Abstract
Purpose
To test whether adherens junction proteins are present in the epithelium and the endothelium of corneal equivalents.
Methods
Corneal cell types were harvested from human eyes and grown separately. Stromal equivalents were constructed by seeding fibroblasts into a collagen gel on which epithelial and endothelial cells were added on each side. Alternatively, bovine endothelial cells were used. At maturity, sections of stromal equivalents were processed for Masson's trichrome or indirect immunofluorescence using antibodies against pan-, N-, or E-cadherins or α- or β-catenins. Alternatively, stromal equivalents were dissected, to separate the proteins from the epithelium, endothelium, and stroma with sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Western blots of the transferred proteins exposed to these primary antibodies were detected with chemiluminescence. Native corneas were processed similarly.
Results
Three or four layers of epithelial cells reminiscent of the native cornea (basal cuboidal and superficial flatter cells) lay over a stromal construct containing fibroblastic cells under which an endothelium is present. Western blots and indirect immunofluorescence revealed that, similarly to the native cornea, the epithelium reacted positively to antibodies against catenins (α and β) and E-cadherin. The endothelium of corneal constructs, whether of human or bovine origin, reacted mildly to catenins and N-cadherin.
Conclusions
This collagen-based corneal equivalent simulated the native cornea. Cells from the epithelial and endothelial layers expressed adherens junction proteins, indicating the presence of cell–cell contacts and the existence of polarized morphology of these layers over corneal equivalents.
Introduction
In contrast to fibroblasts within connective tissues, epithelial cells are polarized and have distinct plasma membrane domains on their apical and basolateral sides. This segregation of lipids and different protein sets on each domain of epithelial cell membranes, referred to as the fence function, is vital: it not only protects cells but also allows them to vectorially transport molecules by absorption or secretion. These processes are efficient because of a gate function that limits the paracellular permeability to water and ions [1]. These functions are fulfilled by tight junctions and associated proteins as well as cell–cell adhesion mechanisms. Adherens junction complexes are also essential in establishing distinct basolateral and apical domains [2]. The polarized organization of epithelial cells is consolidated by subsequent cell–cell and cell–extracellular matrix (ECM) adhesion proteins as well as protein sorting in the exocytic pathway that will deliver specific proteins to appropriate cell domains [2].
Epithelial cells are attached to each other by E-cadherin, an adherens junction protein. Other classical cadherins such as N-, P-, or VE isotypes are found in neural, placental, or vascular endothelial tissues. Cadherins, which are members of a multigene family, mediate calcium-dependent homophilic cell–cell interactions through their N-terminal extracellular domain. On their intracellular C-terminal domain, classical cadherins are linked to the cell actin filaments by catenin complexes [3]. Such linkage between cells and their actin filaments influences cell behavior as cadherins and catenins, originally thought of exclusively as structural proteins, are now known to participate in cell signaling [4,5]. Downregulation of cadherins is observed when a cell migrates from the epithelial layers or transforms into a motile phenotype, since it occurs during development or tumor invasion [6]. Formation of the initial membrane contacts between two epithelial cells creates a distinct cell area in which a pool of mobile E-cadherin is sequestered into immobile puncta along membrane contacts. This initial cadherin binding completely reorganizes the actin cytoskeleton and forms the adhesive belts of cadherin and actin found in mature monolayers [7]. Subsequently, other specific junction proteins assemble themselves to control paracellular diffusion and ensure cohesion with tight junctions and desmosomes [2].
The human cornea protects the delicate structures of the eye in addition to its most powerful lens. Cells from the anterior epithelium express E-cadherin and β-catenin [8]. This epithelium is constantly renewed by the division of transient amplifying and adult stem cells, which are located in the corneal limbus. Limbal stem cells do not express E-cadherin [9], but N-cadherin is expressed in clusters associated with the limbal phenotype in vitro [10]. β-catenin is found in cell membranes, the cytoplasm, and the nucleus of a few limbal cells, where activation of the nuclear Wnt/β-catenin signaling contributes to maintaining the undifferentiated phenotype of cultured limbal stem cells [11]. On the inner side of the human cornea in vivo, the endothelium expresses N-cadherin at the intercellular junction and a diffuse cytoplasmic E-cadherin [12]. When seeded in culture after a brief EDTA-trypsin dissociation treatment, endothelial cells initially cease to express N-cadherin RNA but resume it so that at 21 days after culture, expression of junctional N-cadherin reaches a level similar to the one observed in vivo [12].
