Abstract
Irreversible vision loss is most often caused by the loss of function and subsequent death of retinal neurons, such as photoreceptor cells—the cells that initiate vision by capturing and transducing signals of light. One reason why retinal degenerative diseases are devastating is that, once retinal neurons are lost, they don't grow back. Stem cell-based cell replacement strategy for retinal degenerative diseases are leading the way in clinical trials of transplantation therapy, and the exciting findings in both human and animal models point to the possibility of restoring vision through a cell replacement regenerative approach. A less invasive method of retinal regeneration by mobilizing endogenous stem cells thus is highly desirable and promising for restoring vision. Although many obstacles remain to be overcome, the field of endogenous retinal repair is progressing at a rapid pace with encouraging results in recent years.
Keywords: retinal regeneration, endogenous stem cells, neuron replacement therapy
Introduction
The retina, as the most accessible part of the central nervous system (CNS), is susceptible to degeneration as a result of genetic mutation or acquired conditions. A variety of diseases can cause retinal neuron degeneration, leading to irreversible blindness. These include conditions that cause photoreceptor death, such as age-related macular degeneration (AMD), retinitis pigmentosa, and cone or rod dystrophy, or damage to the optic nerve and retinal ganglion cells, such as glaucoma and optic neuritis. These diseases share common pathophysiological features: permanent loss of retinal neurons.
Recent advancements in pharmacological therapies, for example the anti-angiogenic treatment for patients with neovascular AMD1-2, have been successful in slowing down the progression of certain retinal diseases or prevent further deterioration of function. However, no treatments are available to completely halt neurodegeneration or enable regeneration and re-establishment of retinal functions in patients once the neurons are lost.
With recent progress, stem cell therapy either by transplanting stem cells or by recruiting endogenous stem cell populations is emerging as a new approach that has the potential to reverse vision loss after retinal degeneration or damage. Attempts have been made in human trials to replace those lost through harvesting and transplanting donor stem cells into the eyes of patients with retinal degenerative diseases, and several clinical trials are in progress3-4. The exciting findings in successful restoration of sight in both human and animal models suggest the feasibility of reversing vision loss through a regenerative approach. To this end, new neurons may originate either from an engrafted or endogenous source of stem/progenitor cells. Cell transplantation is still a complex multistep process, even though transplanted stem cells have the capacity to proliferate, differentiate into various cell lineages and repopulate the host retina. Drug-based regenerative therapy that aims at mobilizing the endogenous progenitor cell population to repair the retina may offer many advantages over the transplantation approach. These include less-concerns about immune rejection, neuron integration, tumor formation and disease transmission by implanted cells. The idea of retinal repair through mobilizing endogenous stem cells presents an attractive approach that intends to relieve vision loss in patients by generating and preserving the disease afflicted cells with their own cells. The eye being a relatively small organ presents a special advantage in this approach as it reduces the number of cells required for regenerative therapies – a critical barrier to cell-based approach. To date, the field is rapidly advancing with encouraging results.
Sources of Endogenous Stem Cells/Progenitor Cells
The concept that the adult mammalian CNS contains populations of resident neural stem/progenitor cells was accepted two decades ago5-6. Emerging evidence suggests that Müller cells are dormant stem-like cells found throughout the retina and serve as a source of progenitor cells to regenerate retinal neurons after injury7-8. In addition, ciliary epithelia-derived cells, retinal pigment epithelium (RPE) and bone marrow–derived cells (BMCs) have also been reported as potential sources of progenitor cells that can be mobilized to the injured retina (Fig. 1, 2).
Figure 1.
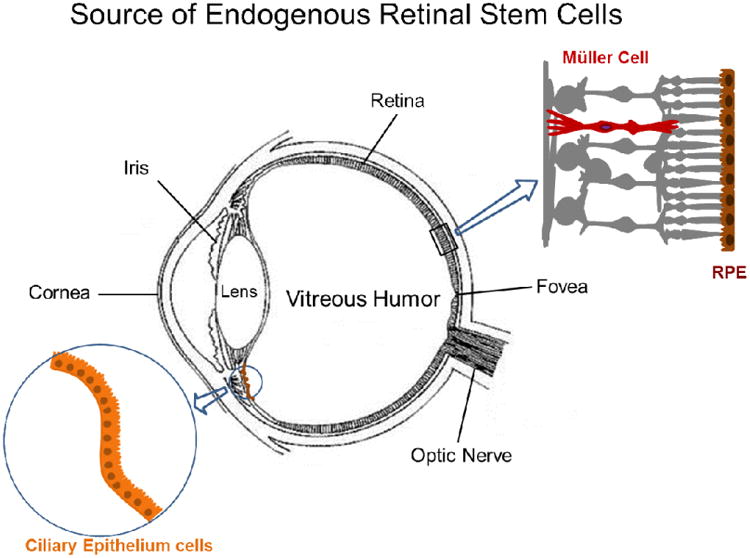
Sources of endogenous retinal stem cells. The retina has been shown to contain a population of endogenous stem cells, including Müller cells in neural retina, retinal pigment epithelium and ciliary epithelium cells in the ciliary margin zone.
Figure 2.
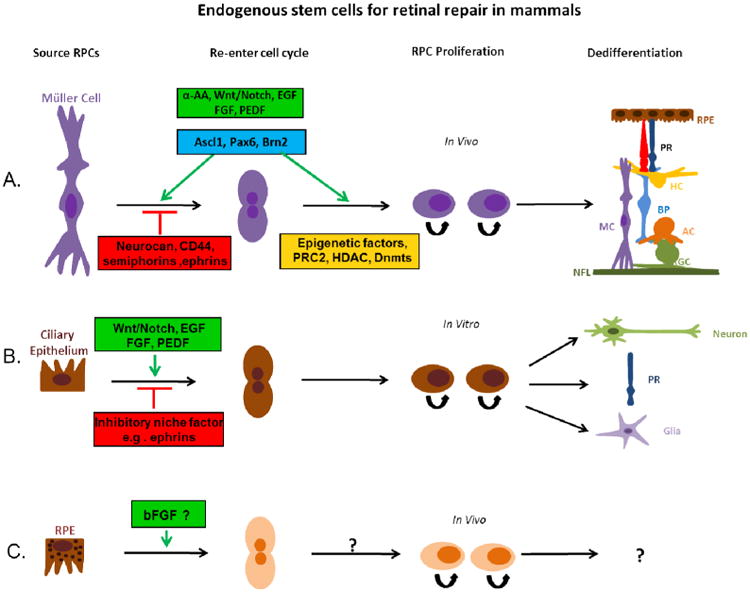
Müller cells, retinal pigment epithelium and Ciliary epithelium cells are reported as retinal stem cells for retinal repair in mammals. (A) Latent Müller cells are stimulated or suppressed to re-enter the cell cycle by different factors (green or red boxes). Müller cells can be induced to proliferate by growth factors and transcription factors listed in the green boxes and differentiate into various retinal cell types. Modification of epigenetic factors may also contribute to the regulation of the stem cell potency of Müller cells. (B) It has been shown that in vitro, ciliary epithelium cells isolated from mature retina can be stimulated (green box) or suppressed (red box) to re-enter the cell cycle, differentiating into new neurons, including photoreceptors (PR), and glia cells. (C) Retinal pigment epithelium has also been shown to possess the ability to proliferate and transdifferentiate into retinal neurons, but the neurogenic potential of these cells in mammals are fairly restricted and appear to be deficient in the regulatory elements that are required for induction of transdifferentiation. EGF: Epidermal Growth Factor; FGF: Fibroblast Growth Factor; PEDF: Pigment Epithelium-Derived Factor; α-AA: α-Aminoadipic Acid; Ascl1: Achaete-Scute Complex-like 1; Pax6: Paired Box Gene 6; Brn2: Brain-2; PRC2: polycomb repressive complex-2; HDAC: Dnmts: DNA methyltransferases; RPC: Retinal Progenitor cell; PR: Photoreceptor; RPE: Retinal Pigment Epithelium; HC: Horizontal cell; BP: Bipolar cell; MC: Müller cell; AC: Amacrine cell; RGC: Retinal Ganglion cell; NFL: Nerve Fiber layer
Lower vertebrates, such as fish and amphibian, are capable of regenerating the retina, and Müller cells are thought to serve as the primary source of retinal progenitors9. After injury, quiescent Müller cells re-enter the cell cycle and de-differentiate to form multipotent progenitors that subsequently generate all retinal neuron types that repair the retina and restore visual function10-16. Over the past decade, efforts have been placed to investigate whether retinal neuroregeneration can be induced from Müller cells of adult mammals, such as mouse and rat.
