Abstract
Background
Previous research on clinical and high-risk samples suggests that signs of autism spectrum disorder (ASD) can be detected between one and two years of age. We investigated signs of ASD at 18 months in a population-based sample and the association with later ASD diagnosis.
Methods
The study sample includes 52 026 children born 2003 through 2008, and is a subset of children that participated in the Norwegian Mother and Child Cohort (MoBa), a population-based longitudinal study, and the Autism Birth Cohort (ABC), a sub-study on ASD. Parents completed all 23 items from the Modified Checklist for Autism in Toddlers (M-CHAT) at 18 months.
Results
The M-CHAT 6-critical-item criterion and the 23-item criterion had a specificity of 97.9% and 92.7% and a sensitivity of 20.8% and 34.1%, respectively. In the 173 children diagnosed with ASD to date, 60 children (34.7%) scored above the cut-off on either of the screening criteria. The items with the highest likelihood ratios were “interest in other children”, “show objects to others”, and “response to name”.
Conclusion
Even though one third of the children who later received an ASD diagnosis were identified through M-CHAT items, the majority scored below cut-off on the screening criteria at 18 months. The results imply that it might not be possible to detect all children with ASD at this age.
Keywords: Autism spectrum disorders, early identification, M-CHAT, longitudinal studies, Autism Birth Cohort Study, Norwegian Mother and Child Cohort Study
Previous research has attempted to identify early markers of autism spectrum disorder (ASD). In retrospective studies, parents of children with an ASD diagnosis commonly report that they were concerned about their child's development at 18 months.1,2 Other studies have investigated home videotapes from the time around the child's first birthday, and report that it is possible to observe behaviours related to ASD at this age in children who later receive an ASD diagnosis.3,4 A third approach has been to study younger siblings of children with ASD. The earliest behavioural signs seem to emerge in the second year of life, and the difference between children with and without ASD increases during this time with respect to social skills and language.5-7
Given the potential for early intervention to alter the developmental course of ASDs, the American Academy of Pediatrics recommends that all 18- and 24-month-olds be screened for ASD during regular health visits.8 The empirical support for universal screening, however, is sparse. There has been limited research on population-based screening for ASD. In one longitudinal population-based study, Baird et al found that the majority of children later diagnosed with ASD were missed on screening at 18 months with the Checklist for Autism in Toddlers (CHAT).9 A revised version of CHAT, the Modified Checklist for Autism in Toddlers (M-CHAT) is now the most frequently used instrument for early screening for ASD.10 M-CHAT has been studied in both high-risk (children referred for diagnostic assessment) and low-risk samples (children attending routine visits to well-baby clinics), performing more accurately in high-risk than low-risk samples.11,12 Information obtained from low-risk samples is limited by the fact that assessments were largely confined to children who screened positive. Limited efforts have been made to identify false negatives, that is, children who are not identified in the screening but who later receive an ASD diagnosis. Researchers in this field have expressed the need for longitudinal studies in population-based samples that also determine the proportion of false negatives.13,14
We investigated the association between parent-reported signs of ASD at 18 months and later ASD diagnosis in a population-based sample.
Methods
Study Sample
Included in the present study are 52 026 children born from August 2003 through 2008 (age range 4 years, 0 months to 9 years, 4 months at the end of follow-up December 31st 2012, mean age 6 years, 8 months). The study sample is derived from the Norwegian Mother and Child Cohort Study (MoBa)15 and the Autism Birth Cohort (ABC) Study16. The MoBa cohort is a prospective population-based pregnancy cohort established by the Norwegian Institute of Public Health. The ABC is a nested case-cohort designed to identify and study cases of ASD in the MoBa. Enrolment of pregnant women in the MoBa began in 1999 and was completed in 2008 (approximately 109 000 children). The participation rate among invited mothers was about 38.5%. Approximately 73% of MoBa participants completed the 18-month questionnaire. The present study includes only children whose mothers had responded to the 18-month MoBa questionnaire and completed all 23 M-CHAT questions. The M-CHAT was included in the 18-month MoBa questionnaire from March 2005. Characteristics of the study sample are presented in Table 1.