A tissue-engineered cornea based on untransformed human cells should mimic the properties of the native cornea. For eventual clinical use, tissue-engineered organs produced in vitro should form a cohesive permeability barrier to limit bacterial invasion [13]. This protective function requires epithelial cells to be polarized since some pathogens, such as Pseudomonas aeruginosa, are less infectious when they have access only to the apical cell border [14]. Therefore, the formation of cell polarity that happens during organ development should also occur in the assemblage of corneal substitutes, aimed to replace donor corneas.
In addition to use as corneal replacement, a corneal equivalent could be a useful model for studying wound healing, physiology, toxicology, and pharmacology [15]. For any of these applications, the formation of polarized epithelial layers over a corneal equivalent is desirable to reproduce the original properties of the cornea. The goal of this study is to explore whether epithelial and endothelial cells on a collagen-based corneal equivalent express adherens junctional proteins such as cadherins or catenins.
Methods
Dissection and cell cultures
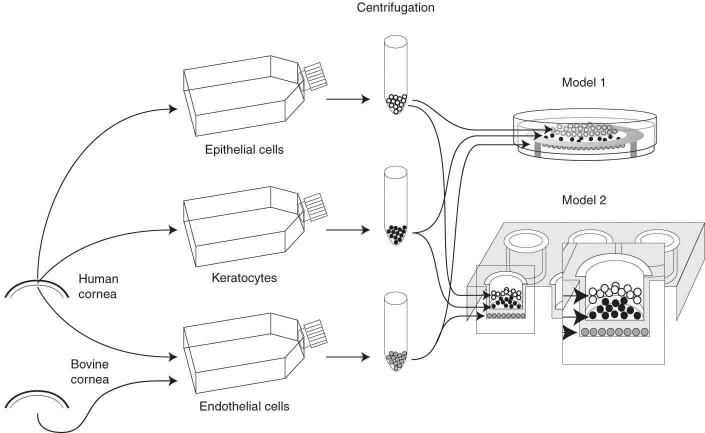
All procedures, approved by an institutional review board, followed the guidelines of ARVO and of the Helsinki declaration for the ethical use and disposal of human tissues in research. For this project, a total of 16 pairs of human eyes, unsuitable for transplantation, were obtained from the eye bank (Banque d'yeux du centre universitaire d'ophtalmologie, CHU de Québec, Québec City, Canada). Donors’ ages ranged from 5 months to 69 years. The general procedures for harvesting the cells and making corneal equivalents are shown in Figure 1. After dissection of the eye and overnight incubation of the cornea in a dispase solution (2.5 mg/ml, 4 °C), a sheet of limbal epithelial cells was lifted with forceps and seeded in culture dishes with irradiated murine Swiss-3T3 fibroblasts as a feeder layer in DME-Ham’s medium (Life Technologies Co, Grand Island, NY). This medium, a 3:1 mixture of Dulbecco-Vogt modification of Eagle’s medium and Ham’s F-12, was supplemented with additives as previously reported [16]. A corneal endothelial sheet was separated from the corneal stroma and seeded in culture with F99 medium (1:1 M199:Hams’ F12) supplemented with 10% fetal bovine serum (FBS), ascorbic acid (20 µg/ml), insulin 20 µg/ml, transferrin (2.5 µg/ml), penicillin (100 UI/ml), and gentamicin (25 µg/ml) [17]. The minced stromal pieces were digested in a collagenase H solution (0.125 U/ml, 6 h, 37 °C). The solution was collected and centrifuged, and the cell pellet was suspended in a lysis buffer (8 M urea, 4% w/v, Nonidet P40) to serve as a control of native human fibroblasts in the electrophoresis experiments, or seeded in DME supplemented with 10% FBS and antibiotics. In some experiments, endothelial cells from bovine corneas were extracted as described above and placed in cultures using DME supplemented with 20% FBS, bovine pituitary extract (25 mg/l) as well as penicillin (100 IU/ml) and gentamicin (25 µg/ml). All cultures were maintained at 8% CO2 and fed every other day with the media described above. Cells from each monolayer cultures were trypsinized before reaching confluence. After centrifugation, cells were suspended, counted, and seeded (density indicated in Table 1). Sometimes an aliquot of the suspension was stored in liquid nitrogen following standard techniques [18]. No cells were used above the eighth passage in making a corneal construct.