Müller cells of adult mammals share many properties of retinal progenitor cells (RPCs). They express the same neurogenic genes, such as Notch and Wnt, as those found in the fish17,18, and can be reprogrammed in a dish to become retinal neural or photoreceptor progenitors19,20. In vivo, it has been shown that, by targeting specific signaling pathways through administering fibroblast growth factor (FGF)21, Notch22,23, Wnt24-26, or sonic hedgehog27, a significant number of Müller cells can be induced to re-enter the cell cycle and display properties of retinal progenitors. While transcription factor Ascl1a was shown to be required for retinal regeneration in the fish12, 14, 28, recent report indicates that overexpressing a single transcription factor, Ascl1, is also sufficient to induce a neurogenic state of mature Müller cells of mice29. These results suggest that some part of the regenerative program occurring in non-mammalian vertebrates remains in the Müller cells of mammalian retina, which may be induced for retinal repair in patients with retinal degeneration.
The ciliary marginal zone of lower vertebrates, such as teleosts and amphibians, is also known to harbor a pool of RPCs capable of producing new retinal neurons throughout life30. A population of multipotent RPCs has been isolated from the ciliary epithelium (CE) of adult rodents and humans that shows the capacity to generate various retinal cell types in vitro31-32. However, their ability to proliferate and generate new retinal neurons, such as photoreceptors, appears to be limited in vivo33-34. Mitogens, including basic FGF, insulin, Wnt3a, and pigment epithelium-derived factor, are found to promote the proliferative potential of CE-derived RPCs35-39. Transcription factors, such as OTX2, Crx and Chx10, increase the photoreceptor progeny of CE-derived RPCs40. Nevertheless, the neurogenic potential of these cells in birds and mammals are fairly restricted. There has been scarce evidence suggesting that these cells contribute to retinal regeneration after injury in adult mammals or birds.
In urodela of amphibians, such as salamanders, RPE cells located between the retina and choroid are capable of transdifferentiating into neurons and regenerating the entire retina41-42. The regenerative process usually starts with the de-differentiation of pigmented cells, which then proceed to depigmentation, re-entrance of the cell cycle, and expression of progenitor cell genes43. In mammals, RPE of embryonic rats has also been shown to possess the ability to transdifferentiate into retinal neurons and develop into neural retina, but only during the earliest developmental stage44. In addition, peripheral RPE cells of adult rats retain the capacity to enter the cell cycle and complete cellular division in vivo, although they divide at a low cycling rate45. Interestingly, RPE cells from adult humans are reported as being capable of generating stable RPE and differentiating into mesenchymal lineages in vitro46. Besides, it has been shown that cultured human RPE cells can differentiate into neurons that are positive for beta-III tubulin, MAP2, and neurofilament proteins; whereas, no photoreceptor or glial marker positive cells were observed in these cultures47. Mammalian RPEs appear to be deficient in the regulatory elements that are required for induction of transdifferentiation45. The neurogenic potential of these cells in birds and mammals are fairly restricted. Evidence suggesting that these cells contribute to retinal regeneration after injury in adult mammals or birds is limited48.
Some studies have described the ability of BMCs to cross lineage boundaries and express tissue-specific proteins in different organs49-51. In mice, it has been shown that endogenous BMCs can migrate to the subretinal space in the damaged retina, presumably to initiate or participate in neural repair52-54. However, no evidence has been suggested that they can transdifferentiate into cells with anatomical or functional characteristics of retinal neurons53-54. To date, Müller cells are the best characterized mammalian cell type that shows retinal progenitor cell properties and generates new retinal neurons after injury in adult mammals55-57.
Niche Signals and Stem Cell Potential
Neuroregenerative potential of RPCs depends on both the intrinsic properties of neural stem cells and the environment, or “niche”, in which stem cells reside. The regenerative properties of Müller cells are evolutionarily conserved. In contrast to lower vertebrates, mammals have lost the ability to regenerate retinal neurons, likely due to the constraints of the non-neurogenic environment of the adult retina58. In rodent retinas, for example, the Müller cells become reactive and hypertrophic in response to injury, but few re-enter the cell cycle—a first step toward Müller cell transdifferentiation into RPCs58. Treating the retina with exogenous activating factors after damage has been shown to induce proliferation of endogenous RPCs. For example, Wnt3a59, epidermal growth factor (EGF)60, FGF61, insulin-like growth factor (IGF)62, retinoic acid59, Notch63, Nmethyl-N-nitrosourea64 and α-aminoadipic acid (α-AA)55 have all been reported to stimulate the proliferation from at least a subpopulation of Müller cells.
Müller cell proliferation has been studied in a variety of species, both in vitro and in vivo, and some of these mitogenic factors are better characterized. In the post hatch chick, a combination of insulin and FGF causes a large percentage of the Müller cells to proliferate. P2Y-receptor activation stimulates Müller cells proliferation in guinea pig, as does the activation of the platelet-derived growth factor receptor65. Perhaps the best-studied mitogenic factor for Müller cells is EGF, which stimulates Müller cells proliferation of mice60, rats66, rabbits67, guinea pigs65, and humans68. Intraocular injection of EGF significantly increases the number of BrdU-positive Müller cells in adult rats after light damage66. Wnt3a also induces BrdU-positive Müller cells in retinal explant cultures, where retinal damage inevitably occurs during culture preparation59.
Several groups have studied retinal damage-induced Müller cell proliferation in mice25,55,56 and reported that mouse Müller cells could be induced to proliferate when neurotoxic damage was coupled with growth factor stimulation. Interestingly, mouse Müller cells can be stimulated to proliferate in the absence of neural death by a subtoxic dose of glutamate or alpha-aminoadipate (α-AA)55. Together, these observations implicate that the non-neurogenic environment of adult mammals may present an inhibitory niche that suppresses the regenerative potential of Müller cells. Similar to Müller cells, CE-derived RSCs have also been found to be capable of re-entering the cell cycle in the presence of certain mitogens, including bFGF, insulin, Wnt3a, and pigment epithelium-derived factor35-39. Likely, there is a large overlap in the molecular pathways that regulate the proliferative and regenerative potentials of RPCs of different sources.
