Abstract
Nitric oxide (NO) plays an important role on several biological functions. Recently, it has been reported the possibility of modifying the NO release profile from the NO donors through its coupling to gold nanoparticles (AuNPs). Thus, AuNPs were synthesized and they were exposed to the NO donor ruthenium complex Cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3 termed (Ru-4PySH)—forming AuNPs-{Ru-4PySH}n cluster. Our results indicate that AuNPs do not modify the maximum effect (ME) and potency (pD2) in the vasodilation induced by Ru-4PySH. Both complexes induce similar vascular relaxation in concentration-dependent way. However, the NO released from the complex AuNPs-{Ru-4PySH}n is lower than Ru-4PySH. Both complexes release only NO0 specie, but AuNPs-{Ru-4PySH}n releases NO in constant way and exclusively in the extracellular medium. In time-course, Ru-4Py-SH was faster than AuNPs-{Ru-4PySH}n in inducing the maximum vasodilation. Inhibition of soluble guanylyl cyclase (sGC) abolished the vasodilation induced by Ru-4PYSH, but not by AuNPs-{Ru-4PySH}n. Non-selective potassium (K+) channel blocker TEA had no effect on the vasodilation induced by AuNPs-{Ru-4PySH}n, but it reduced the potency to Ru-4PySH. In conclusion, our results suggest that AuNPs can reduce the permeability of NO donor Ru-4PySH due to AuNPs-{Ru-4PySH}n cluster formation. AuNPs reduce NO release, but they do not impair the vasodilator effect induced by the NO donor. Ru-4PySH induces vasodilation by sGC and K+ channels activation, while AuNPs-{Ru-4PySH}n activates mainly sGC. Taken together, these findings represent a new pharmacological strategy to control the NO release which could activate selective biological targets.
Keywords: Gold nanoparticle, Vasodilation, Nitric oxide (NO), NO donor ruthenium complex, Soluble guanylyl cyclase, Potassium channels
Introduction
In the recent years, the diatomic free radical nitric oxide (NO) has been shown to be involved in many important biological events, such as platelet adhesion and aggregation, metabolism, neuronal activity, immune response, and vasodilation [1]. Among its diverse functions, NO has been widely implicated in the vascular smooth muscle relaxation [2–5]. The predominant signaling pathway responsible for the vascular relaxation induced by NO appears to involve the activation of soluble guanylyl cyclase (sGC) with the production of cyclic guanosine monophosphate (cGMP) and activation of protein kinase G [6]. Nevertheless, some authors have reported that vascular relaxation to NO may occur through a cGMP independent pathway in the smooth muscle by the direct activation of K+ channels [7, 8].
Recently, it is increasing the number of disease states involving NO imbalance [9, 10] and this has stimulated extensive research activity into the chemistry, biology, and pharmacology of NO. The proposal and achievement of new drugs can represent an advance in this field. In this way, the discovery of the several bioregulatory roles of NO demands new methods for generating NO in a controlled way to facilitate an improved understanding of the physiological and physiopathological function of NO. Bonaventura et al. [11] by comparing the mechanism underlying the relaxation induced by two NO donors sodium nitroprusside (SNP) and a ruthenium complex verified that the ruthenium complex can be used as pharmacological tool without toxicity, in contrast to SNP that is toxic since it releases cyanide.
Beyond the NO donor ruthenium complex represents an exogenous source of NO apparently compatible with biological system, another new strategy is try to modify the NO release from this synthetic drug. Gold nanoparticles (AuNPs) have recently emerged as attractive novel platform for target-specific delivery of therapeutic agents [12]. Gold nanoparticles are essentially inert, non-toxic, and easy synthesis [13]. Rothrock et al. [14] reported the synthesis of gold nanoparticles that was designed to control the NO release. A few years later, Polizzi et al. [15] reported the synthesis of water-soluble NO-releasing gold nanoparticles with significantly enhanced NO payloads.
In this way, we synthesized a NO donor complex, cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3, also called Ru-4PySH, that it was adsorbed to gold nanoparticles (AuNPs) forming AuNPs-{Ru-4PySH}n cluster. In contrast to the NO donor coupled to AuNPs in DMSO obtained by Diaz-Garcia et al. [16], we have proposed its synthesis and studies in aqueous solution. The potential of this nitro ruthenium complex has been explored as NO deliver agent in physiological conditions [17–21]. Thus, the current study aimed to characterize the profile of NO release by ruthenium complexes without (Ru-4PySH) or with gold nanoparticles (AuNPs-{Ru-4PySH}n), and the cellular mechanisms that are activated by these complexes to induce vascular relaxation in rat aorta.