Figure 1.
Schematic diagram of the preparation of corneal equivalent made with collagen. Cells were harvested from human corneas and separately grown in culture dishes. Just before confluence, cells were trypsinized to produce a suspension. Cells were added over the collagen construct cast in a Petri dish (model 1). Alternatively, endothelial cells obtained from bovine corneas were seeded over a culture insert to produce a collagen corneal construct made of human epithelium and keratocytes (model 2).
Table 1. Seeding densities of cells in culture dish and corneal equivalent.
| Culture type | Cell type |
||||
|---|---|---|---|---|---|
| Epithelial | 3T3 | Stromal (keratocyte) | Human endothelial | Bovine endothelial | |
| Culture dish (in 103 cells/cm2) |
6.6-13.3 |
20 |
6.6 |
13.3 |
6.6-10 |
| Corneal equivalent (in 103 cells/stromal equivalent) | 800-1600 | 25 (in 103 cells/ ml of stromal equivalent) | 100-140 | 600 | |
Preparation of a corneal equivalent
Each stromal equivalent was prepared with lyophilized cow skin collagen extracted at Laboratory of Experimental Organogenesis (LOEX) [16,19]. A collagen solution was poured either in a 35-mm bacteriological Petri dish (model 1) or in the well of a culture insert (model 2). Models 1 and 2, which had a surface area of 7 cm2 and 4.2 cm2 contained human or bovine endothelial cells, respectively (Figure 1 and Table 1). As a similar volume of collagen was used in both types of constructs; their respective thicknesses were inversely related to that surface area. Trypsinized fibroblasts were rapidly mixed on ice with cold collagen (2.8 mg/ml in 0.1% sterile acetic acid), DME (2.7X solution), NaOH (0.7 M), and FBS (18.5%). This mixture was poured over an annulus of sterilized filter paper (Whatman, Maidstone, UK) contained within the Petri dish (model 1) or the culture insert (model 2). After casting, equivalents incubated for 2 h (37 °C) to allow gelation, after which DME was added.
The order of the steps to prepare corneal constructs was different: In model 1, the collagen gel was cast first, and endothelial cells were seeded 3 or 4 days afterward over the gel. In model 2, bovine endothelial cells were plated on the upside-down surface of the insert before the collagen gel was poured into its well on the following day. In both cases, a suspension of human (model 1) or bovine endothelial cells (model 2) was poured into a metallic ring placed over 1) the filter paper or 2) over the surface of the insert membrane. Cells attached for 2 h, after which culture medium was poured over the cells. Rings were removed after 8 h, and the corneal constructs were cultured under immersed conditions. Cells from the collagen constructs with endothelial cells were fed with a 1:1 mixture of fibroblasts and endothelial media. The epithelium was seeded 3 or 4 days after seeding endothelial cells, by pouring a suspension of epithelial cells over the bare side of the collagen construct. The medium was switched to a 1:1 mixture of epithelial and endothelial media for 7–10 days, at which time the equivalents were raised to the air–liquid interface, and cultured for another 7–10 days to allow cell differentiation. Then the equivalents were processed for histological analysis or electrophoresis.
Histology and indirect immunofluorescence
Biopsy specimens were fixed in Bouin’s solution and embedded in paraffin. Five-μm-thick sections were stained with Masson’s trichrome. Biopsy specimens were also included in frozen tissue embedding medium (OCT compound, Tissue-Tek, Bayer Canada, Etobicoke, Canada), frozen in liquid nitrogen, and kept at −70 °C until used for indirect immunofluorescence. After fixation in acetone (−20 °C, 10 min), the sections were exposed to a primary antibody for 45 min. Primary antibodies were either polyclonal ones developed in rabbit (Sigma, St. Louis, MO) against pan cadherin, catenins of the α or β types, or monoclonal ones developed in mouse (BD Bioscience, PharMingen, Mississauga, Canada) against human cadherins (N or E). Each primary antibody was diluted in PBS (1X 137 mM NaCl, 2.7 mM KCl, 6.5 mM Na2HPO4, 1.5 mM KH2PO4, 0.9 mM CaCl2 and 0.48 mM MgCl2, pH 7.2) containing 1% bovine serum albumin (BSA). Control sections were incubated in PBS-BSA only. Specimens were exposed for 30 min to a secondary antibody coupled to an Alexa 594 fluorophore (Molecular Probes, Eugene, OR): chicken anti-rabbit immunoglobulin G (IgG) or goat anti-mouse IgG for sections exposed to a polyclonal or monoclonal primary antibody, respectively. Nuclei were counterstained with Hoechst 33,258 (Sigma). Slides were observed with epifluorescence on a Nikon Eclipse T2000-U (Mississauga, Canada) and photographed with a Hamamatsu (Bridgewater, NJ.) B&W digital camera. Negative controls, for which the primary antibody was omitted, did not show any fluorescence.