Recent studies have also begun to unveil the components of the negative niche factors that inhibit the regenerative potential of the adult retina and brain. Among them, ephrin-A and EphA receptors are thought to act as important players69-71. For example, adult neurogenesis is detected in two restricted areas, the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) of the CNS; however, neural stem cells are found widely distributed throughout the adult CNS, including those that are considered the non-neurogenetic areas72. The astrocytes in areas outside of the neurogenic SGZ and SVZ of adult mice express high levels of ephrin-A2 and -A3, which present an inhibitory niche negatively regulating neural stem cell growth73. Adult mice lacking ephrin-A2 and -A3 display active ongoing neurogenesis throughout the CNS.73 Interactions of ephrin-A/EphA family with neurogenic signals, such as Wnt and FGF, have been documented74-76, further supporting a role of ephrin-As in the regulation of neural stem cell behavior. Recent reports indicate that ephrin-As also play a key inhibitory role in the developing retina and adult ciliary epithelium to suppress stem cell proliferation and retinogenesis via suppressing Wnt signaling77. Together, these data indicate a novel mechanism associated with ephrinA/EphAs as endogenous modulators in the control of neurogenesis and regeneration in the adult CNS.
Studies in the brain and retina have suggested that the limitation of retinal neuroregeneration can be attributed to an assortment of factors, including the presence of inhibitory extracellular matrix or cell adhesion molecules. Among these factors, injury-induced inhibitory molecules, such as chondroitin sulfate proteoglycans, neurocan, hyaluronan-binding glycoprotein CD44, semaphorins and ephrins, are particularly abundant in the retina77-80. These molecules have previously been shown to function as inhibitory cues for neurite and axonal extension and block the regeneration of nerve fibers as well as neural stem cell growth78, 81. The retina, as part of the CNS, upregulates these growth inhibitory molecules after injury. Both neurocan and CD44 are expressed in the normal retina, and injury stimulates the increased production of these proteins in reactive retinal astrocytes and Müller cells82. Upregulation of these inhibitory molecules leads to an inhospitable environment for the proliferation of RPCs and neuron regeneration77, and hinders successful RPC transplantation by blocking donor-host integration83. In addition, the most recent study showed that BMP2, BMP4 and sFRP2 secreted by the adult lens and cornea inhibit CE-derived RPCs and contribute to adult RPCs quiescence in the adult mammalian eye84. Thus, to induce regeneration and restore visual function through transplantation, neutralization of these inhibitory proteins may be necessary.
Together, both stimulating the growth pathways and blocking the effects of extracellular inhibitory molecules of neurons have been proved to be beneficial for promoting retinal regeneration after diseases or injury. Although the detailed mechanisms involved remain to be determined, a two-pronged approach might be the most efficient way of promoting retinal regeneration and repair after injury.
Intracellular Signals and Transcriptional Regulation
Niche factors must signal through intracellular pathways to regulate stem cell behavior. The self-renewal of stem cells is tightly controlled through concerted actions of intrinsic transcription factors and networks. Moreover, it has been shown that aging generally has a negative impact on the proliferation and regenerative potential of neural stem cells. Adult neurogenesis in the brain decreases with aging, both as a result of reduced proliferation and differentiation of newborn cells; in parallel with it, an age-associated decline in cognitive performance is often observed. The age of the animal at the time of testing also seems to be a crucial determinant of the ability of Müller cells to re-enter the cell cycle. There is a large decline in the response of Müller cells to mitogens during the second postnatal week in mice56. This loss in responsiveness to EGF correlates with the down-regulation of EGF receptor (EGFR) that occurs over the same period. These studies support the notion that the failure of retinal neurons to regenerate in the adult is not a mere outcome of changes in the extraneuronal milieus; rather, the intrinsic nature of RPCs also contributes critically to their regenerative capacity.
Recent years, some key intracellular signals and transcription factors that control the intrinsic growth programs of retinal progenitors have been identified. Ascl1 is emerging as a key regulator determining the neuronal fate of glial lineage neural stem cells. Forced expression of Ascl1 induced a neurogenic state of mature Müller cells of mice,29 suggesting it as a potential target for stimulating retinal neuroregenerative therapy after disease or injury. Moreover, it is evident that expression of Ascl1 along with two other transcription factors, Brn2 and Myt1l, using viral gene delivery directly converted fibroblasts into neurons85, suggesting a crucial involvement of Ascl1 in neuronal fate determination.
Pax6 gene encodes a transcription factor controlling retinal neurogenesis and regeneration86-88. In the vertebrate retina, Pax6 is highly expressed during retinal development to maintain the multipotency and proliferation capacity of retinal progenitors89. Pax6 is also detected in a subpopulation of Müller cells of adult mice where it may be involved in the molecular response to retinal injury11, 18. It has been showed that photoreceptor injury induces migration of Pax6-positive Müller cell nuclei toward the outer retinal layers. These cells express markers of cell cycle, implicating their potential to re-enter the cell cycle similarly to that is seen in lower vertebrates90. Moreover, Pax6 is upregulated in the Müller cells of mice after N-methyl-D-aspartate glutamate induced neural damage or Wnt3a treatment, as are components of the Notch pathway, Dll1, Notch1 and Nestin56, 59, 91. Studies with the human retina have indicated that many progenitor cell genes (e.g. Pax6 and Sox2) are reactivated in the mammalian Müller cells after damage and mitotic stimulation68.
Crx, NeuroD, Nrl, and Nr2e3 are the major transcription factors known to be involved in photoreceptor genesis during development thus far. Crx and NeuroD are expressed in photoreceptors of the developing and mature retina, and are essential for precise differentiation and maturation92. Nrl is exclusively expressed in rod photoreceptors and is essential for their development and maintenance93. Ex vivo studies show that Nr2e3 acts synergistically with Nrl and inhibits the activation of cone genes by Crx94-95.
Even though with the increased understanding of the roles of the molecular signals in the regulation of retinal regeneration, to date, successful repair of the damaged or diseased retina remains a challenge. The critical issue hampering our understanding of the mechanisms controlling retinal regeneration lies in the complexity of the problem and its potential involvement of multiple factors. In order to develop clinically feasible and applicable therapies, studies are needed to further elucidate the interactive effects of these factors as well as the mechanisms underlying the regulation of the proliferation and regenerative behavior of RPCs.
Epigenetic Regulation of Stem Cell Potential
Epigenetics is one of the most promising and expanding fields in the current biomedical research landscape. The term generally refers to chromatin modifications that persist from one stage of cell division to the next stage. It involves heritable alterations of gene expression without changes in DNA sequence, and contributes to the diversity of gene expression and memory of cell lineage. Epigenetics is believed to play a major role in retinal development and cell specification, partly through stabilizing transcriptional programs in embryonic progenitors and differentiated descendants, and establishing and maintaining gene expression in RPCs in the postnatal life. Thus, epigenetic mechanism is a likely avenue which should be explored to change the plasticity of RPCs and enhance the endogenous regenerative potential of the retina.