Table 1.
Characteristics by diagnosis
| ASD n = 173 n (%) | Non-ASD n = 51 853 n (%) | ||
|---|---|---|---|
| Infant sex | Male | 150 (86.7) | 26 447 (51.0) |
| Female | 23 (13.3) | 25 406 (49.0) | |
| Maternal education (years) | <12 | 10 (5.8) | 2 700 (5.2) |
| 12 | 55 (31.8) | 12 331 (23.8) | |
| 13-16 | 67 (38.7) | 21 971 (42.4) | |
| ≥17 | 35 (20.2) | 13 482 (26.0) | |
| Missing | 6 (3.5) | 1 369 (2.6) | |
| Maternal age (years) | <25 | 21 (12.1) | 4 756 (9.2) |
| 25-29 | 50 (28.9) | 16 915 (32.6) | |
| 30-34 | 71 (41.0) | 20 776 (40.1) | |
| ≥35 | 31 (17.9) | 9 406 (18.1) | |
| Parity | 0 | 100 (57.8) | 24 932 (48.1) |
| 1 | 45 (26.0) | 17 730 (34.2) | |
| ≥2 | 28 (16.2) | 9 191 (17.7) | |
| Year of birth | 2003 | 25 (14.5) | 2 608 (5.0) |
| 2004 | 39 (22.5) | 9 098 (17.5) | |
| 2005 | 32 (18.5) | 10 190 (19.7) | |
| 2006 | 34 (19.7) | 11 126 (21.5) | |
| 2007 | 22 (12.7) | 10 293 (19.9) | |
| 2008 | 21 (12.1) | 8 538 (16.5) | |
Signs of ASD at 18 Months
M-CHAT is a 23-item, yes-no parent-report questionnaire developed to screen for ASD in children aged 16 to 30 months.10 All items were translated into Norwegian by professional translators, and then back-translated into English for quality control purposes. Six critical items were identified by the M-CHAT authors, addressing “imitation”, “interest in other children”, “initiative to joint attention (point for interest)”, “response to joint attention (follow point)”, “show objects to others”, and “response to name”.10 Children who fail any three of the 23 total items or two of the six critical items are categorized as at risk for ASD (screen-positive).10 The M-CHAT authors recommend that a telephone interview by an experienced clinician is used for follow-up and further evaluation of screen-positive children. Consistent with the American Academy of Pediatrics’ recommendations on early screening for ASD, we did not include a telephone interview.8
ASD Cases
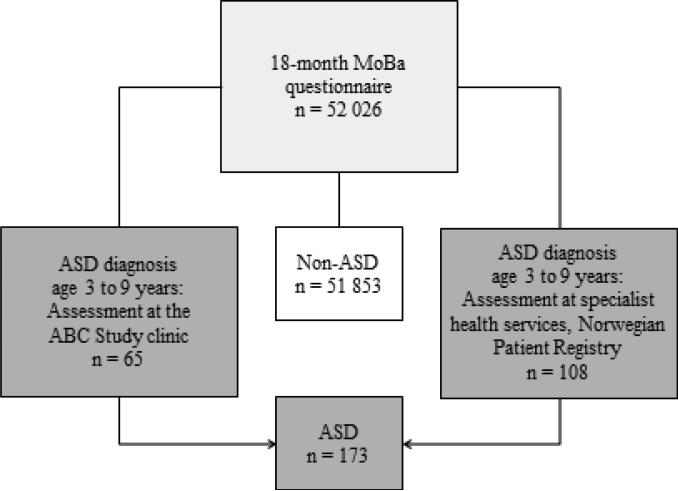
ASD cases in the current study were identified either by assessment at the ABC Study clinic or by assessment at regional specialist health services and registered in the Norwegian Patient Registry (NPR) (Figure 1).
Figure 1.