Electrophoresis and western blot of corneal equivalents
Some corneal equivalents were incubated (1 h, 37 °C) in a HEPES-buffered dispase (2.5 mg/ml) solution. The endothelial and epithelial sheets were separated with forceps from the stromal equivalents under a dissecting microscope. Each layer was separately processed with a Polytron homogenizer (PT3100, Kinematica, Littau, Switzerland). The homogenate was prepared on ice in a cold lysis buffer supplemented with a 1X protease inhibitor cocktail (Complet, Boehringer, Laval, Canada). The lysate protein concentration was measured on a microplate reader at 540 nm using the Micro BCA colorimetric assay (Pierce, Rockford, IL). Mini polyacrylamide gels (t=0.75 mm) were cast with separating and stacking gels of 10% and 5% acrylamide, respectively. Cell lysates were reduced (5 min, 95 °C) in a sample buffer containing 10% β-mercapto-ethanol. Lanes were loaded with a lysate containing 15 µg protein (1 µg protein/µl) and with protein standards (Kaleidoscope, Biorad, Hercules, CA). Proteins separated at constant voltage (100 V) were transferred overnight at 4 °C on nitrocellulose (Hybond C-extra, Amersham Biosciences, Piscataway, NJ) or polyvinylidene difluoride (Biorad) membranes. Gels and membranes were stained with Coomassie blue and Ponceau S to confirm transfer. After blocking with a 5% skimmed milk TBS-Tween-20, membranes were exposed to the same primary antibodies and then to a peroxidase-conjugated goat anti-rabbit (Sigma) or a goat anti-mouse IgG (Chemicon, Temecula, CA) antibody. Detection was done with chemiluminescence (ECL, Amersham). Experiments were repeated at least twice.
Results
The macroscopic view of the three-dimensional corneal construct with epithelial and endothelial cells remained translucent during the 20 to 25 days of culture (Figure 2). Several times, collagen gels ruptured in the last 2 weeks of culture, presumably because of myofibroblast-induced contraction of the collagen in the thickness axis [15].
Figure 2.

Macroscopic view of a corneal equivalent in its Petri dish after 25 days in culture.
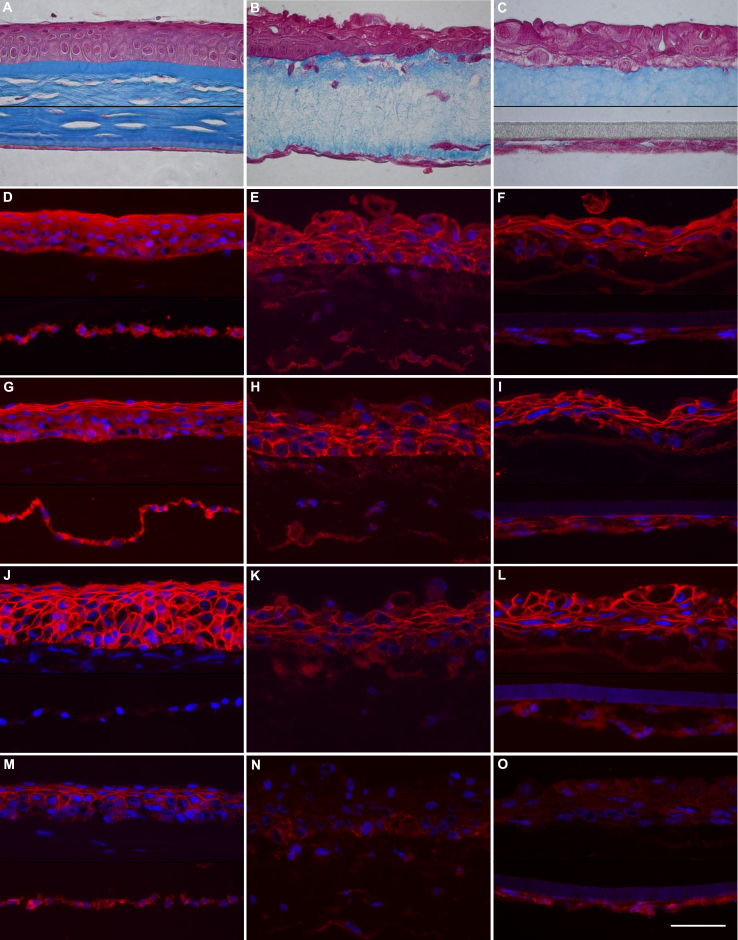