Epigenetic regulation includes histone modifications, DNA methylation, and other mechanisms, which work together to establish and maintain the global and local condensed or decondensed chromatin states to determine gene expression96-98. Disruption of epigenetic machineries is known to provoke aberrant gene expression patterns that give rise to developmental defect. Histone modifications, including histone methylation and acetylation, are areas of intensive interest. In part, this is because chemical compounds that manipulate these processes have been recently identified and some have been shown to affect retinal neurons survival99-100.
The histone methyltransferase complex, termed polycomb repressive complexes (PRCs), controls key steps in developmental transitions and cell fate choices99, 101. PRC2 methyltransferase activity, for example, catalyzes the addition of histone H3 lysine 27 trimethylation (H3K27me3) to specific genomic loci, which act as docking sites for recruiting additional repressive complexes. PRC2 regulates the progression of retinal progenitors from proliferation to differentiation. In Xenopus, the PRC2 core components are enriched in retinal progenitors and downregulated in differentiated cells. Knockdown of the PRC2 core component Ezh2 leads to reduced retinal progenitor proliferation, in part due to upregulation of the Cdk inhibitor p15Ink4b. In addition, although PRC2 knockdown does not alter eye patterning or the expression of retinal progenitor genes, such as Sox2, it does cause suppression of proneural bHLH gene expression. These studies indicate that PRC2 is crucial for the initiation of neural differentiation in the retina. Consistent with these observations, knocking down or blocking PRC2 function constrains the generation of most retinal neural cell types and promotes a Müller glial cell fate decision99.
It is thought that histone acetylation promotes a more open chromatin structure that allows for gene transcription, while histone methylation in general stabilizes transcriptional programs in progenitor cells and their differentiated descendants. Thus, histone modification is crucial for establishing and maintaining gene expression in cell's postnatal life102-103. Accordingly, alterations in histone acetylation may cause behavioral changes of RPCs, such as death and aberrant differentiation. In contrast, mutations associated with histone methylation are likely to result in long-term consequences on cell survival and function. Histone deacetylation generally represses gene promoters and is used to silence genes during differentiation. Loss of histone deacetylatase 1 (HDAC1) in zebrafish leads to retinal overproliferation and inhibition of differentiation through activation of the Wnt and Notch pathways104. In mouse retinal explants, HDAC inhibition resulted in defects in rod differentiation but also, unlike in zebrafish, in a reduction in proliferation105.
Another major form of epigenetic regulation is DNA methylation. Although the role of cell-specific DNA methylation in the retina is still unclear, one potential mechanism may be that it helps direct proper lineage decisions and differentiation of retinal precursor cells. Recent study demonstrates that DNA methyltransferases (Dnmts) are involved in development of the vertebrate eyes. High levels of mouse Dnmts expression are observed during early stages of retinal differentiation106. In the zebra fish embryo, knockdown of the maintenance Dnmts by a translation-blocking antisense morpholino results in a profound disorganization of all retinal layers107.
Epimutation, defined as abnormal transcriptional repression or activation of genes caused by mutations in epigenetic modulators108, is generally considered reversible in comparison to genetic mutations. The potential to pharmacologically modify gene expression through the manipulation of epigenetic regulation is currently an area of intense interest. The research in this field may unveil novel pathways underlying stem cell regulation and lead to new epigenetic drug targets for boosting the regenerative potential of endogenous stem cells for treating and reversing vision loss.
Functional Restoration of Retinal Neurons
Translation of stem cell biology into clinical application for retinal degenerative disorders via endogenous stem cells must overcome three major obstacles: The first obstacle concerns the development of methods to mobilize endogenous stem cells to sufficiently proliferate and restore the lost cell numbers; the second obstacle regards directing the targeted differentiation of endogenous stem cells into retinal progenitors capable of regenerating desired retinal cell types in vivo; finally, to enable restoration of sight newly-generated neurons must integrate into the neural circuitry, form synaptic contacts with the existing neurons, and establish functional connectivity. By repopulating an injured retina with newly-generated neurons in fish, these cells have been shown to develop functional connections with the existing circuitry and restore sight109. Emerging studies on stem cell-based therapy for targeted neuron replacements, such as photoreceptors and retinal ganglion cells, indicate that directed neurodifferentiation by endogenous stem cells could also be achieved in adult mammals.
Photoreceptors are photosensitive cells, and their degeneration is a major cause of blindness worldwide, partly due to their incapability of regeneration or self-repair. Stem-cell therapy for photoreceptor replacement provide an exciting prospect for restoring sight in those whose vision is significantly impaired by retinal disease affecting primarily the photoreceptors. Currently, no treatments are available that can effectively reverse vision loss due to photoreceptor degeneration. Various sources of cells, including neural precursors110, embryonic and postnatal RPCs111, neural stem cell lines112 and bone marrow stem cells113, have been tested for their ability to differentiate and replace photoreceptors. An appropriate source of precursor cells is a key for photoreceptor cell replacement therapy. Post-mitotic photoreceptor precursor cells could easily be derived from RPCs isolated from neonatal mouse retinas (P1 – P5)114. However, equivalent human retinal progenitor cells would have to be derived from second-trimester fetus114. Aside from ethical considerations, such tissues are in limited provision and might not provide a consistent source of cells for retinal cell transplantation114.
Considerable progress has been made in differentiating embryonic stem cells (ESCs) in vitro toward a neural retinal precursor phenotype that is competent to generate photoreceptor-like cells111, 115. Opsin- and rhodopsin-positive cells are obtained after subretinal grafting of human ESCs, indicating the potential of human ESCs to differentiate into retinal cells, while the subretinal microenvironment supports their differentiation toward a photoreceptor cell fate116. New rod and cone photoreceptors have also been successfully generated from ESCs from mouse, monkey and human117-122. Most recent study has demonstrated that retinal stem cells isolated from the adult retina have the potential of producing functional photoreceptor cells that can integrate into the retina, morphologically resembling endogenous photoreceptors, and forming synapses with resident retinal neurons123. Both structural integration of grafted cells and improvement of pupillary reflex have been reported after transplantation of photoreceptor precursors into a mouse model of retinal degeneration124.
Currently, many labs have reported an increase in proliferation of mammalian Müller cells—an endogenous source of RPCs—and their migration into the injured areas of the retina25, 55, 91, 125. However, it remains unclear if the newly-developed neurons can integrate and allow restoration or improvement of visual function. A number of studies using a transplantation approach further support the extraordinary potential of cell-replacement therapy in functionally refurbishing damaged retinal tissues. These include the studies directed towards the creation of new photoreceptors118, 126 or RPE by grafted stem cells127-129. A variety of different cell types have been tested in their ability to restore retinal function. ESCs, RPCs and photoreceptor precursor cells have all been shown to form new functioning photoreceptors and improve retinal function following transplantation into the degenerative retinal hosts118, 124. Moreover, there has been evidence that transplanted cells are capable of forming synaptic contacts with local retinal neurons, suggesting that functional communication between newly generated cells with native retinal neurons can be developed for improvement of visual function.