MoBa responders to the 18-month questionnaire and source of ASD diagnosis
Potential ASD cases in the MoBa (identified via the 3-, 5- or 7-year MoBa questionnaire or referred from parents and professionals)16 were asked to participate in a one-day clinical assessment at the ABC Study clinic at Nic Waals Institute, a child psychiatry clinic in Oslo. The children were assessed by a research-trained team of experienced clinicians (clinical psychologists and child psychiatrists). An ASD diagnosis was made if the children met the DSM-IV criteria for Autistic Disorder, Asperger Syndrome, or Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS). Signs of autism were assessed using the Autism Diagnostic Interview – Revised17 and the Autism Diagnostic Observation Schedule18.
Through linkage with the Norwegian Patient Registry we were able to identify MoBa participants who had not been assessed at the ABC Study clinic, but had received ASD diagnoses from Norwegian specialist health services. The case definition included the ICD-10 diagnoses F84.0 Childhood Autism, F84.1 Atypical Autism, F84.5 Asperger Syndrome, F84.8 Other Pervasive Developmental Disorder, and F84.9 Pervasive Developmental Disorder, Unspecified.
In a separate validation study,19 60 children registered with an ASD diagnosis in the patient registry were assessed at the ABC Study clinic in order to validate the register cases. Fifty-eight children were found to meet the DSM-IV criteria for ASD, generating a positive predictive value of 97% (95% CI 88%, 100%). The participation rate in the validation study was 36%.
As the ABC and MoBa are ongoing studies, more children will be diagnosed with ASD in the future. ASD case-identification will continue at least until 2016 when all MoBa children will have turned 7 years.
Participants in MoBa and the ABC Study signed informed consent approved by the Regional Committee for Medical and Health Research Ethics prior to entry.
Statistical Analyses
Sensitivity, specificity, and PPV were calculated based on two-by-two contingency tables. Fisher's exact test was used to assess the difference in the proportion failing each M-CHAT item in the ASD group and the non-ASD group. Likelihood ratio (LR) was calculated for all individual M-CHAT items as well as for the 6-critical-item criterion and the 23-item criterion. The LR indicates how many times more likely people with the condition are to have a positive test compared to individuals without the condition: LR = Sensitivity / (1 – Specificity). Data analyses were performed using SPSS software for Windows (version 20.0).
Results
The Relationship between Score on M-CHAT at 18 Months and Later ASD Diagnosis
In this sample of 52 026 children, 173 children have received an ASD diagnosis to date. The frequencies of children, who scored above and below cut-off on the M-CHAT 6-critical-item criterion and 23-item criterion in the ASD group and the non-ASD group, respectively, are presented in Table 2, together with the sensitivity, specificity, PPV, and LR.
Table 2.
Sensitivity, specificity, positive predictive value (PPV), and likelihood ratio (LR) for M-CHAT 6-critical-item criterion and 23-item criterion
| ASD n=173 | Non-ASD n=51 853 | Sensitivity [95% CI] | Specificity [95% CI] | PPV [95% CI] | LR | |
|---|---|---|---|---|---|---|
| Above cut-off | 36 (20.8%) | 1067 (2.1%) | ||||
| 6-critical-item criterion | 20.8% [15.0, 27.6] | 97.9% [97.8, 98.1] | 3.3% [2.3, 4.5] | 10.1 | ||
| Below cut-off | 137 (79.2%) | 50 786 (97.9%) | ||||
| Above cut-off | 59 (34.1%) | 3804 (7.3%) | ||||
| 23-item criterion | 34.1% [27.1, 41.7] | 92.7% [92.4, 92.9] | 1.5% [1.2, 2.0] | 4.6 | ||
| Below cut-off | 114 (65.9%) | 48 049 (92.7%) | ||||
The M-CHAT 6-critical-item criterion and the 23-item criterion, administered at 18 months, had a specificity of 97.9% and 92.7%, sensitivity of 20.8% and 34.1%, and PPV of 3.3% and 1.5%, respectively, in relation to diagnoses of ASD at a later age. Sixty children with ASD (34.7%) failed either the 6-critical-item criterion or the 23-item criterion; all except one of the children in the ASD group who failed the 6-critical-item criterion also failed the 23-item criterion.