Materials and methods
The UV–visible spectra were recorded in the Hitachi U-3501. Infrared (IR) spectra were recorded on a Protegé 460 series FT-IR spectrometer, by using solid samples pressed in KBr pellets. The NO measurements were performed by using the ISO-NO NO-meter and the DUO-18 acquisition board. The sensitivity of this apparatus ranges between 1 nmol/L and 20 μmol/L, with a 2-mm sensor, which directly detects NO concentration by amperometric technique. The setup was calibrated following the procedure previously reported [22–24]. The recordings were performed in organ chamber with aortic rings in Krebs solution maintained at pH 7.4 gassed with 95 % O2 and 5 % CO2 at 37 °C under ambient light to guarantee similar conditions of the functional studies.
Synthesis and characterization of the complexes
The recrystallized complex salt Cis-[Ru(bpy)2(NO)(L)].(PF6)3 was prepared as previously described by Sauaia et al. [25]. The ligand “L” was replaced by thiol 4-aminepirydine (4PySH) forming Cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3 also called Ru-4PySH. The Ru-4PySH complex was characterized by UV–Vis and infrared spectroscopy, and then compared to the published results [25].
Gold nanoparticles (AuNPs) were synthesized by the technique described by Turkevitch and adapted by Frens [26]. This method consists in the reduction of HAuCl4 by citrate anion in aqueous solution and the obtainment of AuNPs was characterized by plasmon band formation in 520 nm wavelength and ruby red color. We obtained AuNPs around 10 nm measured by intensity of size distribution using Zeta Sizer (Malvern Instruments). AuNPs and thiol 4-aminopirydine (4PySH) exhibit high chemical affinity [27]. Therefore, the AuNPs-{Ru-4PySH}n cluster formation was monitored by UV–Vis spectroscopy of 3.32 mL AuNPs before (plasmon band in 520 nm at time zero) and after the addition of 10 μL Ru-4PySH 1.38 mmol/L.
After 60 min of interaction and stabilization, the sample (AuNPs plus Ru-4PySH) assumed violet staining and a new size measurement was performed. The size of AuNPs along Ru-4PySH was in the range from 10 nm to around 1,000 nm. The AuNPs-{Ru-4PySH}n complex was always prepared 60 min before the use. The vasodilator effect of both complexes was evaluated in ambient light conditions.
Vessel preparation
Male Wistar rats (180–200 g) were killed by decapitation, and all the procedures were performed in accordance with the Ethical Animal Committee of the University of São Paulo, Brazil. The thoracic aorta was quickly removed, dissected free, and cut into 4-mm long. In order to avoid the interference of the endothelium factors, in the present study, we investigated the vascular relaxation induced by Ru-4PySH, simple AuNPs, and AuNPs-{Ru-4PySH}n in denuded aortic rings which were placed between two stainless-steel stirrups and connected to an isometric force transducer (Letica Scientific Instruments; Barcelona, Spain) to measure tension. The aortic rings were placed in a 10 mL organ chamber containing Krebs solution with the following composition (mmol/L): NaCl 130, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, NaHCO3 14.9, glucose 5.5, CaCl2 1.6. The solution was maintained at pH 7.4 gassed with 95 % O2 and 5 % CO2 at 37 °C at ambient light. The aortic rings were initially stretched to a basal tension of 1.5 g before allowing them to equilibrate for 60 min in the bath fluid. Endothelial integrity was qualitatively assessed by the degree of relaxation caused by acetylcholine (1 μmol/L) in the presence of contractile tone induced by 0.1 μmol/L phenylephrine. Because our studies required endothelium-denuded aortas, the rings were discarded if there was any degree of relaxation.
Experimental protocols
Vasodilator effect induced by Ru-4PySH, AuNPs-{Ru-4PySH}n, and simple AuNPs
To examine the vasorelaxation induced by the NO donors, the aortic rings were contracted with 0.1 μmol/L phenylephrine (EC50). When the contraction had reached the plateau, Ru-4PySH (0.3 nmol/L to 10 μmol/L), AuNPs-{Ru-4PySH}n (0.3 nmol/L to 10 μmol/L), or AuNPs control (in similar concentrations of AuNPs-{Ru-4PySH}n) were cumulatively added.
Time-course for vascular relaxation induced by Ru-4PySH and AuNPs-{Ru-4PySH}n
Ru-4PySH and AuNPs-{Ru-4PySH}n (5 μmol/L) were added to the organ chamber to induce the maximum vasodilator effect when a stable contraction in response to 0.1 μmol/L phenylephrine was achieved.