Compared with photoreceptors, replacing lost RGCs is a more challenging task. This is because successful replacement of RGCs requires not only the survival, migration and integration of donor cells into the ganglion cell layer and differentiation into RGC-like cells. These cells must also extend long axons which navigate through the optic disc, entering the optic nerve through the lamina cribosa. Newly generated RGC axons must be properly myelinated in the optic nerve and continuously extend and make the right way (cross or not cross) into the chiasm, and finally establish functional and topographical connections to the central visual targets. Due to these challenges, efforts at transplantation-based replacement of RGCs are still lagging behind.
ESCs133-134, RPCs135 and Müller cell–derived stem cells131 have been investigated for their potential of replacing RGCs in treating retinal degenerative diseases caused by RGC dysfunction. Interestingly, these results suggest that the stem cells are capable of migrating and integrating into the retina depleted of RGCs or populated by apoptotic RGCs, expressing RGC markers and extending neurites136. iPSCs-derived RGC-like cells with electrophysiological properties similar to RGCs have also been generated from mouse fibroblasts through adenoviral gene delivery137. However, these cells showed limited ability to integrate into the normal retina after transplantation. Some evidence suggests that a glaucomatous retina presents even a less permissive environment to the integration of transplanted cells138. Mesenchymal stem cells139, on the other hand, failed completely to migrate into the injured eye after intravenous engraftment; nevertheless, some neuroprotective effect was observed following transplantation into a rat model of glaucoma140-141. To date, evidence for synaptic integration and functional improvement by stem cell-derived RGCs remains elusive.
Conclusions
One reason why retinal damage or degeneration is so devastating is that, once neurons are lost, they do not grow back. Intense efforts and substantial progress have been made in this field in the last decade. Emerging evidence suggests that the mammalian retina contains a subpopulation of stem-like cells, primarily Müller cells, as well as CE derived RPCs, in addition to RPE and BMC populations, which may retain the capacity for neuronal regeneration under a certain condition. Limitation of retinal regeneration in adult mammals reflects both intrinsic inability of retinal neurons to reinitiate robust regeneration and lack of a permissive environment for such growth.
The regenerative strategy by mobilizing endogenous stem cells to participate in retinal repair has several advantages over cell transplantation therapy as it does not need to face the shortage of donor cells nor diseases or disorders that may be transmitted via implant; it avoids potential immune rejections. Moreover, endogenously derived RPCs are generally thought to be better programmed to differentiate into retinal neurons and integrate into the existing neural circuitry than exogenously transplanted stem cells. Recent research has shown that adult human and mouse Müller cells can be induced to re-enter the cell cycle and regenerate new neurons in vitro and in vivo following stimulation by a single compound, although the number and diversity of regenerative neurons is still limited. Practically, a drug therapy for stimulating residential RPCs derived from the patient's own retina would be clinically more viable than transplanting exogenous cells. This is because the (drug therapy) method reduces the concerns over the ethical issues associated with the use of embryonic stem cells, while injection of a drug solution into the eye is a clinically established procedure and considered less invasive than cell transplantation. Patient's native Müller (stem-like) cells are likely to be competent to generate retinal specific cells. Currently, the primary challenge of inducing retinal regeneration from Müller progenitors falls onto the limited number of Müller cells that can be activated to re-enter the cell cycle and participate in regeneration and repairing process. The tumorogenic potential of Müller progenitors is much less of an issue as compared to transplanted hESCs and iPSCs. To our knowledge, there hasn't been any report of tumor formation by Müller progenitors in vitro or in vivo upon mitogen stimulation. Although barriers to regenerative cell survival, migration, and integration as well as long-term efficacy and safety concerns remain to be overcome, endogenous retinal repair is progressing at a rapid pace and may soon turn the endogenous stem cell approach into a viable therapy.
Acknowledgments
All authors have read the journal's policy on conflicts of interest and have none to declare.
Abbreviations
- CNS
central nervous system
- AMD
age-related macular degeneration
- RGCs
retinal ganglion cells
- PCs
Progenitor cells
- RPCs
retinal progenitor cells
- CE
ciliary epithelium
- BMCs
bone marrow–derived cells
- FGF
fibroblast growth factor
- CMZ
ciliary marginal zone
- EGF
epidermal growth factor
- IGF
insulin-like growth factor
- α-AA
α-aminoadipic acid
- PRCs
polycomb repressive complexes
- H3K27me3
histone H3 lysine 27 trimethylation
- HDAC1
histone deacetylatase 1
- Dnmts
DNA methyltransferases
- ESCs
Embryonic stem cells
- hESCs
human embryonic stem cells
- iPSCs
induced pluripotent stem cells
- MSCs
mesenchymal stem cells
- RPE
retinal pigment epithelium
- PR
photoreceptor
- HC
horizontal cell
- BP
bipolar cell
- MC
Müller cell
- AC
amacrine cell
- NFL
never fiber layer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenfeld PJ. Bevacizumab versus ranibizumab for AMD. N Engl J Med. 2011 May 19;364(20):1966–7. doi: 10.1056/NEJMe1103334. [DOI] [PubMed] [Google Scholar]
- 2.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011 May 19;364(20):1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer AO, MacLaren RE. Translating induced pluripotent stem cells from bench to bedside: application to retinal diseases. Current gene therapy. 2013 Apr;13(2):139–51. doi: 10.2174/1566523211313020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medina RJ, Archer DB, Stitt AW. Eyes open to stem cells: safety trial may pave the way for cell therapy to treat retinal disease in patients. Stem Cell Res Ther. 2011;2(6):47. doi: 10.1186/scrt88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 6.Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8591–5. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010 Apr;16(4):193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia. 2003 Jul;43(1):70–6. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 9.Jones BW, Watt CB, Frederick JM, et al. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol. 2003 Sep 8;464(1):1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- 10.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007 Jun 27;27(26):7028–40. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008 Jan 30;28(5):1109–17. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008 Nov;87(5):433–44. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010 Nov;12(11):1101–7. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006 Jun 7;26(23):6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol. 2010 Oct 15;518(20):4196–212. doi: 10.1002/cne.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006 Nov 1;299(1):283–302. doi: 10.1016/j.ydbio.2006.07.029. Epub 2006 Jul 29. [DOI] [PubMed] [Google Scholar]
- 18.Roesch K, Jadhav AP, Trimarchi JM, et al. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008 Jul 10;509(2):225–38. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia M, Forster V, Hicks D, Vecino E. Effects of muller glia on cell survival and neuritogenesis in adult porcine retina in vitro. Invest Ophthalmol Vis Sci. 2002 Dec;43(12):3735–43. [PubMed] [Google Scholar]