The comparative histology and immunochemistry of adherens junction proteins of a native cornea (Figure 3A,D,G,J,M) may be compared with the corresponding ones of corneal equivalents of model 1 (Figure 3B,E,H,K,N) or model 2 (Figure 3C,F,I,L,O). Compared to native corneal stroma (Figure 3A), the gel of both types of corneal constructs (Figure 3B,C) seemed less fibrous and contained fewer fibroblasts dispersed in the collagen matrix stained with Masson’s trichrome. The epithelial layers of corneal constructs (Figure 3B,C) comprised three to four layers of epithelial cells that were not as organized as the ones from native tissue (Figure 3A). Human (Figure 3B) or bovine endothelial cells (Figure 3C) attached directly to the collagen gel (model 1) or to the synthetic membrane of the insert (model 2), respectively. The basal epithelial cells over collagen constructs were columnar and got flatter toward the surface, especially in the model containing human endothelial cells (Figure 3B). The epithelium and the endothelium of the native corneas presented intense cytoplasmic staining (Figure 3D,G) with increased reactivity toward cell borders in the suprabasal layers of the corneal epithelium against α- or β-catenins. In the epithelium of the corneal constructs, the cytoplasmic staining against catenins (Figure 3E,F,H,I) was less intense and was concentrated near the cytoplasmic sides of the cell borders. The endothelium of the corneal constructs was positive for catenins, although this reactivity was weak.
Figure 3.
Histology and immunology of native and reconstructed corneas. Native (A, D, G, J and M) and reconstructed corneas were sectioned for microscopic observation. Corneal constructs contained human endothelium directly seeded under the collagen gel (B, E, H, K and N) or bovine endothelium growing on a synthetic membrane (C, F, I, L and O). As shown on the paraffin sections, stained with Masson’s trichrome (A–C), cells are stained pink, and the collagen tissue is blue. Immunofluorescent images of cryosections (D–O) have been exposed to the antibodies against the following antigens: α-catenin (D–F), β-catenin (G–I), E-cadherin (J–L), and N-cadherin (M–O). Cell nuclei were stained blue with Hoechst dye (D–R). Scale bar = 50 µm.
When present, reactivity to E- and N-cadherin was near the cell borders. In the native corneal epithelium, strong labeling of E-cadherin was detected in all layers (Figure 3J) whereas lighter labeling of N-cadherin was present only in the suprabasal layers of the epithelium (Figure 3M). The endothelium was N-cadherin positive but E-cadherin negative. The epithelium of the corneal constructs reacted to E-cadherin (Figure 3K,L) but not to N-cadherin, whereas the bovine corneal endothelium reacted lightly to N-cadherin (Figure 3O). As expected, neither the corneal stroma nor the keratocytes reacted to any of these antibodies.