Effect of the extracellular NO scavenger oxyhemoglobin (HbO2), NO0 scavenger hydroxocobalamin, or NO− scavenger l-cysteine on the vascular relaxation induced by Ru-4PySH and AuNPs-{Ru-4PySH}n
The oxidation of hemoglobin was performed as previously described [28]. Oxyhemoglobin (HbO2 10 μmol/L), hydroxocobalamin (0.1 mmol/L), or the NO− scavenger l-cysteine (1 mmol/L) was added 30 min before the addition of the contractile agonist 0.1 μmol/L phenylephrine. Then, cumulative concentration–response curves to Ru-4PySH or AuNPs-{Ru-4PySH}n (0.3 nmol/L to 10 μmol/L) were obtained.
Effect of the soluble guanylyl cyclase inhibitor (ODQ) and potassium (K+) channels blocker (TEA) on the relaxation induced by Ru-4PySH and AuNPs-{Ru-4PySH}n
To examine the contribution of soluble guanylyl cyclase or K+ channels to the relaxation induced by Ru-4PySH and AuNPs-{Ru-4PySH}n, ODQ (1 μmol/L), or tetraethylammonium (TEA 1 mmol/L), non-selective K+ channel blocker was incubated for 30 min before the addition of phenylephrine (0.1 μmol/L). Then, cumulative concentration-response curves for Ru-4PySH or AuNPs-{Ru-4PySH}n (0.3 nmol/L to 10 μmol/L) were obtained.
Statistical analysis
Data are expressed as mean ± SEM. Each set of experiments were studied at least five aortic rings isolated from different rats. The pharmacological parameters, maximum effect (ME) and potency (pD2), obtained from concentration–response curves for Ru-4PySH and AuNPs-{Ru-4PySH}n were used to express the data. ME was considered as the maximal amplitude response reached in the concentration-effect curves for relaxant agents. The concentrations of the agents producing a half-maximal relaxation amplitude (EC50) were determined after logit transformation of the normalized concentration–response curves, and they were reported as the negative logarithm (pD2) of the mean of individual values for each tissue using the GraphPad Prism version 3.0 (GraphPad Software Corporation, San Diego, CA). Statistical significance was tested by one way ANOVA (post-test: Newman–Keuls) and Student’s t test, and values of P < 0.05 were considered to be significant.
Drugs
Phenylephrine, acetylcholine, hydroxocobalamin, l-Cysteine, tetraethylammonium (TEA), ODQ, and MTT were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). Hemoglobin was purchased from Calbiochem–Novabiochem (La Jolla, CA, USA).
Reagents
RuCl3.3H2O and thiol 4-aminopirydine were purchased as a highly pure reagent from Sigma-Aldrich Chemical and used as supplied; sodium nitrite purchased from Mallinckrodt. Double distilled H2O was used for all experiments. All chemical preparations and measurements were carried out under an argon atmosphere and protected from light.
Results and discussion
In the last years, it has been demonstrated that several ruthenium-derived NO donors induce vascular relaxation, without vascular cytotoxicity on different conditions and by different mechanisms [11, 17, 18, 20, 21, 29]. In the present study, we have verified that AuNPs, Ru-4PySH, and AuNPs-{Ru-4PySH}n in the concentrations that induce the maximum effect did not present cytotoxicity to the smooth muscle cells (data not shown). These results are according to data previously reported by Bonaventura and colleagues for other NO donor complex [29].
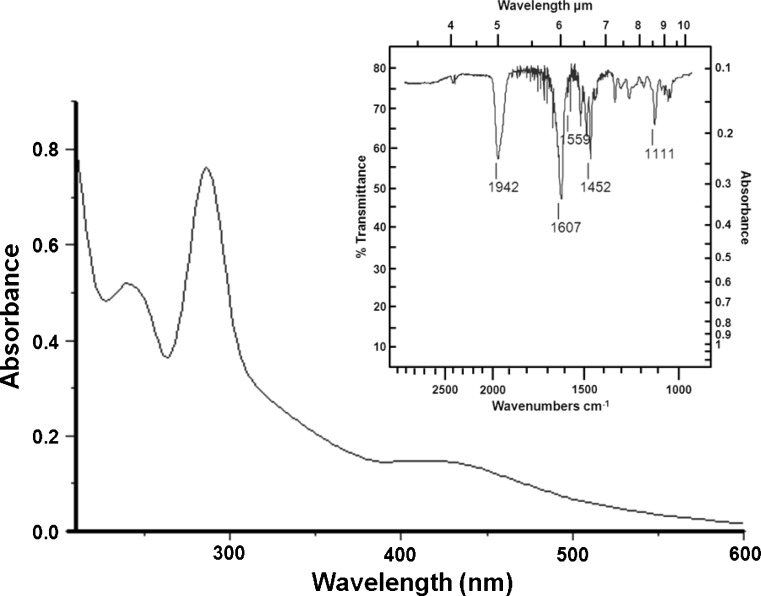
The ruthenium complex Ru-4PySH was characterized by elemental analysis, UV–Visible spectrum (UV–Vis) and infrared spectrum (FT-IR). FT-IR is frequently used to characterize the oxidation state of the metal and NO ligands in ruthenium complexes [30]. The NO frequency of Cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3 (Ru-4PySH) reported in this study is 1,942 cm−1 which indicates that formally a linear NO+ is coordinated to the ruthenium(II) center. Thus, the ruthenium complex was characterized in situ by UV–Vis spectrum and it was attributed to the formation of Cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3 simplified termed (Ru-4PySH) (Fig. 1).
