Abstract
Purpose
It was recently reported that residential altitude is inversely associated with all-cause mortality among incident dialysis patients; however, no adjustment was made for key case-mix and laboratory variables. We re-examined this question in a contemporary patient database with comprehensive clinical and laboratory data.
Methods
In a contemporary 8-year cohort of 144,892 maintenance dialysis patients from a large dialysis organization, we examined the relationship between residential altitude and all-cause mortality. Using data from the U.S. Geological Survey (USGS), the average residential altitudes per approximately 43,000 U.S. zip codes were compiled and linked to the residential zip codes of each patient.
Results
Mortality risks for these patients were estimated by Cox proportional hazard ratios (HR). The study population's mean±SD age was 61±15 years, and patients included 45% women and 57% diabetics. In fully-adjusted analysis, those residing in the highest altitude strata (≥6000 ft) had a lower all-cause mortality risk in fully-adjusted analyses: death HR: 0.92 (95% CI, 0.86–0.99), as compared to patients in the reference group (<250 ft).
Conclusions
Residential altitude is inversely associated in all-cause mortality risk in maintenance dialysis patients notwithstanding the unknown and unmeasured confounders.
Keywords: Altitude, hypoxia, dialysis, mortality, environment
Introduction
It is known that living at higher altitudes can induce hypoxia related events. Because the kidney is a key mediator of hypoxia-induced erythropoiesis,1 dialysis patients may be especially susceptible to anemia under hypoxic conditions. Only one study has examined the relationship between altitude and all-cause mortality in dialysis patients. This study, which used 1995–2004 patient follow-up data from the United States Renal Data Survey (USRDS), reported an inverse association between residential altitude and all-cause mortality in incident dialysis patients.2 Relative to patients living at <76 m (250 ft), a 15% reduction in mortality risk was seen in patients residing in the highest altitudinal strata (≥6000 ft). The apparent reduction in mortality risk in ESRD patients at high altitudes was attributed largely to the induction of hypoxia-induced factors, which may induce more effective erythropoiesis3 and regulate enzymes associated with cardiovascular risk.4–6
A recognized limitation of this study, however, was the unavailability of several baseline case-mix and laboratory variables that may strongly confound the altitude-mortality relationship in maintenance dialysis patients. For instance, the analyses did not account for markers of chronic inflammation or protein-energy malnutrition (PEM), which may attenuate erythropoiesis7–10 and also diminish anemia responsiveness to EPO administration.11, 12 Moreover, information on important modifiers of survival in dialysis patients, such as access modality, was unavailable.
This study, with detailed information on patient malnutrition-inflammation complex syndrome (MICS) status, as well as previously unavailable case-mix and laboratory variables, will re-examine the altitude-mortality relationship in a contemporary cohort of dialysis patients followed over 8 years (2001–2009). We hypothesize that higher residential altitudes are associated with significantly different mortality risk among maintenance dialysis patients.
Methods
Human Subjects and Data
All individuals with Stage 5 CKD who underwent dialysis treatment in one of the outpatient dialysis facilities of a US based dialysis organization, i.e., DaVita, were eligible for entry into the cohort from July 1, 2001 to June 30, 2006 and were followed for the outcome of interest until June 30, 2009 for a total of 32 consecutive calendar quarters. The creation and analyses of this non-concurrent, dynamic cohort of dialysis patients have been described previously.13, 14 To minimize measurement variability and to address the effect of short-term variation in dietary and fluid intake on weight or laboratory measurements, we averaged all repeated measures for each patient during any given calendar quarter, i.e., over 13 consecutive weeks or 3 months. The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research. The requirement for a written consent form was waived because of the large number and anonymity of the patients studied, and the non-intrusive nature of the research.
Dialysis Treatment
Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the first day that the patient entered the cohort. The first (baseline) study quarter for each patient was the calendar quarter in which patient's vintage was >45 days during at least half of the time of that quarter. The administered dialysis dose was measured by single-pooled Kt/V using urea kinetic modeling equations that are described elsewhere.15
Laboratory Values
Most blood samples were collected pre-dialysis with the exception of the post-dialysis serum urea nitrogen, which was obtained to calculate urea kinetics. Blood samples were drawn using uniform techniques in all dialysis clinics and were transported within 24 hours to a single laboratory center (DaVita Laboratory in Deland, Florida), where the laboratory values were measured by automated and standardized methods. Most laboratory values were measured monthly, including serum creatinine, urea, albumin, calcium, phosphorus, bicarbonate, alkaline phosphatase, and total iron-binding capacity (TIBC). Serum ferritin and intact parathyroid hormone (PTH) were measured at least quarterly. Hemoglobin was measured weekly to bi-weekly in most patients.