Analysis of M-CHAT Items
Table 3 shows the percentages of children in the ASD group and the non-ASD group who failed each M-CHAT item. There was a statistically significant difference between the ASD group and the non-ASD group in the proportion failing each item except for “Does your child ever seem oversensitive to noise (e.g. plugging ears)?” and “Does your child look at your face to check your reaction when faced with something unfamiliar?”. The items corresponding to the highest LRs were “interest in other children”, “show objects to others”, and “response to name”.
Table 3.
Percentage of children in the ASD and non-ASD groups that failed each item on the M-CHAT
| Topic of M-CHAT item | ASD n = 173 | Non-ASD n = 51 853 | P-value | LR |
|---|---|---|---|---|
| 1. Enjoy being swung/bounced | 2.3 | 0.6 | 0.026 | 3.6 |
| 2. Interest in other children* | 5.2 | 0.4 | <0.001 | 14.6 |
| 3. Enjoy climbing | 6.9 | 0.8 | <0.001 | 8.7 |
| 4. Enjoy peek-a-boo | 2.3 | 0.6 | 0.018 | 4.1 |
| 5. Pretend play | 16.2 | 1.9 | <0.001 | 8.3 |
| 6. Point to ask | 21.4 | 3.8 | <0.001 | 5.7 |
| 7. Point for interest* | 22.0 | 3.3 | <0.001 | 6.7 |
| 8. Functional play | 9.2 | 1.1 | <0.001 | 8.8 |
| 9. Show objects to others* | 14.5 | 1.3 | <0.001 | 11.0 |
| 10. Eye contact | 6.4 | 2.0 | 0.001 | 3.1 |
| 11. Oversensitive to noise | 11.6 | 12.4 | 0.807 | 0.9 |
| 12. Response to smile | 1.2 | 0.2 | 0.037 | 6.7 |
| 13. Imitation* | 19.1 | 6.1 | <0.001 | 3.1 |
| 14. Response to name* | 5.2 | 0.4 | <0.001 | 11.6 |
| 15. Follow point* | 22.5 | 3.5 | <0.001 | 6.5 |
| 16. Walk | 17.9 | 2.4 | <0.001 | 7.5 |
| 17. Follow gaze | 11.6 | 2.0 | <0.001 | 5.9 |
| 18. Unusual finger movements | 14.5 | 7.8 | 0.003 | 1.8 |
| 19. Attract attention to activity | 12.7 | 3.1 | <0.001 | 4.1 |
| 20. Appear deaf | 4.6 | 1.3 | 0.002 | 3.5 |
| 21. Understand what others say | 9.8 | 1.1 | <0.001 | 8.9 |
| 22. Stare at nothing | 24.9 | 13.4 | <0.001 | 1.9 |
| 23. Check parent's reaction | 15.0 | 10.8 | 0.080 | 1.4 |
The six critical items
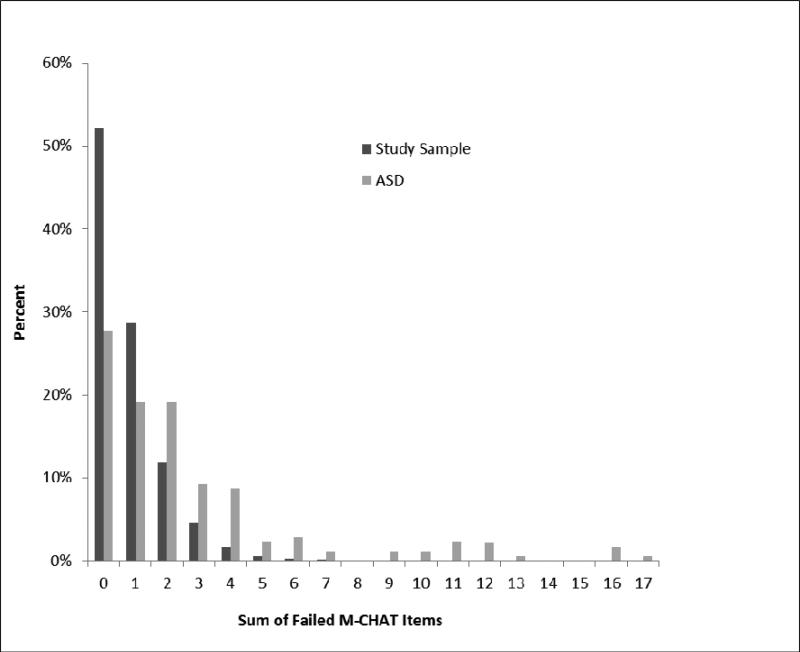
The respective distributions of total numbers of failed M-CHAT items in the study sample and for children with ASD are presented in Figure 2.