- 20.Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V. Adult human Muller glia cells are a highly efficient source of rod photoreceptors. Stem Cells. 2011 Feb;29(2):344–56. doi: 10.1002/stem.579. [DOI] [PubMed] [Google Scholar]
- 21.Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002 Nov 1;22(21):9387–98. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Debbio CB, Balasubramanian S, Parameswaran S, Chaudhuri A, Qiu F, Ahmad I. Notch and Wnt signaling mediated rod photoreceptor regeneration by Muller cells in adult mammalian retina. PLoS One. 2010;5(8):e12425. doi: 10.1371/journal.pone.0012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007 Dec 1;312(1):300–11. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007 Apr 11;27(15):4210–9. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Hunter DJ, Rooker S, et al. Wnt signaling promotes Muller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci. 2013 Jan;54(1):444–53. doi: 10.1167/iovs.12-10774. [DOI] [PubMed] [Google Scholar]
- 26.Sanges D, Romo N, Simonte G, et al. Wnt/beta-Catenin Signaling Triggers Neuron Reprogramming and Regeneration in the Mouse Retina. Cell Rep. 2013 Jul 25;4(2):271–86. doi: 10.1016/j.celrep.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun. 2007 Nov 16;363(2):347–54. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15858–63. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollak J, Wilken MS, Ueki Y, et al. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013 Jun;140(12):2619–31. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubota R, Hokoc JN, Moshiri A, McGuire C, Reh TA. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002 Mar 31;134(1-2):31–41. doi: 10.1016/s0165-3806(01)00287-5. [DOI] [PubMed] [Google Scholar]
- 31.MacNeil A, Pearson RA, MacLaren RE, Smith AJ, Sowden JC, Ali RR. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells. 2007 Oct;25(10):2430–8. doi: 10.1634/stemcells.2007-0035. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Sta Iglesia DD, Kielczewski JL, et al. Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Invest Ophthalmol Vis Sci. 2007 Apr;48(4):1674–82. doi: 10.1167/iovs.06-1034. [DOI] [PubMed] [Google Scholar]
- 33.Gualdoni S, Baron M, Lakowski J, et al. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. Stem Cells. 2010 Jun;28(6):1048–59. doi: 10.1002/stem.423. [DOI] [PubMed] [Google Scholar]
- 34.Cicero SA, Johnson D, Reyntjens S, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6685–90. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Das AV, Soto-Leon F, Ahmad I. Growth factor-responsive progenitors in the postnatal mammalian retina. Dev Dyn. 2005 Feb;232(2):349–58. doi: 10.1002/dvdy.20290. [DOI] [PubMed] [Google Scholar]
- 36.Abdouh M, Bernier G. In vivo reactivation of a quiescent cell population located in the ocular ciliary body of adult mammals. Exp Eye Res. 2006 Jul;83(1):153–64. doi: 10.1016/j.exer.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Kubo F, Nakagawa S. Hairy1 acts as a node downstream of Wnt signaling to maintain retinal stem cell-like progenitor cells in the chick ciliary marginal zone. Development. 2009 Jun;136(11):1823–33. doi: 10.1242/dev.029272. [DOI] [PubMed] [Google Scholar]
- 38.Inoue T, Kagawa T, Fukushima M, et al. Activation of canonical Wnt pathway promotes proliferation of retinal stem cells derived from adult mouse ciliary margin. Stem Cells. 2006 Jan;24(1):95–104. doi: 10.1634/stemcells.2005-0124. [DOI] [PubMed] [Google Scholar]
- 39.De Marzo A, Aruta C, Marigo V. PEDF promotes retinal neurosphere formation and expansion in vitro. Adv Exp Med Biol. 2010;664:621–30. doi: 10.1007/978-1-4419-1399-9_71. [DOI] [PubMed] [Google Scholar]
- 40.Inoue T, Coles BL, Dorval K, et al. Maximizing functional photoreceptor differentiation from adult human retinal stem cells. Stem Cells. 2010 Mar 31;28(3):489–500. doi: 10.1002/stem.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikegami Y, Mitsuda S, Araki M. Neural cell differentiation from retinal pigment epithelial cells of the newt: an organ culture model for the urodele retinal regeneration. J Neurobiol. 2002 Feb 15;50(3):209–20. doi: 10.1002/neu.10031. [DOI] [PubMed] [Google Scholar]
- 42.Susaki K, Chiba C. MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: Is FGF2 an induction factor? Pigment Cell Res. 2007 Oct;20(5):364–79. doi: 10.1111/j.1600-0749.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- 43.Araki M. Regeneration of the amphibian retina: role of tissue interaction and related signaling molecules on RPE transdifferentiation. Dev Growth Differ. 2007 Feb;49(2):109–20. doi: 10.1111/j.1440-169X.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain Res. 1995 Apr 24;677(2):300–10. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
- 45.Al-Hussaini H, Kam JH, Vugler A, Semo M, Jeffery G. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vis. 2008;14:1784–91. [PMC free article] [PubMed] [Google Scholar]
- 46.Salero E, Blenkinsop TA, Corneo B, et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012 Jan 6;10(1):88–95. doi: 10.1016/j.stem.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Amemiya K, Haruta M, Takahashi M, Kosaka M, Eguchi G. Adult human retinal pigment epithelial cells capable of differentiating into neurons. Biochem Biophys Res Commun. 2004 Mar 26;316(1):1–5. doi: 10.1016/j.bbrc.2004.01.172. [DOI] [PubMed] [Google Scholar]
- 48.Wohl SG, Schmeer CW, Isenmann S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog Retin Eye Res. 2012 May;31(3):213–42. doi: 10.1016/j.preteyeres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001 Apr 5;410(6829):701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 50.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001 May 4;105(3):369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 51.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002 Nov 15;111(4):589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Reca RG, Atmaca-Sonmez P, et al. Retinal pigment epithelium damage enhances expression of chemoattractants and migration of bone marrow-derived stem cells. Invest Ophthalmol Vis Sci. 2006 Apr;47(4):1646–52. doi: 10.1167/iovs.05-1092. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Atmaca-Sonmez P, Schanie CL, Ildstad ST, Kaplan HJ, Enzmann V. Endogenous bone marrow derived cells express retinal pigment epithelium cell markers and migrate to focal areas of RPE damage. Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4321–7. doi: 10.1167/iovs.06-1015. [DOI] [PubMed] [Google Scholar]
- 54.Machalinska A, Klos P, Baumert B, et al. Stem Cells are mobilized from the bone marrow into the peripheral circulation in response to retinal pigment epithelium damage--a pathophysiological attempt to induce endogenous regeneration. Curr Eye Res. 2011 Jul;36(7):663–72. doi: 10.3109/02713683.2011.576796. [DOI] [PubMed] [Google Scholar]
- 55.Takeda M, Takamiya A, Jiao JW, et al. alpha-Aminoadipate induces progenitor cell properties of Muller glia in adult mice. Invest Ophthalmol Vis Sci. 2008 Mar;49(3):1142–50. doi: 10.1167/iovs.07-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19508–13. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ooto S, Akagi T, Kageyama R, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004 Sep 14;101(37):13654–9. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bringmann A, Iandiev I, Pannicke T, et al. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009 Nov;28(6):423–51. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007 Apr 11;27(15):4210–9. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueki Y, Reh TA. EGF stimulates Muller glial proliferation via a BMP-dependent mechanism. Glia. 2013 May;61(5):778–89. doi: 10.1002/glia.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romo P, Madigan MC, Provis JM, Cullen KM. Differential effects of TGF-beta and FGF-2 on in vitro proliferation and migration of primate retinal endothelial and Muller cells. Acta Ophthalmol. 2011 May;89(3):e263–8. doi: 10.1111/j.1755-3768.2010.01968.x. [DOI] [PubMed] [Google Scholar]