Fig. 1.
UV–Vis spectrum of Cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3 (Ru-4PySH) in phosphate buffer pH 7.4. Inset: Infrared spectrum. NO frequency of Cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3 (Ru-4PySH) in 1,942 cm−1 indicates that formally a linear NO+ is coordinated to the ruthenium(II) center
Gold nanoparticles were characterized in situ by UV–Vis spectrum by plasmon band formation in 520-nm wavelength with maximum absorbance intensity around 1 at time zero (Fig. 2). The complex AuNPs-{Ru-4PySH}n was obtained by spontaneous interaction between AuNPs and Ru-4PySH. It was monitored at 1, 2, 5, 10, 20, 30, and 60 min by UV–Vis spectroscopy (Fig. 2). It shows a decrease in the absorbance in 520 nm followed by an increase in the intensity absorbance in wavelength higher than 650 nm. After 30 min, the interaction was stabilized and it was not different from 60 min. The decreased peak in 520 nm (UV–Vis) indicates consumption of single AuNPs due its possible interaction with thiol group from Ru-4PySH.
Fig. 2.
UV–Vis spectrum of 3.32 mL AuNPs before (plasmon band in 520 nm at time zero) and after the addition of 10 μL Cis-[Ru(bpy)2(NO)(4PySH)].(PF6)3 (Ru-4PySH) 1.38 mmol/L. Time-dependent spontaneous interaction between AuNPs and Ru-4PySH shown by decrease plasmon band intensity in 520 nm and increase absorbance intensity in wavelengths higher than 650 nm with stabilization between 30 and 60 min
The representative scheme of the steps to obtain the complexes Ru-4PySH and AuNPs-{Ru-4PySH}n is shown in the Fig. 3.
Fig. 3.
Representative scheme of the steps to obtainment of the complexes Ru-4PySH and AuNPs-{Ru-4PySH}n. 4PySH thiol 4-aminepirydine, L ligant, Ru Cis-[Ru(bpy)2(NO)(L)].(PF6)3; Ru-4PySH [Ru(bpy)2(NO)(4PySH)].(PF6)3, AuNPs gold nanoparticles, AuNPs-{Ru-4PySH}n, AuNPs + [Ru(bpy)2(NO)(4PySH)].(PF6)3, n undetermined number of Ru-4PySH that interact with AuNPs
The NO released from the ruthenium complexes Ru-4PySH and AuNPs-{Ru-4PySH}n was analyzed by a selective NO-sensor, which directly detects NO concentrations by amperometric technique. As shown in the Fig. 4, the NO released from the AuNPs-{Ru-4PySH}n complex was lower than NO released from Ru-4PySH, but its NO release was constant up to 500 s.
Fig. 4.
Time-dependent NO release from Ru-4PySH and AuNPs-{Ru-4PySH}n (5 μmol/L) in Krebs solution at pH 7.4 in the presence of the aortic rings
Vascular relaxation induced by Ru-4PySH, AuNPs-{Ru-4PySH}n, and simple AuNPs
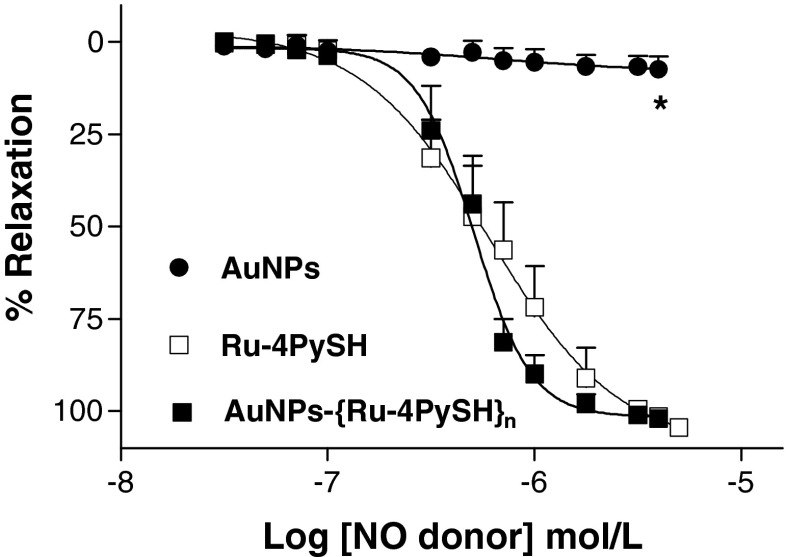
As shown in the Fig. 5, the relaxation induced by the complexes Ru-4PySH and AuNPs-{Ru-4PySH}n was concentration-dependent in denuded aortic rings contracted with 0.1 μmol/L phenylephrine. Ru-4PySH and AuNPs-{Ru-4PySH}n demonstrated similar maximum effect and potency as shown by the values of ME and pD2, respectively (Table 1). On the other hand, AuNPs did not induce vascular relaxation. The absence of vasodilator effect for single AuNPs is also an indicative of non-cytotoxicity. Gold particles have been also used in medicine to improve the therapeutic effects of drugs designed to treat arthritis. Currently, due to its inert chemical and physical properties, gold cores are considered to be biocompatible and non-toxic which has enlarged its use for disease diagnosis and therapy [31, 32].