Figure 2.
Sum of failed M-CHAT items in the total study sample and for children with ASD
Comment
The results of the current study suggest that screening with M-CHAT alone in a general population at 18 months is not effective in identifying the majority of children who ultimately receive a diagnosis of ASD. A very high proportion of the children in the ASD group scored below cut-off on M-CHAT at 18 months. The question is whether signs of ASD had not yet appeared in these children at 18 months, whether the screening instrument was not sensitive enough to pick up the signs, or whether parents had not been able to recognize and report signs of ASD at this age. Three issues need to be addressed: (1) a child's age at the time of screening, (2) the concept of ‘developmental disorder’, and (3) the ability of parents to recognize signs of ASD.
At 18 months of age, when parents respond to the M-CHAT items on the MoBa questionnaire, many of the skills that are used to discriminate between children with ASD and children with typical development are still emerging. It is likely that this is a time when the behavioural signs are not yet clear for all children who later receive an ASD diagnosis. Baird et al note that in their general population sample screened with CHAT, only 19 of 50 children who later received a childhood autism diagnosis were identified at 18 months.9 With respect to the M-CHAT, Pandey et al found better identification at 24 months than at 18 months,12 and Nygren et al20 found good identification at 30 months.
ASD is a developmental disorder, which implies that the signs manifest themselves only when the child has reached an age when certain behaviours are expected to be present. The signs will therefore become increasingly evident with increasing age, and might not be fully manifest or recognizable at 18 months in all children with ASD, particularly in children who have IQ and language skills within the normal range. Bolton et al21 found that differences in skill development became more widespread and pronounced as development progressed in children with ASD. Surén et al19 found increasing prevalence rates of ASD with increasing age in a study of children registered in the Norwegian Patient Registry. More children with normal IQs and less severe symptoms have received ASD diagnoses in recent years.22 It is likely that the clinical characteristics of children with ASD, such as IQ and symptom severity, will influence the proportion of children who have clear signs of ASD at an early age and who are identified through screening. This may explain some of the differences in results between clinical and population-based samples.
Some studies have found discrepancies between clinical observations and parental report of signs in children with ASD under the age of two years, and that parents are less likely than clinicians to detect some of the more subtle aspects of abnormal social and communicative behaviours.23,24 The wording of questions might also affect parental report, as parents might report more accurately if they have multiple choices based on concrete behaviours rather than generally-phrased yes-no questions.25 Other studies indicate that early signs detected by parents are based more on signs of general developmental delay than on signs specific to ASD,1,26 and that M-CHAT might more accurately identify children with ASD who have lower intellectual and adaptive functioning and miss children with stronger general development.27-29
Studies using M-CHAT in clinical samples have found some overlap of behavioural signs in ASD and other developmental disorders.30,31 In our study sample, as many as 7.3% (3804) of the children in the non-ASD group scored above cut-off on the M-CHAT 23-item criterion at 18 months. Greater specificity in the screening instrument would enhance the efficiency of the diagnostic process and lead to more appropriate allocation of oversubscribed clinical resources. Although the specificity was above 90% for both pre-defined M-CHAT screening criteria in the current study, the proportion of children who scored above cut-off on M-CHAT who actually have ASD is low. The PPV is highly dependent on the prevalence rate of the condition in the study sample, and a test that has good PPV in a clinical sample may have poor PPV in a population-based sample.32 Two recent studies, which investigated the use of M-CHAT at the 18-months health check-up in the United States and Japan, respectively, emphasize the importance of using a two-stage screening procedure whereby the M-CHAT is followed by a telephone interview assessment to avoid a large number of false positives.29,33
Strengths and Limitations
The principal strengths of this study are the population-based design, the prospective data collection, and the combination of screening, referrals, and registry linkage to identify ASD cases in the study sample. In contrast to most clinical studies, both children with mild and severe ASD symptoms are included. Information about signs of ASD was collected prospectively, before the diagnoses were made. The diagnoses were determined when the children were three years or older, an age when ASD diagnoses are reported to be fairly stable.34 Large population-based cohorts are challenged by modest participation rates, losses to follow-up, and selection bias. Although the ABC Study does not escape these problems, the participation rate is relatively high, and its basis in MoBa combined with linkage to nationwide registries are comparative advantages.16 Losses to follow-up and cases that are not picked up by the screening instruments are likely to be picked up at a later stage through the Norwegian Patient Registry.