Integration of Altitude Data
Using data from the U.S. Geological Survey (USGS), the average residential altitudes of approximately 43,000 U.S. zip codes were compiled and linked to the residential zip codes of each DaVita patient.
Statistical Methods
Two analytical methods were used to assess the relationship between residential altitude and all-cause mortality: 1) categorical hazard analyses, as described in our prior studies,2, 3 using residential altitude stratified into five groups: <250 (reference), 250–<2000, 2000–<4000, 4000–<6000, and ≥6000 ft and 2) proportional-hazards regression models with restricted cubic splines using continuous measurements of residential altitude, as used in similar reports.14, 16 In the spline analyses, we used two degrees of freedom to generate one knot at 267 ft, corresponding to our second lowest altitudinal strata (250–<2000).
For each analysis, three models were examined based on the level of multivariate adjustment: (a) an unadjusted model that included mortality as the outcome and entry calendar quarter (q1 through q20) as covariates; (b) a case-mix adjusted model that included the above plus age, sex, dialysis modality (hemodialysis vs. peritoneal), race/ethnicity (African-Americans, non-Hispanic Caucasians, Asians, Hispanics, and Other), diabetes mellitus, dialysis vintage, mode of access (arteriovenous fistula, graft, catheter, or other), primary insurance, marital status, dialysis dose (single pool kt/v), erythropoietin dose, and the following comorbidities: alcohol dependence, current smoker, atherosclerotic heart disease, congestive heart failure, chronic obstructive pulmonary disorder, cerebrovascular disease, peripheral vascular disease, and cancer; and (c) a MICS adjusted model which included all of the covariates in the case-mix model as well as serum albumin, calcium, bicarbonate, creatinine, ferritin, hemoglobin, lymphocyte, normalized protein nitrogen appearance, phosphorus, white blood cell count, alkaline phosphatase, parathyroid hormone, and body mass index.
We assessed variations in patient characteristics according to altitudinal strata by means of a nonparametric test for trend across ordered groups. We also compared characteristics of the highest altitude stratum (≥6000 ft) with those the reference group (<250 ft) with a t-test for unequal variances (Table 1). With respect to our sample, patients who were transplanted or left DaVita clinics were censored at the time of the event. We performed simple imputation for variable data that was unavailable, though missing data was less than 1% for most laboratory and demographic variables. All analyses were carried out using Stata version 10.1 (Stata Corporation, College Station, Texas).
Table 1.
Baseline characteristics stratified by residential altitude (feet)
| Variable | Total | <250 | 250–<2000 | 2000–<4000 | 4000–<6000 | 6000+ | Trend | 6000+ vs. <250 |
|---|---|---|---|---|---|---|---|---|
| n=144,892 | (n=61,253) | (n= 71,403) | (n= 5,804) | (n= 5,094) | (n= 1,338) | P* | P† | |
| Age (years) | 61±15 | 61±15 | 61±16 | 60±15 | 60±15 | 59±16 | <0.001 | <0.001 |