Fig. 5.
Effect of AuNPs, Ru-4PySH, and AuNPs-{Ru-4PySH}n on denuded aortic rings contracted with phenylephrine (PE 0.1 μmol/L). In the maintained contractions, Ru-4PySH (0.3 nmol/L to 10 μmol/L, n = 10), AuNPs-{Ru-4PySH}n (0.3 nmol/L to 10 μmol/L, n = 10), or AuNPs (in proportional concentration to AuNPs-{Ru-4PySH}n, n = 5) was cumulatively added. Data are mean ± SEM of n experiments performed on preparations obtained from different animals. Asterisk denotes difference from Ru-4PySH and AuNPs-{Ru-4PySH}n (P < 0.001)
Table 1.
Effect of the extracellular NO scavenger oxyhemoglobin (HbO2, 10 μmol/L), NO0 scavenger hydroxocobalamin (0.1 mmol/L), NO− scavenger l-cysteine (1 mmol/L), non-selective potassium channel blocker tetraethylammonium (TEA, 1 μmol/L), and soluble guanylyl cyclase inhibitor (ODQ 1 μmol/L) on vascular relaxation induced by NO donors Ru-4PySH and AuNPs-{Ru-4PySH}n in denuded aortic rings
| Ru-4PySH | AuNPs-{Ru-4PySH}n | |||||
|---|---|---|---|---|---|---|
| ME (%) | pD2 | n | ME (%) | pD2 | n | |
| Control | 104.6 ± 0.9 | 6.22 ± 0.13 | 10 | 102.2 ± 2.2 | 6.34 ± 0.07 | 10 |
| HbO2 | 91.4 ± 4.2 | 4.70 ± 0.15* | 5 | – | – | 5 |
| Hydroxocobalamin | 94.4 ± 7.2 | 5.00 ± 0.26* | 5 | 62.7 ± 9.8* | 4.62 ± 0.19* | 6 |
| l-cysteine | 101.7 ± 1.9 | 6.14 ± 0.04 | 5 | 103.9 ± 6.0 | 6.23 ± 0.05 | 5 |
| TEA | 108.6 ± 3.9 | 5.51 ± 0.12* | 7 | 99.9 ± 3.9 | 6.22 ± 0.05 | 7 |
| ODQ | – | – | 7 | 25.4 ± 8.0* | – | 7 |
* P < 0.001, denotes difference from control
Time-course for vascular relaxation induced by Ru-4PySH and AuNPs-{Ru-4PySH}n
The time to reach the maximum relaxation was longer for AuNPs-{Ru-4PySH}n (750 s, n = 5) than for Ru-4PySH (450 s, n = 5) as shown in the Fig. 6.
Fig. 6.
Time-course for Ru-4PySH and AuNPs-{Ru-4PySH}n-induced relaxation. Denuded thoracic aortic rings were pre-contracted with 0.1 mmol/L phenylephrine and 0.1 and 5 μmol/L Ru-4PySH or AuNPs-{Ru-4PySH}n were added. Data are mean ± SEM of five experiments performed on preparations obtained from different animals
Our work indicates that the coupling of AuNPs with Ru-4PySH contributes to reduce the termed “burst release” [33], characterized by an initial large bolus of NO released before its rate reaches a stable profile. Consequently, time-course for vascular relaxation induced by AuNPs-{Ru-4PySH}n also was increased as compared to Ru-4PySH.