The main limitation of the current study is the fact that ascertainment of ASD cases in the cohort is not yet complete. However, this is likely to have led to an overestimation rather than an underestimation of sensitivity, specificity, and PPV. We expect that the ASD cases identified in the future will on average be higher functioning and have less severe signs than the children who are identified to date, and therefore, that they are less likely to have scored above cut-off on the M-CHAT at 18 months.
The authors of the M-CHAT recommend that a telephone interview should always be conducted to follow up the parent-report questionnaire. However, not all policy papers or previous studies recommended or administered the follow-up interview when examining the properties of the M-CHAT.35 In a large population-based study like MoBa, the administration of a follow-up interview is infeasible. It is also important to note that the use of a follow-up interview might reduce the number of false positives and increase the specificity of the instrument, but it could not reduce the false negatives if it only followed positive screens. The Norwegian translation of the M-CHAT has never previously been used in large-scale studies in Norway, but it is no reason to think that Norwegian parents will interpret the items differently than parents in other countries in Europe and North America. The M-CHAT items are embedded in a larger questionnaire that focuses on child development and behaviour at 18 months. The M-CHAT items are presented together in a separate section of the questionnaire. In our opinion, rather than interfering with the completion of the M-CHAT items, it is likely that the questions leading up to the M-CHAT items helped the parents to tune in to their child's behaviour. We chose to include only participants who completed all 23 M-CHAT items in the analyses presented here. However, we also conducted the same analyses on the whole sample, including the participants who had one or more items missing, and the results were similar. The percentages of children with missing items were similar in the ASD group (5.5%, n=10) and in the non-ASD group (6.8%, n=3770).
Finally, we have no way to assess the impact of social and cultural factors, such as parental awareness of early developmental markers associated with ASD, on results presented here. Some studies have found an association between age of identification of ASD and community and family characteristics such as access to services,36,37 parents’ socio-economic status,38 and ethnic background.39,40 Compared to some other countries, Norway has relatively small differences in socioeconomic status and ethnicity in the population. Although many parts of the country are rural, the health- and welfare-system is well-developed. The majority of children attend a public child care center from the age of 1 or 2 years. Most children with developmental or behavior problems are referred for assessment at a specialist clinic, but a diagnosis is not essential in order to receive intervention in child care centers and schools, which differs from the United States. It is unclear whether this affects the number of children who receive an ASD diagnosis at different ages or the time interval from apparent signs of ASD to diagnosis. The reported prevalence rate of ASD in older children in Norway19 is comparable to the United Kingdom41 and the United States42.
Future research should explore what characterizes children with and without signs of ASD reported by parents at 18 months, and the association between early signs and later functional outcomes such as symptom severity and cognitive functioning. Future research should also address whether timing and composition of screening instruments can be optimized for maximal public health impact.
Acknowledgments
The authors would like to acknowledge and thank all participating families. The Norwegian Mother and Child Cohort Study is funded by the Norwegian Ministry of Health and Care Services, the Norwegian Ministry of Education and Research, the Research Council of Norway / FUGE (grant no. 151918), the National Institute of Neurological Disorders and Stroke (NIH/NINDS), Bethesda, MD, USA (grant no.NS47537), and the National Institute of Environmental Health Sciences (NIH/NIEHS), Research Triangle Park, NC, USA (contract no.NO-ES-75558). The Autism Birth Cohort study is funded by the National Institute of Neurological Disorders and Stroke, (NIH/NINDS), Bethesda, MD, USA (grant no.NS47537). The present study was supported by a grant from the Norwegian Research Council (grant no.196452).
