directly produced the observed size increases.
It is generally supposed that strabismus arises from neural causes, peripheral mechanical causes, or a combination of these factors. In some cases the clinical cause is obviously mechanical, as in traumatic extraocular muscle avulsion, thyroid ophthalmopathy, or when multipositional MRI demonstrates heterotopy or instability of the extraocular muscle pulleys.12,19,20 The neural hypothesis supposes either peripheral denervation or misinnervation of individual extraocular muscles or a disorder of fusional vergence leading to abnormal central commands in the presence of otherwise normal cranial nerves and extraocular muscles. The congenital cranial dysinnervation disorders, such as Duane syndrome18,21 and congenital fibrosis syndromes22–25 are obvious examples of strabismus caused by abnormal peripheral innervation of extraocular muscles.18,22,24–26 However, the most common and perplexing clinical examples of strabismus are infantile and developmentally acquired forms of esotropia and exotropia in which there is no apparent extraocular muscle peripheral denervation or dysinnervation. These seem plausibly to be the results of pathology of central vergence commands, perhaps, as in the case of accommodative or sensory deprivation esotropia, interacting with optical or environmental factors.
Animal models have confirmed that abnormal visual experience in infancy from alternating monocular occlusion or optical decorrelation can induce abnormal cross-axis eye movements, dissociated vertical deviation, and A- or V-pattern esotropia or exotropia in the absence of gross structural abnormalities of extraocular muscles27 or their peripheral innervations5,28; however, a few reports, such as that of Brueckner and colleagues,29 support a genetic or molecular influence via visual sensory deprivation altering the development of extraocular muscle structure and myosin expression. These authors provided anatomical evidence that during development, visual input to the oculomotor system affects extraocular muscle–specific myosin expression. They found that extraocular muscle phenotypes can be significantly altered due by visual experience during the critical period of visual development. Visual sensory deprivation studies have demonstrated evidence of changes in extraocular muscle fiber types in strabismic monkeys.30
Recently, Altick and colleagues31 found that 22 of 87 (25%) muscle-specific genes in humans were significantly down-regulated in extraocular muscles removed at strabismus surgery. These alterations, mainly decreases in expression of contractility genes and increases of extracellular matrix–associated genes, reflect changes in extraocular muscle structure associated with strabismus. It is unclear how these genetic modifications relate to the functional increases in extraocular muscle contractility observed by MRI in the current study, but they further support the proposition that the extraocular muscles themselves are altered in strabismus.
Although studies in primates have been invaluable in understanding human strabismus,4,27 the current functional MRI study in humans suggests that the clinical situation may be even more complex. Basic experiments have demonstrated that primate congenital and developmental strabismus can arise from abnormal maturation of vergence and gaze circuits in the brain.5 The current functional MRI study demonstrates that human concomitant esotropia also is associated with functional anatomical changes in horizontal rectus muscles. Given the plasticity of extraocular muscle phenotypes to changes in their neural inputs, such functional anatomical changes are not surprising and could easily be the consequences of abnormal innervational patterns. Changes in extraocular muscle phenotypes could even develop together with abnormal innervational commands within complex neuromuscular feedback loops. The understanding of human concomitant esotropia may require parallel investigations incorporating direct investigations of extraocular muscle functional anatomy (such as MRI) or force generation simultaneous with single unit neural recordings, as well as further development of direct measures of extraocular muscle function in strabismic patients.
Acknowledgments
Supported by: US Public Health Service, National Eye Institute grants EY08313 and EY00331 and the Shaw Family Endowment Fund. Kirsta Schoeff was Elsa and Louis Kelson and Jack Rubin Memorial Fellow. Joseph Demer is Leonard Apt Professor of Ophthalmology. Zia Chaudhuri was supported by the BOYSCAST Fellowship of the Department of Science and Technology, Government of India.
Footnotes
Presented at the 38th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, San Antonio, Texas, March 24–28, 2012.