Effect of oxyhemoglobin, hydroxocobalamin, and l-cysteine on vascular relaxation induced by Ru-4PySH and AuNPs-{Ru-4PySH}n
One of the purposes of this work was to evaluate whether the resizing of the AuNPs along with Ru-4PySH, from 10 nm to around 1,000 nm, forming AuNPs-{Ru-4PySH}n would modify the localization where the NO is released. The incubation with the extracellular NO scavenger oxyhemoglobin (HbO2 10 μmol/L) shifted to the right the concentration-effect curve for Ru-4PySH complex reducing the potency (Fig. 7b), but it did not reduce its ME (Fig. 7a and Table 1). On the other hand, HbO2 abolished the relaxation induced by AuNPs-{Ru-4PySH}n (Fig. 7a, b and Table 1).
Fig. 7.
Effect of extracellular NO scavenger oxyhemoglobin (HbO2, 10 μmol/L), NO0 scavenger hydroxocobalamin (0.1 mmol/L) or NO− scavenger l-cysteine (1 mmol/L) on the relaxation induced by the NO donors Ru-4PySH (a) or AuNPs-{Ru-4PySH}n on denuded aortic rings pre-contracted with phenylephrine (PE 0.1 μmol/L) after incubation or in the absence of scavenger (control). Data are mean ± SEM of n = 5–10 experiments performed on preparations obtained from different animals. Asterisk denotes difference of maximum effect (ME) (a) and potency (pD 2) (b) from control (P < 0.001)
Our results show that the relaxation induced by Ru-4PySH was less potent in presence of HbO2 as compared to the control in absence of the extracellular NO scavenger. It indicates that Ru-4PySH induces vascular relaxation due to intracellular and extracellular NO release similarly to other NO donors [11, 34]. However, HbO2 abolished the vascular relaxation induced by AuNPs-{Ru-4PySH}n. It suggests that due to its large size, AuNPs-{Ru-4PySH}n probably does not permeate the plasma membrane. Thus, it releases NO only in the extracellular medium.
Another important point for the vasodilator effect is the NO specie released by the NO donors as free radical (NO0) or nitroxyl ions (NO−). Incubation with the NO0 scavenger hydroxocobalamin shifted to the right the concentration-effect curve for Ru-4PySH complex reducing the potency (Fig. 7b), but it did not reduce its ME (Fig. 7a and Table 1). Nevertheless, hydroxocobalamin abolished the relaxation induced by AuNPs-{Ru-4PySH}n (Fig. 7a, b and Table 1). On the other hand, the NO− scavenger l-cysteine (1 mmol/L) had no effect on vasodilator effect induced by both NO donors (Fig. 7a, b and Table 1). These data indicate that NO0 plays an important role in vasodilator effect induced by both NO donors, but its role is more important for AuNPs-{Ru-4PySH}n than Ru-4PySH.
Effect of ODQ and TEA on vascular relaxation induced by Ru-4PySH and AuNPs-{Ru-4PySH}n
In relation to the cellular mechanism induced by both complexes Ru-4PySH and AuNPs-{Ru-4PySH}n, some pharmacological tools were used. ODQ was used as a selective sGC inhibitor, the main target to the vasodilator effect of NO responsible by cGMP production [35].
As shown in Fig. 8a and Table 1, the sGC-inhibitor ODQ abolished the vascular relaxation induced by Ru-4PySH. However, ODQ only partially reduced the vascular relaxation induced by AuNPs-{Ru-4PySH}n. On the other hand, the K+ channel blocker TEA reduced the potency to Ru-4PySH, but it did not modify the ME (Fig. 8a and Table 1). In contrast, TEA did not alter the ME neither pD2 for AuNPs-{Ru-4PySH}n (Fig. 8b and Table 1).
Fig. 8.
Effect of non-selective potassium (K+) channel blocker tetraethylammonium (TEA 1 μmol/L) or soluble guanylyl cyclase inhibitor (ODQ 1 μmol/L) on the relaxation of denuded aortic rings induced by Ru-4PySH (a) or AuNPs-{Ru-4PySH}n (b) in the absence (control) or after incubation. Data are mean ± SEM of n = 7–10 experiments performed on preparations obtained from different animals. Asterisk denotes difference from control (P < 0.001)
Our results show that the sGC inhibition abolished the vascular relaxation induced by Ru-4PySH, but it did not abolish the vasodilation induced by AuNPs-{Ru-4PySH}n. These data suggest that NO released from both complexes Ru-4PySH and AuNPs-{Ru-4PySH}n distinctly activate sGC. On the other hand, the use of non-selective K+ channel blocker TEA inhibited the vascular relaxation induced by Ru-4PySH as observed for the other NO donors Terpy and SNP [11]. However, TEA and the selective K+ channel blockers (data not shown) had no effect on vascular relaxation induced by AuNPs-{Ru-4PySH}n.