Reference list
- 1.De Giacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. European Child & Adolescent Psychiatry. 1998;7(3):131–136. doi: 10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- 2.Werner E, Dawson G, Munson J, Osterling J. Variation in early developmental course in autism and its relation with behavioral outcome at 3-4 years of age. Journal of Autism and Developmental Disorders. 2005;35(3):337–350. doi: 10.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- 3.Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- 4.Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9-12 months of age. Journal of Autism and Developmental Disorders. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- 5.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2-3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37(1):12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- 7.Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120(5):1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 9.Baird G, Charman T, Baron-Cohen S, Cox A, Swettenham J, Wheelwright S, et al. A screening instrument for autism at 18 months of age: A 6-year follow-up study. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(6):694–702. doi: 10.1097/00004583-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2001;31(2):131–144. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman JM, Robins DL, Ventola PE, Pandey J, Boorstein HC, Esser EL, et al. The Modified Checklist for Autism in Toddlers: A follow-up study investigating the early detection of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(5):827–839. doi: 10.1007/s10803-007-0450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey J, Verbalis A, Robins DL, Boorstein H, Klin AM, Babitz T, et al. Screening for autism in older and younger toddlers with the Modified Checklist for Autism in Toddlers. Autism: The International Journal of Research and Practice. 2008;12(5):513–535. doi: 10.1177/1362361308094503. [DOI] [PubMed] [Google Scholar]
- 13.Williams J, Brayne C. Screening for autism spectrum disorders: What is the evidence? Autism: The International Journal of Research and Practice. 2006;10(1):11–35. doi: 10.1177/1362361306057876. [DOI] [PubMed] [Google Scholar]
- 14.Al-Qabandi M, Gorter JW, Rosenbaum P. Early autism detection: Are we ready for routine screening? Pediatrics. 2011;128(1):e211–217. doi: 10.1542/peds.2010-1881. [DOI] [PubMed] [Google Scholar]
- 15.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). International Journal of Epidemiology. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 16.Stoltenberg C, Schjølberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, et al. The Autism Birth Cohort: A paradigm for gene-environment-timing research. Molecular Psychiatry. 2010;15(7):676–680. doi: 10.1038/mp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 18.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule - Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 19.Surén P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, et al. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics. 2012;130(1):e152–158. doi: 10.1542/peds.2011-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nygren G, Sandberg E, Gillstedt F, Ekeroth G, Arvidsson T, Gillberg C. A new screening programme for autism in a general population of Swedish toddlers. Research in Developmental Disabilities. 2012;33(4):1200–1210. doi: 10.1016/j.ridd.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Bolton PF, Golding J, Emond A, Steer CD. Autism spectrum disorder and autistic traits in the Avon Longitudinal Study of Parents and Children: Precursors and early signs. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(3):249–260. doi: 10.1016/j.jaac.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Keyes KM, Susser E, Cheslack-Postava K, Fountain C, Liu K, Bearman PS. Cohort effects explain the increase in autism diagnosis among children born from 1992 to 2003 in California. International Journal of Epidemiology 2012. 41(2):495–503. doi: 10.1093/ije/dyr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone WL, Hoffman EL, Lewis SE, Ousley OY. Early recognition of autism. Parental reports vs. clinical observation. Archives of Pediatrics & Adolescent Medicine. 1994;148(2):174–179. doi: 10.1001/archpedi.1994.02170020060010. [DOI] [PubMed] [Google Scholar]