References
- 1.Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–9. doi: 10.1016/s0002-9394(01)01099-6. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky MS, Fray KH. Dissociated horizontal deviation after surgery for infantile esotropia: Clinical characteristics and proposed pathophysiologic mechanisms. Arch Ophthalmol. 2007;125:1683–92. doi: 10.1001/archopht.125.12.1683. [DOI] [PubMed] [Google Scholar]
- 3.Das V. Investigating mechanisms of strabismus in non-human primates. J AAPOS. 2008;12:324–5. doi: 10.1016/j.jaapos.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi AC, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Inv Ophthalmol Vis Sci. 2011;52:6697–705. doi: 10.1167/iovs.11-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tychsen LT, Richards M, Wong A, et al. Spectrum of infantile esotropia in primates: Behavior, brains, and orbits. J AAPOS. 2008;11:375–80. doi: 10.1016/j.jaapos.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark RA, Demer JL. Functional morphometry of horizontal rectus extraocular muscles during ocular duction. Inv Ophthalmol Vis Sci. 2012;53:7375–9. doi: 10.1167/iovs.12-9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S, Nishida Y, Isberg B, Lennerstrand G. MRI measurements of normal extraocular muscles and other orbital structures. Graefes Arch Clin Exp Ophthalmol. 2000;238:393–404. doi: 10.1007/s004170050370. [DOI] [PubMed] [Google Scholar]
- 8.Kushner BJ. Does extraocular muscle form denote function? Arch Ophthalmol. 2010;128:1604–9. doi: 10.1001/archophthalmol.2010.301. [DOI] [PubMed] [Google Scholar]
- 9.Miller JM, Bockisch CJ, Pavlovski DS. Missing lateral rectus force and absence of medial rectus co-contraction in ocular convergence. J Neurophysiol. 2002;87:2421–33. doi: 10.1152/jn.00566.2001. [DOI] [PubMed] [Google Scholar]
- 10.Miller JM, Davison RC, Gamlin PD. Motor nucleus activity fails to predict extraocular muscle forces in ocular convergence. J Neurophysiol. 2011;105:2863–73. doi: 10.1152/jn.00935.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demer JL. A 12 year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–47. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 12.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical strabismus management: Principles and techniques. Philadelphia: WB Saunders; 1999. pp. 84–98. [Google Scholar]
- 13.Demer JL, Dusyanth A. T2 fast spin echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15:17–23. doi: 10.1016/j.jaapos.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 15.Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of extraocular muscles: A new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–88. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 16.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–85. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 17.Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011;2011:904–8. doi: 10.1001/archophthalmol.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane’s retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202. doi: 10.1167/iovs.06-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Cur Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–78. [PubMed] [Google Scholar]
- 21.Miyake N, Demer JL, Shaaban S, et al. Expansion of the CHN1 strabismus phenotype. Inv Ophthalmol Vis Sci. 2011;52:6321–8. doi: 10.1167/iovs.11-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–39. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 23.Demer JL, Clark RA, Lim K-H, Engle EC. Magnetic resonance imaging of innervational and extraocular muscle abnormalities in Duane-radial ray syndrome. Invest Ophthalmol Vis Sci. 2007;48:5505–11. doi: 10.1167/iovs.07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demer JL, Clark RA, Tischfield MA, Engle EC. Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Invest Ophthalmol Vis Sci. 2010;51:4600–11. doi: 10.1167/iovs.10-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim KH, Engle EC, Demer JL. Abnormalities of the oculomotor nerve in congenital fibrosis of the extraocular muscles and congenital oculomotor palsy. Invest Ophthalmol Vis Sci. 2007;48:1601–6. doi: 10.1167/iovs.06-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tischfield MA, Baris HN, Gupta ML, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and neuronal circuitry. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narasimhan A, Tychsen LT, Poukens V, Demer JL. Horizontal rectus muscle anatomy in naturally and artificially strabismic monkeys. Invest Ophthalmol Vis Sci. 2007;48:2576–88. doi: 10.1167/iovs.06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das V, Mustari M. Correlation of cross-axis eye movements and motoneuron activity in non-human primates with “A” pattern strabismus. J AAPOS. 2007;48:665–74. doi: 10.1167/iovs.06-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brueckner JK, Ashby LP, Prichard JR, Porter JD. Vestibuloocular pathways modulate extraocular muscle myosin expression patterns. Cell Tissue Res. 1999;295:477–84. doi: 10.1007/s004410051253. [DOI] [PubMed] [Google Scholar]
- 30.Cheng G, Mustari MJ, Khanna S, Porter S. Comprehensive evaluation of the extraocular muscle critical period by expression profiling in the dark-reared rat and monocularly-deprived monkey. Inv Ophthalmol Vis Sci. 2003;44:3842–55. doi: 10.1167/iovs.03-0170. [DOI] [PubMed] [Google Scholar]
- 31.Altick AL, Feng CY, Schlauch K, Johnson LA, von Bartheld CS. Differences in gene expression between strabismic and normal human extraocular muscles. Inv Ophthalmol Vis Sci. 2012;53:5168–77. doi: 10.1167/iovs.12-9785. [DOI] [PMC free article] [PubMed] [Google Scholar]