The main finding of this work was that the association of the NO donor Ru-4PySH to AuNPs forming AuNPs-{Ru-4PySH}n cluster did not modify the efficacy and potency of these compounds in inducing vascular relaxation, although it modified the NO release and the cellular mechanism. For our surprise, this procedure reduced the NO release. These results are different of the results obtained by Polizzi et al. [15]. In accordance to our finding, Rothrock et al. [14] reported that at 37 °C the NO donor coupled to AuNPs released low levels of NO. These results suggest the possible chemical and thermal mechanisms involved in the NO release.
As reported by Thomas et al. [36], the steady-state NO concentrations seem to be a key determinant of its biological function. Precise cellular responses are differentially regulated by specific NO concentration. The direct effects of NO are those chemical reactions that occur in low concentrations enough to allow NO to react directly with a biological target molecule.
The NO0 specie has been described to activate mainly sGC, while NO− specie could activate directly K+ channels [7, 37]. In addition, the present work shows that the amount and duration of NO0 released by NO donor also determine its pharmacological properties. The degree of activation of K+ channels and sGC may depend on the amount of NO0 released from NO donor. Although we did not quantify the NO molar concentration released by both NO donors, in accordance our findings, Hall and Garthwaite [38] reported that low NO concentration, a range of 100 pmol/L (or below), seems contribute to selective in vivo sGC activation.
Conclusion
Taken together, our results suggest that Ru-4PySH induces vasodilator effect by activation of sGC and K+ channels while AuNPs-{Ru-4PySH}n activates mainly sGC, possibly because the AuNPs-{Ru-4PySH}n releases low NO levels, but only NO0 specie in constant way. It would activate sGC–cGMP-mediated processes and possibly independent of K+ channel activation. In conclusion, this study provides evidence that NO donor coupled to AuNPs represents a new pharmacological strategy to control the NO release and, consequently, modify its cellular mechanisms without impairing its effectiveness.
Acknowledgments
We thank Dr. Michele Paulo for assistance in MTT Assay. This work was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflict to interest
None declared.
References
- 1.Gao Y. The multiple actions of NO. Pflugers Arch - Eur J Physiol. 2010;459:829–839. doi: 10.1007/s00424-009-0773-9. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Ballot B, Wood KS. Regulation of soluble guanylate cyclase activity by phorpyrins and metallophorpyrins. J Biol Chem. 1984;259:6201–6207. [PubMed] [Google Scholar]
- 3.Ignarro LJ, Buga GM, Wood KS, Byrns RE. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ignarro LJ. Endothelium-derived nitric oxide pharmacology and relationship to the actions of organic nitrate esters. Pharm Res. 1989;6:651–659. doi: 10.1023/A:1015926119947. [DOI] [PubMed] [Google Scholar]
- 5.Moncada S, Palmer RMJ, Gryglewski RJ. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986;83:9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohse MJ, Forstermann U, Schmitt HHHW. Pharmacology of NO: cGMP signal transduction. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;358:111–112. doi: 10.1007/PL00005230. [DOI] [PubMed] [Google Scholar]
- 7.Bolotina V, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 8.Homer KL, Wanstall JC. Cyclic GMP- independent relaxation of rat pulmonary artery by spermine NONOate, a diazeniumdiolate nitric oxide donor. Br J Pharmacol. 2000;131:673–682. doi: 10.1038/sj.bjp.0703613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bateman RM, Sharpe MD, Ellis CG. Bench-to-bedside review: microvascular dysfunction in sepsis–hemodynamics, oxygen transport, and nitric oxide. Crit Care. 2003;7:359–373. doi: 10.1186/cc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–418. doi: 10.1253/circj.CJ-08-1102. [DOI] [PubMed] [Google Scholar]
- 11.Bonaventura D, De Lima RG, Vercesi JA, Da Silva RS, Bendhack LM. Comparison of the mechanisms underlying the relaxation induced by two nitric oxide donors: sodium nitroprusside and new ruthenium complex. Vasc Pharmacol. 2007;46:215–222. doi: 10.1016/j.vph.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 13.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 14.Rothrock AR, Donkers RL, Schoenfisch MH. Synthesis of nitric oxide-releasing gold nanoparticles. J Am Chem Soc. 2005;127:9362–9363. doi: 10.1021/ja052027u. [DOI] [PubMed] [Google Scholar]