- 24.Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Rowberry JP, Macari S, Campbell D, Chawarska K. The first year inventory: Comparing parent report and clinical observation in high and low risk for ASD infants at 12 months.. Poster presented at: 12th International Meeting for Autism Research (IMFAR); Toronto, Canada. May 17-19, 2012. [Google Scholar]
- 26.Baghdadli A, Picot MC, Pascal C, Pry R, Aussilloux C. Relationship between age of recognition of first disturbances and severity in young children with autism. European Child & Adolescent Psychiatry. 2003;12(3):122–127. doi: 10.1007/s00787-003-0314-6. [DOI] [PubMed] [Google Scholar]
- 27.Eaves LC, Wingert H, Ho HH. Screening for autism: Agreement with diagnosis. Autism: The International Journal of Research and Practice. 2006;10(3):229–242. doi: 10.1177/1362361306063288. [DOI] [PubMed] [Google Scholar]
- 28.Snow AV, Lecavalier L. Sensitivity and specificity of the Modified Checklist for Autism in Toddlers and the Social Communication Questionnaire in preschoolers suspected of having pervasive developmental disorders. Autism: The International Journal of Research and Practice. 2008;12(6):627–644. doi: 10.1177/1362361308097116. [DOI] [PubMed] [Google Scholar]
- 29.Kamio Y, Inada N, Koyama T, Inokuchi E, Tsuchiya K, Kuroda M. Effectiveness of using the Modified Checklist for Autism in Toddlers in two-stage screening of autism spectrum disorder at the 18-month health check-up in Japan. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1864-1. doi: 10.1007/s10803-013-1864-1. [DOI] [PubMed] [Google Scholar]
- 30.Ventola P, Kleinman J, Pandey J, Wilson L, Esser E, Boorstein H, et al. Differentiating between autism spectrum disorders and other developmental disabilities in children who failed a screening instrument for ASD. Journal of Autism and Developmental Disorders. 2007;37(3):425–436. doi: 10.1007/s10803-006-0177-z. [DOI] [PubMed] [Google Scholar]
- 31.Canal-Bedia R, García-Primo P, Martín-Cilleros MV, Santos-Borbujo J, Guisuraga-Fernández Z, Herráez-García L, et al. Modified Checklist for Autism in Toddlers: Cross-cultural adaption and validation in Spain. Journal of Autism and Developmental Disorders. 2011;41(10):1342–1351. doi: 10.1007/s10803-010-1163-z. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ. Epidemiology: An introduction. Oxford University Press Inc; New York, NY: 2002. [Google Scholar]
- 33.Chlebowski C, Robins DL, Barton ML, Fein D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics. 2013;131(4):e1121–1127. doi: 10.1542/peds.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 35.Yama B, Freeman T, Graves E, Yuan S, Campbell MK. Examination of the properties of the Modified Checklist for Autism in Toddlers (M-CHAT) in a population sample. Journal of Autism and Developmental Disorders. 2012;42(1):23–34. doi: 10.1007/s10803-011-1211-3. [DOI] [PubMed] [Google Scholar]
- 36.Daniels AM, Mandell DS. Children's compliance with American Academy of Pediatrics’ well-child care visit guidelines and the early detection of autism. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1831-x. doi:10.1007/s10803-013-1831-x. [DOI] [PubMed] [Google Scholar]
- 37.Lauritsen MB, Astrup A, Pedersen CB, Obel C, Schendel DE, Schieve L, et al. Urbanicity and autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1875-y. doi:10.1007/s10803-013-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fountain C, King MD, Bearman PS. Age of diagnosis for autism: Individual and community factors across 10 birth cohorts. Journal of Epidemiology & Community Health. 2011;65(6):503–510. doi: 10.1136/jech.2009.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandell DS, Listerud J, Levy SE, Pinto-Martin JA. Race differences in the age at diagnosis among Medicaid-eligible children with autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(12):1447–1453. doi: 10.1097/00004583-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Tek S, Landa RJ. Differences in autism symptoms between minority and non-minority toddlers. Journal of Autism and Developmental Disorders. 2012;42(9):1967–1973. doi: 10.1007/s10803-012-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The Special Needs and Autism Project (SNAP). Lancet. 2006;368(9531):210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- 42.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorders – Autism and Developmental Disabilities Monitoring Network, United States, 2008. MMWR Surveillance Summary. 2012;61(3):1–19. [PubMed] [Google Scholar]