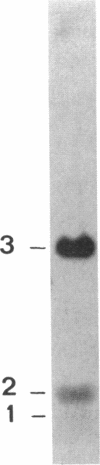
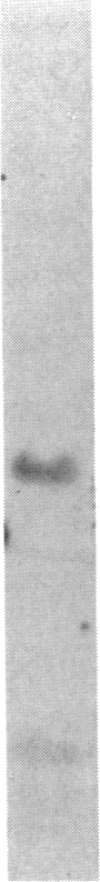
Abstract
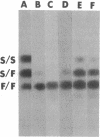
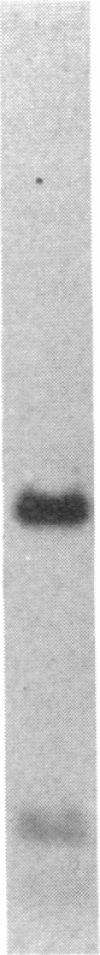
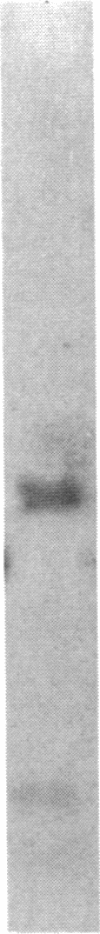
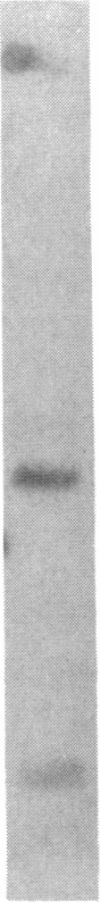
Three independently isolated and unstable mutants of the maize alcohol dehydrogenase 1 gene (Adh1) have arisen by insertion of the Mu transposing element into the first intervening sequence of the progenitor Adh1 allele. The mutants have been selected for their decreased levels of alcohol dehydrogenase 1 activity. The original mutants were unstable, giving rise to both revertant alleles and altered mutant alleles. From one of the original mutants, two derivative mutants have been recovered and described. We analyzed the effect of Mu insertion in all five of these mutants by measuring relative levels of run-off transcripts from the progenitor and mutant alleles and by comparing levels of run-off transcripts corresponding to regions lying 5' and 3' to the insertion sites. In this paper we present evidence that early transcriptional events are affected, but that, in spite of the inclusion of a 1.4-kilobase transposing DNA element, processing of transcripts occurs normally.
Full text
PDF

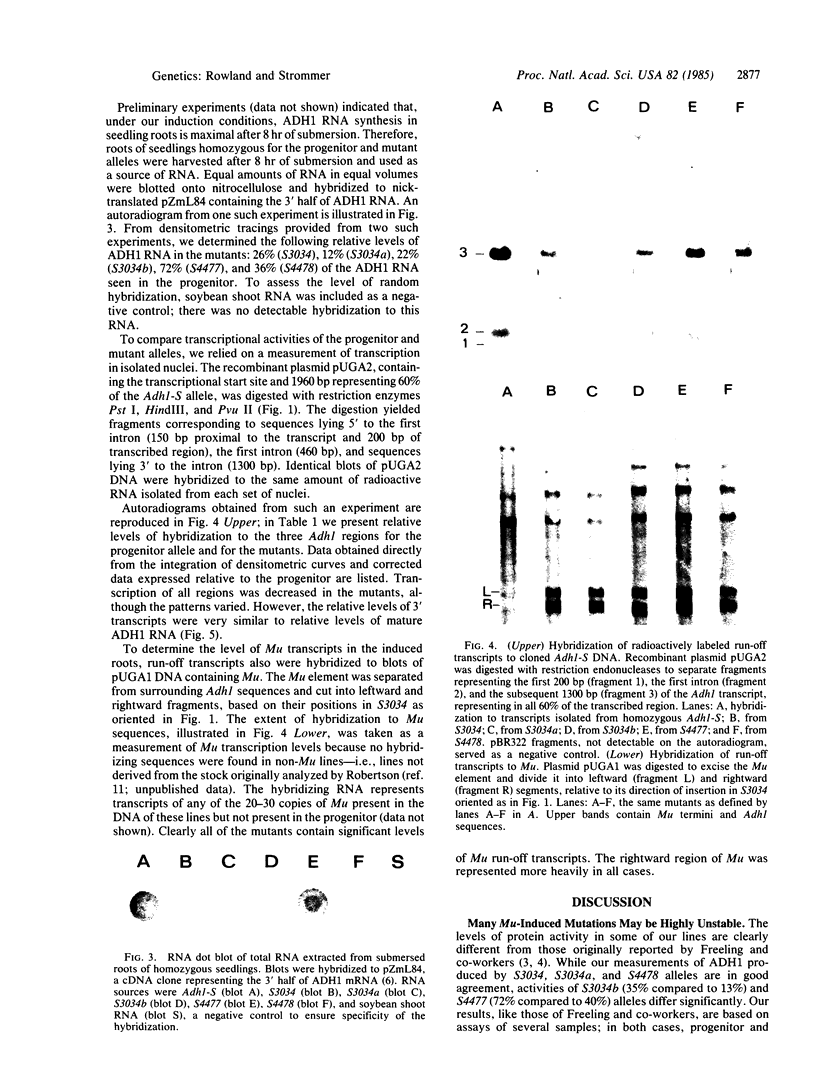
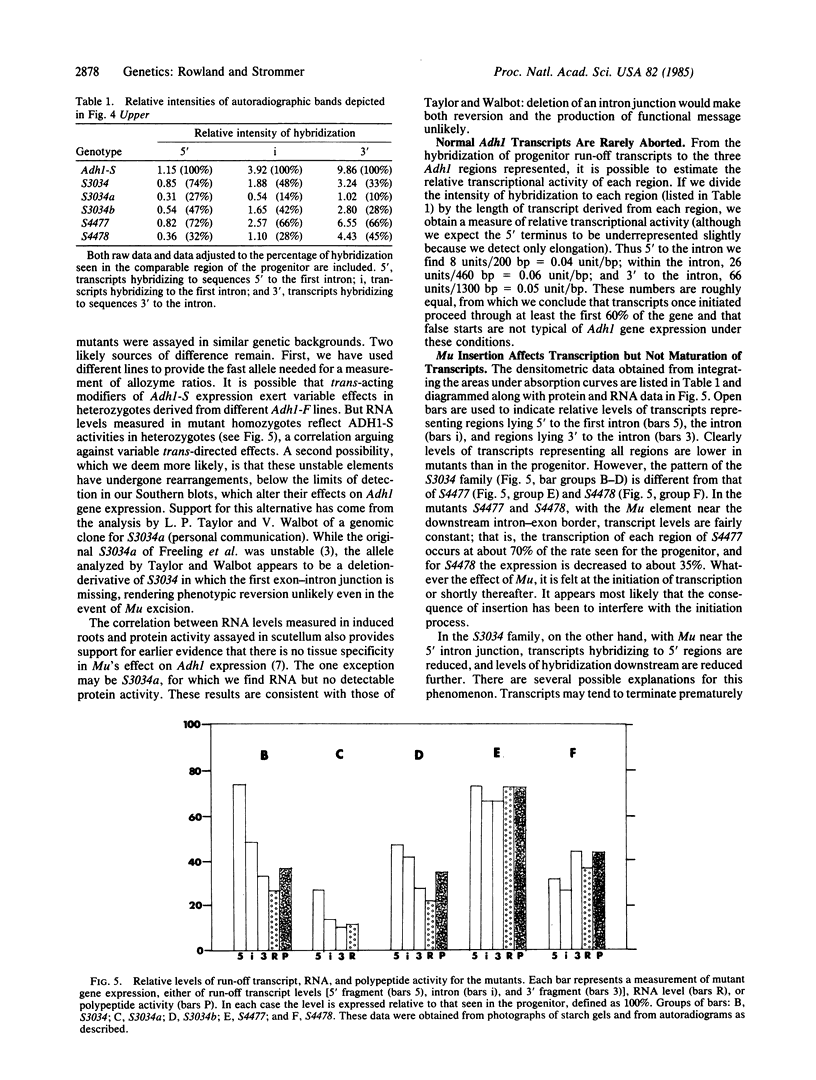

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Ayala F. J., Powell J. R., Tracey M. L., Mourão C. A., Pérez-Salas S. Enzyme variability in the Drosophila willistoni group. IV. Genic variation in natural populations of Drosophila willistoni. Genetics. 1972 Jan;70(1):113–139. doi: 10.1093/genetics/70.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R. F., Thompson D. V., Talbot D. R., Swanson J., Bennetzen J. L. Nucleotide sequence of the maize transposable element Mul. Nucleic Acids Res. 1984 Aug 10;12(15):5955–5967. doi: 10.1093/nar/12.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Swanson J., Taylor W. C., Freeling M. DNA insertion in the first intron of maize Adh1 affects message levels: cloning of progenitor and mutant Adh1 alleles. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4125–4128. doi: 10.1073/pnas.81.13.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruskin A. M., Tyner A. L., Wells D. E., Showman R. M., Klein W. H. Accumulation in embryogenesis of five mRNAs enriched in the ectoderm of the sea urchin pluteus. Dev Biol. 1981 Oct 30;87(2):308–318. doi: 10.1016/0012-1606(81)90154-8. [DOI] [PubMed] [Google Scholar]
- Ferl R. J., Brennan M. D., Schwartz D. In vitro translation of maize ADH: evidence for the anaerobic induction of mRNA. Biochem Genet. 1980 Aug;18(7-8):681–691. doi: 10.1007/BF00484585. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Vodkin L. O. An insertion sequence blocks the expression of a soybean lectin gene. Cell. 1983 Jun;33(2):465–475. doi: 10.1016/0092-8674(83)90428-2. [DOI] [PubMed] [Google Scholar]
- Harding J. D., MacDonald R. J., Przybyla A. E., Chirgwin J. M., Pictet R. L., Rutter W. J. Changes in the frequency of specific transcripts during development of the pancreas. J Biol Chem. 1977 Oct 25;252(20):7391–7397. [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: II. CHARACTERIZATION OF RNA SYNTHESIZED IN VITRO. Plant Physiol. 1980 Feb;65(2):309–313. doi: 10.1104/pp.65.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. S. Mutator activity in maize: timing of its activation in ontogeny. Science. 1981 Sep 25;213(4515):1515–1517. doi: 10.1126/science.213.4515.1515. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. Comparisons of Relative Activities of Maize Adh(1) Alleles in Heterozygotes-analyses at the Protein (Crm) Level. Genetics. 1973 Aug;74(4):615–617. doi: 10.1093/genetics/74.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Hoopes B. C., McClure W. R., Kleckner N. Three promoters near the termini of IS10: pIN, pOUT, and pIII. Cell. 1983 Sep;34(2):673–682. doi: 10.1016/0092-8674(83)90400-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]