- 62.Ikeda T, Waldbillig RJ, Puro DG. Truncation of IGF-I yields two mitogens for retinal Muller glial cells. Brain Res. 1995 Jul 17;686(1):87–92. doi: 10.1016/0006-8993(95)00473-4. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Sugano E, Isago H, Murayama N, Tamai M, Tomita H. Notch signaling pathway regulates proliferation and differentiation of immortalized Muller cells under hypoxic conditions in vitro. Neuroscience. 2012 Jul 12;214:171–80. doi: 10.1016/j.neuroscience.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 64.Wan J, Zheng H, Chen ZL, Xiao HL, Shen ZJ, Zhou GM. Preferential regeneration of photoreceptor from Muller glia after retinal degeneration in adult rat. Vision Res. 2008 Jan;48(2):223–34. doi: 10.1016/j.visres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A. P2Y receptor-mediated stimulation of Muller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest Ophthalmol Vis Sci. 2003 Mar;44(3):1211–20. doi: 10.1167/iovs.02-0260. [DOI] [PubMed] [Google Scholar]
- 66.Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006 Aug 1;54(2):94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- 67.Sagar SM, Edwards RH, Sharp FR. Epidermal growth factor and transforming growth factor alpha induce c-fos gene expression in retinal Muller cells in vivo. J Neurosci Res. 1991 Aug;29(4):549–59. doi: 10.1002/jnr.490290416. [DOI] [PubMed] [Google Scholar]
- 68.Bhatia B, Jayaram H, Singhal S, Jones MF, Limb GA. Differences between the neurogenic and proliferative abilities of Muller glia with stem cell characteristics and the ciliary epithelium from the adult human eye. Exp Eye Res. 2011 Dec;93(6):852–61. doi: 10.1016/j.exer.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theus MH, Ricard J, Bethea JR, Liebl DJ. EphB3 limits the expansion of neural progenitor cells in the subventricular zone by regulating p53 during homeostasis and following traumatic brain injury. Stem Cells. 2010 Jul;28(7):1231–42. doi: 10.1002/stem.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Depaepe V, Suarez-Gonzalez N, Dufour A, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005 Jun 30;435(7046):1244–50. doi: 10.1038/nature03651. Epub 2005 May 15. [DOI] [PubMed] [Google Scholar]
- 71.Holmberg J, Armulik A, Senti KA, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005 Feb 15;19(4):462–71. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008 May;26(5):1221–30. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc Natl Acad Sci U S A. 2008 Jun 24;105(25):8778–83. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stolfi A, Wagner E, Taliaferro JM, Chou S, Levine M. Neural tube patterning by Ephrin, FGF and Notch signaling relays. Development. 2011 Dec;138(24):5429–39. doi: 10.1242/dev.072108. [DOI] [PubMed] [Google Scholar]
- 75.Yokote H, Fujita K, Jing X, et al. Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):18866–71. doi: 10.1073/pnas.0509741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim BK, Cho SJ, Sumbre G, Poo MM. Region-specific contribution of ephrin-B and Wnt signaling to receptive field plasticity in developing optic tectum. Neuron. 2010 Mar 25;65(6):899–911. doi: 10.1016/j.neuron.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Fang Y, Cho KS, Tchedre K, et al. Ephrin-A3 suppresses Wnt signaling to control retinal stem cell potency. Stem Cells. 2013 Feb;31(2):349–59. doi: 10.1002/stem.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007 Feb;17(1):120–7. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004 Feb;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 80.Kita EM, Bertolesi GE, Hehr CL, Johnston J, McFarlane S. Neuropilin-1 biases dendrite polarization in the retina. Development. 2013 Jul;140(14):2933–41. doi: 10.1242/dev.088286. [DOI] [PubMed] [Google Scholar]
- 81.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003 Jan;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 82.Krishnamoorthy R, Agarwal N, Chaitin MH. Upregulation of CD44 expression in the retina during the rds degeneration. Brain Res Mol Brain Res. 2000 Apr 14;77(1):125–30. doi: 10.1016/s0169-328x(00)00035-8. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J Neurosci. 2007 Apr 25;27(17):4499–506. doi: 10.1523/JNEUROSCI.0200-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balenci L, Wonders C, LK-T B, Clarke L, van der Kooy D. Bmps and Sfrp2 Maintain the Quiescence of Adult Mammalian Retinal Stem Cells. Stem Cells. 2013 Jul 10; doi: 10.1002/stem.1470. [DOI] [PubMed] [Google Scholar]
- 85.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010 Feb 25;463(7284):1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nabeshima A, Nishibayashi C, Ueda Y, Ogino H, Araki M. Loss of cell-extracellular matrix interaction triggers retinal regeneration accompanied by Rax and Pax6 Activation. Genesis. 2013 Jun;51(6):410–9. doi: 10.1002/dvg.22378. [DOI] [PubMed] [Google Scholar]
- 87.Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999 Sep;15(9):371–7. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 88.Insua MF, Simon MV, Garelli A, de Los Santos B, Rotstein NP, Politi LE. Trophic factors and neuronal interactions regulate the cell cycle and Pax6 expression in Muller stem cells. J Neurosci Res. 2008 May 15;86(7):1459–71. doi: 10.1002/jnr.21606. [DOI] [PubMed] [Google Scholar]
- 89.Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr Opin Cell Biol. 2001 Dec;13(6):706–14. doi: 10.1016/s0955-0674(00)00274-x. [DOI] [PubMed] [Google Scholar]
- 90.Joly S, Pernet V, Samardzija M, Grimm C. Pax6-positive Muller glia cells express cell cycle markers but do not proliferate after photoreceptor injury in the mouse retina. Glia. 2011 Jul;59(7):1033–46. doi: 10.1002/glia.21174. [DOI] [PubMed] [Google Scholar]
- 91.Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006 Nov 1;299(1):283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 92.Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997 Nov 14;91(4):531–41. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 93.Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001 Dec;29(4):447–52. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 94.Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005 Jan 5;25(1):118–29. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haider NB, Jacobson SG, Cideciyan AV, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000 Feb;24(2):127–31. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- 96.Muhchyi C, Juliandi B, Matsuda T, Nakashima K. Epigenetic regulation of neural stem cell fate during corticogenesis. Int J Dev Neurosci. 2013 Oct;31(6):424–33. doi: 10.1016/j.ijdevneu.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 97.Hu XL, Wang Y, Shen Q. Epigenetic control on cell fate choice in neural stem cells. Protein Cell. 2012 Apr;3(4):278–90. doi: 10.1007/s13238-012-2916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao Y, Jammes H, Rasmussen MA, et al. Epigenetic regulation of gene expression in porcine epiblast, hypoblast, trophectoderm and epiblast-derived neural progenitor cells. Epigenetics. 2011 Sep 1;6(9):1149–61. doi: 10.4161/epi.6.9.16954. [DOI] [PubMed] [Google Scholar]
- 99.Aldiri I, Moore KB, Hutcheson DA, Zhang J, Vetter ML. Polycomb repressive complex PRC2 regulates Xenopus retina development downstream of Wnt/beta-catenin signaling. Development. 2013 Jul;140(14):2867–78. doi: 10.1242/dev.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He S, Li X, Chan N, Hinton DR. Review: Epigenetic mechanisms in ocular disease. Molecular vision. 2013;19:665–74. [PMC free article] [PubMed] [Google Scholar]