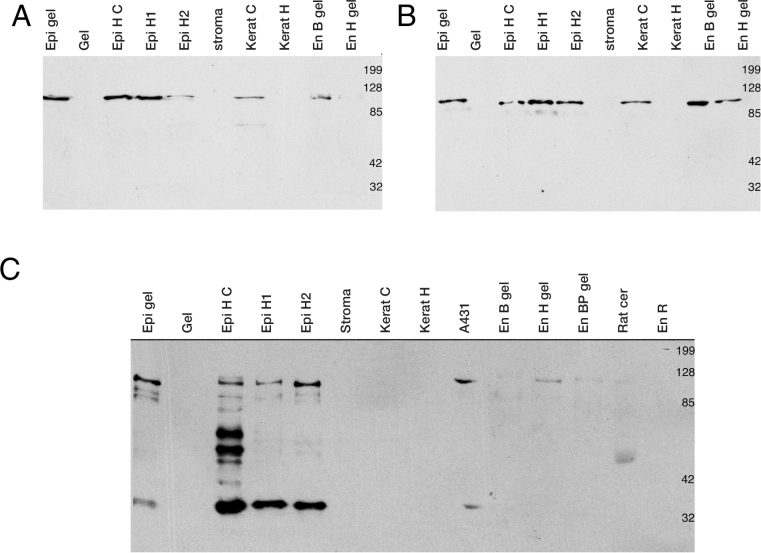
Western blot analysis confirmed the specific reactivity for α- and β-catenins in the corneal constructs (Figure 4A,B). The epithelial and endothelial cells as well as the cultured fibroblasts, but not native keratocytes, expressed α- and β-catenins that migrated on the polyacrylamide gel at 102 kDa and 94 kDa (Figure 4A,B). E- (Figure 4C) and N-cadherins (not shown) were detected at 120 kDa and 130 kDa as expected although other bands were revealed by the antibodies. Epithelial cells, harvested from a native cornea (Figure 4C, lanes “Epi H1,” and “Epi H2”), a cultured monolayer (lane “Epi H C”), or a three-dimensional construct (“Epi gel”) expressed E-cadherin (120 kDa), but were not positive against N-cadherin. In contrast, lysates from the human endothelial cells grown on a stromal equivalent expressed N-cadherin but not E-cadherin (Figure 4C). As expected, there was no reactivity against any tested cadherin in the collagen gel of the stromal equivalent, in the digested stroma, or in the native keratocytes or cultured fibroblasts.
Figure 4.
Western blots from cells or tissues of corneal donors or equivalents. Proteins were obtained from human corneal epithelium in culture over a stromal equivalent (“Epi gel”) or in monoculture (“Epi H C”), or collected from donors (lanes “Epi H1,” and “Epi H2”). The collagen gel is identified as “Gel.” The lane “Stroma” contains corneal stroma treated in collagenase H whereas lanes “Kerat C” and “Kerat H” contain keratocytes in culture or obtained from the donor cornea, respectively. “En B gel” and “En H gel” are lysates from either bovine or human endothelial cells growing on the stromal equivalent. “EnBP gel” is a pool of bovine endothelial cells growing on corneal equivalents that were not seeded with epithelial cells. “En R” refers to endothelial cells isolated from rabbit corneas. A431 and rat cerebrum (Rat cer) lysates were used as positive controls for E- and N-cadherin antibodies. Blots of α-catenin (A), β-catenin (B), and E-cadherin (C).
Discussion
Stromal equivalents were produced by seeding human fibroblasts in a liquid solution of collagen. After gelling, untransformed epithelial and endothelial cells were laid on each side of the stromal construct to mimic the native cornea. Epithelial cells had a structure that was reminiscent of the native cornea: columnar cells near the basal epithelium and flattened superficial cells. As previously shown, this model of collagen gel allows epithelial cells 1) to secrete basement membrane components such as laminin, type VII collagen, and fibronectin and 2) to express a pattern of integrin that is reminiscent of the one observed in a healing native cornea [16]. In the present study, we have shown that these epithelial cells express cadherins and catenins, proteins that are essential for the development of cell polarity.
As shown with protein immunoblotting and in situ immunofluorescence, there is reactivity against E-cadherin and α- and β-catenins in the epithelial cells of native and reconstructed corneas. This is in contrast to the finding of an early study in which normal human corneal epithelium did not express E-cadherin [20]. Presumably, either corneal E-cadherin did not express a binding site for the integrin αEβ7 or exposed an epitope for their antibody (5H9). However, the positive reaction of the corneal epithelium to E-cadherin herein reported is supported by other studies on eye bank tissues [8] or immortalized human corneal epithelium [21-23]. The absence of a N-cadherin band in the western blot of the fresh epithelium was surprising, but not necessary in contradiction with immunofluorescence of the fresh epithelium. The procedures performed to obtain this lysate may selectively retain cells from the basal layers, which were N-cadherin negative on immunofluorescence. The expression of N-cadherin seems associated with the maintenance of a limbal epithelial phenotype in vitro [10].