- 15.Polizzi MA, Stasko NA, Schoenfisch MH. Water-soluble nitric oxide-releasing gold nanoparticles. Langmuir. 2007;23:4938–4943. doi: 10.1021/la0633841. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Garcia AM, Fernández-Oliva M, Ortiz M, Cão R (2009) Interaction of nitric oxide with gold nanoparticles capped with a ruthenium (II) complex. Dalton Trans 7870–7872 [DOI] [PubMed]
- 17.Bonaventura D, De Oliveira FS, Togniolo V, Tedesco AC, Da Silva RS, Bendhack LM. A macrocyclic nitrosyl ruthenium complex is a NO donor that induces rat aorta relaxation. Nitric Oxide. 2004;10:83–91. doi: 10.1016/j.niox.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Bonaventura D, De Oliveira FS, Da Silva RS, Bendhack LM. Decreased vasodilation induced by a new nitric oxide donor in two kidney, one clip hypertensive rats is due to impaired K+ channel activation. Clin Exp Pharmacol Physiol. 2005;32:478–481. doi: 10.1111/j.1440-1681.2005.04215.x. [DOI] [PubMed] [Google Scholar]
- 19.Da Rocha ZN, Marchesi MSP, Molin JC, Lunardi CN, Miranda KM, Bendhack LM, Ford PC, Da Silva RS (2008) The inducing NO-vasodilation by chemical reduction of coordinated nitrite ion in cis-[Ru(NO2)L(bpy)2]+ complex. Dalton Trans 4282–4287 [DOI] [PubMed]
- 20.Pereira AC, Ford PC, Da Silva RS, Bendhack LM. Ruthenium-nitrite complex as pro-drug releases NO in a tissue and enzyme-dependent way. Nitric Oxide. 2011;24:192–198. doi: 10.1016/j.niox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues GJ, Lunardi CN, Lima RG, Santos CX, Laurindo FRM, Da Silva RS, Bendhack LM. Vitamin C improves the effect of a new nitric oxide donor on the vascular smooth muscle from renal hypertensive rats. Nitric Oxide. 2008;18:176–183. doi: 10.1016/j.niox.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Kudo S, Bourassa JL, Boggs SE, Sato Y, Ford PC. In situ nitric oxide (NO) measurement by modified electrodes: NO labilized by photolysis of metal nitrosyl complexes. Anal Biochem. 1997;247:193–202. doi: 10.1006/abio.1997.2097. [DOI] [PubMed] [Google Scholar]
- 23.Mori V, Bertotti M. Nitric oxide solutions: standardisation bychronoamperometry using a platinum disc microelectrode. Analyst. 2000;125:1629–1632. doi: 10.1039/b003482g. [DOI] [Google Scholar]
- 24.Wink DA, Darbyshire JF, Nims RW, Saavedra JE, Ford PC. Reactions of the bioregulatory agent nitric-oxide in oxygenated aqueous-media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993;6:23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 25.Sauaia MG, Oliveira FS, De Lima RG, Cacciari AL, Tfouni E, Da Silva RS (2005) Syntheses, characterization and photochemical properties of new NO0–ruthenium(II) complexes. Inorg Chem Commun 347–349
- 26.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 27.Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. Science. 2007;318:430–433. doi: 10.1126/science.1148624. [DOI] [PubMed] [Google Scholar]
- 28.Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- 29.Bonaventura D, Oliveira FS, Lunardi CN, Vercesi JA, Da Silva RS, Bendhack LM. Characterization of the mechanisms of action and nitric oxide species involved in the relaxation induced by the ruthenium complex. Nitric Oxide. 2006;15:387–394. doi: 10.1016/j.niox.2006.04.260. [DOI] [PubMed] [Google Scholar]
- 30.Bordini J, Hughes DL, Neto JDM, Da Cunha CJ. Nitric oxide photorelease from ruthenium salen complexes in aqueous and organic solutions. Inorg Chem. 2002;41:5410–5416. doi: 10.1021/ic011273d. [DOI] [PubMed] [Google Scholar]
- 31.Kayser O, Lemke A, Hernandez-Trejo N. The impact of nanobiotechnology on the development of new drug delivery systems. Curr Pharm Biotechnol. 2005;6:3–5. doi: 10.2174/1389201053167158. [DOI] [PubMed] [Google Scholar]
- 32.Mieszawska AJ, Mulder WJM, Fayad ZA, Cormode DP. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol Pharm. 2013;10:831–847. doi: 10.1021/mp3005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–136. doi: 10.1016/S0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 34.De Figueiredo LF, Nelson SH, Mathru M, Silva MR, Kramer GC. Effects of hemoglobin-based blood substitutes on vasoactivity of rat aortic rings. Artif Organs. 2001;25:928–933. doi: 10.1046/j.1525-1594.2001.06900.x. [DOI] [PubMed] [Google Scholar]
- 35.Soloviev A, Lehen'kyi V, Zelensky S, Hellstrand P. Nitric oxide relaxes rat tail artery smooth muscle by cyclic GMP-independent decrease in calcium sensitivity of myofilaments. Cell Calcium. 2004;36:165–173. doi: 10.1016/j.ceca.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wanstall JC, JeVery TK, Gambino A, Lovren F, Triggle CR. Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol. 2001;134:463–472. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]