- 101.Stojic L, Jasencakova Z, Prezioso C, et al. Chromatin regulated interchange between polycomb repressive complex 2 (PRC2)-Ezh2 and PRC2-Ezh1 complexes controls myogenin activation in skeletal muscle cells. Epigenetics Chromatin. 2011;4:16. doi: 10.1186/1756-8935-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barth TK, Imhof A. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem Sci. 2010 Nov;35(11):618–26. doi: 10.1016/j.tibs.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Lee S, Lee SK. Crucial roles of histone-modifying enzymes in mediating neural cell-type specification. Curr Opin Neurobiol. 2010 Feb;20(1):29–36. doi: 10.1016/j.conb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamaguchi M, Tonou-Fujimori N, Komori A, et al. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005 Jul;132(13):3027–43. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- 105.Chen B, Cepko CL. Requirement of histone deacetylase activity for the expression of critical photoreceptor genes. BMC Dev Biol. 2007;7:78. doi: 10.1186/1471-213X-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nasonkin IO, Lazo K, Hambright D, Brooks M, Fariss R, Swaroop A. Distinct nuclear localization patterns of DNA methyltransferases in developing and mature mammalian retina. The Journal of comparative neurology. 2011 Jul 1;519(10):1914–30. doi: 10.1002/cne.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rai K, Nadauld LD, Chidester S, et al. Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development. Mol Cell Biol. 2006 Oct;26(19):7077–85. doi: 10.1128/MCB.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banno K, Kisu I, Yanokura M, et al. Epimutation and cancer: a new carcinogenic mechanism of Lynch syndrome (Review) Int J Oncol. 2012 Sep;41(3):793–7. doi: 10.3892/ijo.2012.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fleisch VC, Fraser B, Allison WT. Investigating regeneration and functional integration of CNS neurons: lessons from zebrafish genetics and other fish species. Biochim Biophys Acta. 2011 Mar;1812(3):364–80. doi: 10.1016/j.bbadis.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 110.Ng L, Lu A, Swaroop A, Sharlin DS, Forrest D. Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. J Neurosci. 2011 Aug 3;31(31):11118–25. doi: 10.1523/JNEUROSCI.1709-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gonzalez-Cordero A, West EL, Pearson RA, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013 Aug;31(8):741–7. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–36. [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y, Wang W. Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci. 2010 Jul;51(7):3742–8. doi: 10.1167/iovs.08-3314. [DOI] [PubMed] [Google Scholar]
- 114.West EL, Pearson RA, MacLaren RE, Sowden JC, Ali RR. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009;175:3–21. doi: 10.1016/S0079-6123(09)17501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11331–6. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006 Feb;24(2):246–57. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- 117.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006 Aug 22;103(34):12769–74. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009 Jan 9;4(1):73–9. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5(1):e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008 Feb;26(2):215–24. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 121.Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013 Jun;140(12):2576–85. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Homma K, Okamoto S, Mandai M, et al. Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors. Stem Cells. 2013 Jun;31(6):1149–59. doi: 10.1002/stem.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li T, Lewallen M, Chen S, Yu W, Zhang N, Xie T. Multipotent stem cells isolated from the adult mouse retina are capable of producing functional photoreceptor cells. Cell Res. 2013 Jun;23(6):788–802. doi: 10.1038/cr.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006 Nov 9;444(7116):203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 125.Nickerson PE, McLeod MC, Myers T, Clarke DB. Effects of epidermal growth factor and erythropoietin on Muller glial activation and phenotypic plasticity in the adult mammalian retina. J Neurosci Res. 2011 Jul;89(7):1018–30. doi: 10.1002/jnr.22629. [DOI] [PubMed] [Google Scholar]
- 126.Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog Retin Eye Res. 2004 Mar;23(2):149–81. doi: 10.1016/j.preteyeres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 127.Okamoto S, Takahashi M. Induction of retinal pigment epithelial cells from monkey iPS cells. Invest Ophthalmol Vis Sci. 2011;52(12):8785–90. doi: 10.1167/iovs.11-8129. [DOI] [PubMed] [Google Scholar]
- 128.Park UC, Cho MS, Park JH, et al. Subretinal transplantation of putative retinal pigment epithelial cells derived from human embryonic stem cells in rat retinal degeneration model. Clin Exp Reprod Med. 2011 Dec;38(4):216–21. doi: 10.5653/cerm.2011.38.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Juuti-Uusitalo K, Vaajasaari H, Ryhanen T, et al. Efflux protein expression in human stem cell-derived retinal pigment epithelial cells. PLoS One. 2012;7(1):e30089. doi: 10.1371/journal.pone.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hertz J, Qu B, Hu Y, Patel RD, Valenzuela DA, Goldberg JL. Survival and Integration of Developing and Progenitor-Derived Retinal Ganglion Cells Following Transplantation. Cell Transplant. 2013 Apr 29; doi: 10.3727/096368913X667024. [DOI] [PubMed] [Google Scholar]
- 131.Singhal S, Bhatia B, Jayaram H, et al. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012 Mar;1(3):188–99. doi: 10.5966/sctm.2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Phillips MJ, Wallace KA, Dickerson SJ, et al. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012 Apr;53(4):2007–19. doi: 10.1167/iovs.11-9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aoki H, Hara A, Niwa M, Motohashi T, Suzuki T, Kunisada T. Transplantation of cells from eye-like structures differentiated from embryonic stem cells in vitro and in vivo regeneration of retinal ganglion-like cells. Graefes Arch Clin Exp Ophthalmol. 2008 Feb;246(2):255–65. doi: 10.1007/s00417-007-0710-6. [DOI] [PubMed] [Google Scholar]
- 134.Jagatha B, Divya MS, Sanalkumar R, et al. In vitro differentiation of retinal ganglion-like cells from embryonic stem cell derived neural progenitors. Biochem Biophys Res Commun. 2009 Mar 6;380(2):230–5. doi: 10.1016/j.bbrc.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 135.Cho JH, Mao CA, Klein WH. Adult mice transplanted with embryonic retinal progenitor cells: new approach for repairing damaged optic nerves. Mol Vis. 2012;18:2658–72. [PMC free article] [PubMed] [Google Scholar]
- 136.Mellough CB, Cui Q, Harvey AR. Treatment of adult neural progenitor cells prior to transplantation affects graft survival and integration in a neonatal and adult rat model of selective retinal ganglion cell depletion. Restor Neurol Neurosci. 2007;25(2):177–90. [PubMed] [Google Scholar]
- 137.Meng F, Wang X, Gu P, Wang Z, Guo W. Induction of retinal ganglion-like cells from fibroblasts by adenoviral gene delivery. Neuroscience. 2013 Jul 13; doi: 10.1016/j.neuroscience.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Bull ND, Limb GA, Martin KR. Human Muller stem cell (MIO-M1) transplantation in a rat model of glaucoma: survival, differentiation, and integration. Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3449–56. doi: 10.1167/iovs.08-1770. [DOI] [PubMed] [Google Scholar]
- 139.Zwart I, Hill AJ, Al-Allaf F, et al. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009 Apr;216(2):439–48. doi: 10.1016/j.expneurol.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 140.Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci. 2010 Feb;51(2):960–70. doi: 10.1167/iovs.09-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010 Apr;51(4):2051–9. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]