We also found that the endothelial cells in this cocultured corneal construct expressed variable levels of adherens junction proteins. Whereas native human endothelial cells weakly expressed α- and β-catenins, as well as N-cadherin, cultured human endothelial seemed to express opposite levels of N-cadherin on fluorescence microscopy (Figure 3 N) or western blot. The endothelial cells from bovine corneas cultured over a collagen equivalent expressed α and β-catenins and, weakly, N-cadherin. The weak reactivity against N-cadherin in the human corneal endothelium over the corneal construct raises the possibility that the endothelial cells in this model might have adopted a fibroblastic morphology, as already shown to occur with endothelial cells from human or animal corneas when cultured in vitro [24,25]. Although the monocultures of the endothelium we used had a nice mosaic of hexagonal cells at the eighth passage or lower, we cannot rule out the possibility that the human endothelial cells had become fibroblastic in the corneal constructs. However, this possibility is unlikely since the human endothelial cells cultured in the monolayer conserved their hexagonal shape and formed a nice mosaic until their eighth passage. In addition, for reasons that could be related to the conditions of cell dissociation or culture, the expression of N-cadherin may have been inhibited on this particular corneal construct. When endothelial cells are cultured, the levels of N-cadherin expression reach a level similar to the one observed in vivo only at 21 days after seeding [12]. Finally, when the disruption of endothelial cell junctions by EDTA is followed by a specific sequence of basic fibroblast growth factor (bFGF) and transforming growth factor beta 1 (TGFβ1) application in culture, these cells adopt a fibroblastic morphology while N-cadherin is relocated from the cell junction to the cytoplasm [26].
Endothelial cells obtained from young human corneas were also used. The dispase enzymatic treatment that we used may have exhausted these cells since a mechanical dissociation technique is highly successful in the culture of endothelial cells from adult donors. Mature human corneal endothelium is generally believed to have a limited capacity of division in vivo. However, these cells, which are arrested in the G1 phase of the cell cycle, have retained proliferative potential, as demonstrated by positive reactions against cyclins D, E, and A [27,28]. Cell adhesion mechanisms could be involved in repressing this proliferation. Human endothelium from ex vivo corneas proliferates after mechanical damage occurs over a large surface or to cell junctions by chelating calcium with EDTA [29]. The difficulty in inducing human endothelial cells to replicate in vitro has certainly limited the availability of human endothelial cells to seed over our stromal equivalents and prompted us to use a seeding density lower than we had wished. To evaluate if we could visualize adherens junction proteins with bovine endothelial cells that proliferate better in culture and could be seeded at higher density, we used them in model 2. We generally observed higher reactivity on the bovine endothelium of corneal constructs compared with the human endothelium.
Not surprisingly, there was no reaction for any of these antibodies with fibroblasts from native corneas or from stromal equivalents. The presence of protein from the adherens junction in the epithelial and endothelial layers growing on the corneal equivalent suggests that these layers are polarized. Therefore, these cell layers could fulfill functions analogous to the ones they perform in native tissues, such as protecting the cornea, as normal cell polarity protects at least partially against Pseudomonas aeruginosa, an opportunistic pathogen responsible for corneal ulcers [14].
Acknowledgments
This study was supported by the Vision Research Network of the Fonds de la recherche en santé du Québec (FRSQ) and the Canadian Institutes of Health Research (MOP-53170).
References
- 1.González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WJ, Adams CL, Chen YT, Smith SJ. Epithelial cell polarity from the outside looking in. News Physiol Sci. 2003;18:143–6. doi: 10.1152/nips.01435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–41. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 4.Soler C, Grangeasse C, Baggetto LG, Damour O. Dermal fibroblast proliferation is improved by beta-catenin overexpression and inhibited by E-cadherin expression. FEBS Lett. 1999;442:178–82. doi: 10.1016/s0014-5793(98)01648-2. [DOI] [PubMed] [Google Scholar]
- 5.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–8. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 6.Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- 7.Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–19. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawashima M, Kawakita T, Higa K, Satake Y, Omoto M, Tsubota K, Shimmura S, Shimazaki J. Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium. Mol Vis. 2010;16:2727–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Lu R, Bian F, Zhang X, Qi H, Chuang EY, Pflugfelder SC, Li D-Q. The beta-catenin/Tcf4/survivin signaling maintains a less differentiated phenotype and high proliferative capacity of human corneal epithelial progenitor cells. Int J Biochem Cell Biol. 2011;43:751–9. doi: 10.1016/j.biocel.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higa K, Shimmura S, Miyashita H, Kato N, Ogawa Y, Kawakita T, Shimazaki J, Tsubota K. N-cadherin in the maintenance of human corneal limbal epithelial progenitor cells in vitro. Invest Ophthalmol Vis Sci. 2009;50:4640–5. doi: 10.1167/iovs.09-3503. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:4734–41. doi: 10.1167/iovs.10-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y-T, Hayashida Y, Kheirkhah A, He H, Chen S-Y, Tseng SCG. Characterization and comparison of intercellular adherent junctions expressed by human corneal endothelial cells in vivo and in vitro. Invest Ophthalmol Vis Sci. 2008;49:3879–86. doi: 10.1167/iovs.08-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkovetz DF, Katz J. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 2003;5:613–9. doi: 10.1016/s1286-4579(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 14.Fleiszig SM, Evans DJ, Do N, Vallas V, Shin S, Mostov KE. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–7. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Germain L, Carrier P, Auger FA, Salesse C, Guérin SL. Can we produce a human corneal equivalent by tissue engineering? Prog Retin Eye Res. 2000;19:497–527. doi: 10.1016/s1350-9462(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 16.Germain L, Auger FA, Grandbois E, Guignard R, Giasson M, Boisjoly HM, Guérin SL. Reconstructed human cornea produced in vitro by tissue engineering. Pathobiology. 1999;67:140–7. doi: 10.1159/000028064. [DOI] [PubMed] [Google Scholar]
- 17.Engelmann K, Bednarz J, Bohnke M. Endothelial cell transplantation and growth behavior of the human corneal endothelium. Ophthalmologe. 1999;96:555–62. doi: 10.1007/s003470050452. [DOI] [PubMed] [Google Scholar]
- 18.Freshney IR. Culture of Animal Cells: a Manual of Basic Techniques. 3rd ed. New York: Wiley-Liss; 1994. p. 254–65. [Google Scholar]
- 19.Gallop PM, Seifter S. Preparation and properties of soluble collagens. Methods Enzymol. 1963;VI:635–41. [Google Scholar]
- 20.Scott RA, Lauweryns B, Snead DM, Haynes RJ, Mahida Y, Dua HS. E-cadherin distribution and epithelial basement membrane characteristics of the normal human conjunctiva and cornea. Eye (Lond) 1997;11:607–12. doi: 10.1038/eye.1997.163. [DOI] [PubMed] [Google Scholar]
- 21.Kimura K, Teranishi S, Kawamoto K, Nishida T. Protective effect of dexamethasone against hypoxia-induced disruption of barrier function in human corneal epithelial cells. Exp Eye Res. 2011;92:388–93. doi: 10.1016/j.exer.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Kimura K, Teranishi S, Nishida T. Interleukin-1β-induced disruption of barrier function in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:597–603. doi: 10.1167/iovs.08-2606. [DOI] [PubMed] [Google Scholar]
- 23.Robertson DM, Zhu M, Wu Y-C. Cellular distribution of the IGF-1R in corneal epithelial cells. Exp Eye Res. 2012;94:179–86. doi: 10.1016/j.exer.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Böhnke M, Vogelberg K, Engelmann K. Detection of neurone-specific enolase in long-term cultures of human corneal endothelium. Graefes Arch Clin Exp Ophthalmol. 1998;236:522–6. doi: 10.1007/s004170050115. [DOI] [PubMed] [Google Scholar]
- 25.Petroll WM, Hsu JK, Bean J, Cavanagh HD, Jester JV. The spatial organization of apical junctional complex-associated proteins in feline and human corneal endothelium. Curr Eye Res. 1999;18:10–9. doi: 10.1076/ceyr.18.1.10.5392. [DOI] [PubMed] [Google Scholar]
- 26.Zhu YT, Chen HC, Chen SY, Tseng SC. Nuclear p120-catenin Unlocks Mitotic Block of Contact-inhibited Human Corneal Endothelial Monolayers without Disrupting Adherent Junction. J Cell Sci. 2012;14:14. doi: 10.1242/jcs.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996;37:1566–75. [PubMed] [Google Scholar]
- 28.Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996;37:645–55. [PubMed] [Google Scholar]
- 29.Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:2930–5. [PubMed] [Google Scholar]