| Gender (% female) | 45 | 45 | 46 | 45 | 44 | 45 | 0.896 | 0.879 |
| Diabetes mellitus (%) | 57 | 55 | 57 | 60 | 60 | 57 | <0.001 | 0.180 |
| Dialysis Modality (%) | ||||||||
| Hemodialysis | 93 | 94 | 93 | 85 | 93 | 88 | <0.001 | <0.001 |
| Peritoneal Dialysis | 7 | 6 | 7 | 15 | 7 | 12 | <0.001 | <0.001 |
| Race and Ethnicity (%) | ||||||||
| White | 44 | 40 | 47 | 46 | 54 | 56 | <0.001 | <0.001 |
| Black | 31 | 37 | 29 | 12 | 9 | 8 | <0.001 | <0.001 |
| Hispanic | 14 | 12 | 14 | 34 | 15 | 9 | <0.001 | 0.007 |
| Asian | 3 | 4 | 3 | 1 | 2 | 1 | <0.001 | <0.001 |
| Other | 7 | 6 | 6 | 6 | 18 | 25 | <0.001 | <0.001 |
| Vintage (%): | ||||||||
| <6 months | 13 | 12 | 13 | 12 | 11 | 13 | 0.791 | 0.811 |
| 6–<24 months | 31 | 30 | 31 | 31 | 31 | 33 | =0.001 | 0.033 |
| 2–<5 years | 35 | 35 | 35 | 36 | 35 | 34 | 0.403 | 0.317 |
| >=5 years | 22 | 22 | 22 | 21 | 22 | 21 | 0.003 | 0.136 |
| Primary Insurance (%) | ||||||||
| Medicare | 62 | 61 | 64 | 68 | 51 | 61 | 0.495 | 0.600 |
| Medicaid | 5 | 4 | 6 | 4 | 3 | 3 | <0.001 | 0.270 |
| Private Insurance | 9 | 12 | 7 | 5 | 17 | 13 | <0.001 | 0.203 |
| Other | 15 | 16 | 14 | 12 | 19 | 14 | 0.047 | 0.064 |
| Marital Status (%) | ||||||||
| Married | 41 | 40 | 41 | 45 | 43 | 46 | <0.001 | <0.001 |
| Divorced | 7 | 6 | 7 | 8 | 6 | 6 | <0.001 | 0.495 |
| Single | 23 | 24 | 22 | 21 | 20 | 20 | <0.001 | 0.002 |
| Widowed | 12 | 12 | 33 | 11 | 10 | 11 | 0.170 | 0.104 |
| Catheter Access (%) | 42 | 41 | 43 | 49 | 37 | 40 | 0.724 | 0.004 |
| Kt/V (dialysis dose) | 1.52±0.36 | 1.52±0.36 | 1.52±0.36 | 1.55±0.34 | 1.57±0.34 | 1.57±0.34 | <0.001 | <0.001 |
| Residual Renal Function (ml/min) | 2.95±2.64 | 2.89±2.49 | 2.98±2.76 | 3.55±3.13 | 3.15±2.79 | 2.97±2.45 | 0.133 | 0.813 |
| Comorbidities (%) | ||||||||
| AHD | 21 | 21 | 21 | 19 | 21 | 22 | 0.660 | 0.462 |
| CHF | 27 | 26 | 27 | 23 | 23 | 24 | <0.001 | 0.144 |
| COPD | 5 | 5 | 6 | 6 | 5 | 7 | <0.001 | 0.002 |
| CVD | 7 | 7 | 7 | 7 | 7 | 7 | 0.327 | 0.991 |
| PVD | 11 | 11 | 11 | 10 | 13 | 17 | <0.001 | <0.001 |
| Non-ambulatory State | 3 | 3 | 3 | 2 | 2 | 1 | 0.161 | <0.001 |
| Malignancy | 4 | 4 | 4 | 5 | 4 | 6 | 0.453 | 0.017 |
| Alcohol Dependence | 1 | 1 | 1 | 1 | 1 | 2 | <0.001 | 0.078 |
| Drug Use | 1 | 1 | 1 | 1 | <1 | <1 | <0.001 | <0.001 |
| Current Smoker | 5 | 4 | 5 | 6 | 5 | 6 | <0.001 | 0.003 |
| Serum or Blood Levels | ||||||||
| ferritin (ng/mL) | 503±486 | 502±483 | 513±496 | 500±436 | 388±421 | 491±484 | <0.001 | 0.425 |
| hemoglobin (g/dL) | 12.0±1.4 | 12.0±1.4 | 12.0±1.4 | 12.2±1.4 | 12.3±1.4 | 12.4±1.4 | <0.001 | <0.001 |
| TIBC (mg/dL) | 211±47 | 209±49 | 210±47 | 217±46 | 222±49 | 222±51 | <0.001 | 0.009 |
| albumin (g/dL) | 3.66±0.47 | 3.67±0.47 | 3.65±0.47 | 3.67±0.46 | 3.70±0.44 | 3.67±0.47 | 0.020 | 0.631 |
| calcium (mg/dL) | 9.2±0.7 | 9.2±0.7 | 9.2±0.7 | 9.2±0.7 | 9.1±0.7 | 9.2±0.8 | <0.001 | 0.006 |
| intact PTH (pg/ml) | 348±366 | 358±372 | 346±367 | 308±356 | 310±299 | 334±339 | <0.001 | 0.036 |
| phosphorus (mg/dL) | 5.6±1.5 | 5.6±1.5 | 5.6±1.5 | 5.5±1.5 | 5.3±1.4 | 5.4±1.4 | <0.001 | <0.001 |
| creatinine (mg/dL) | 8.1±3.4 | 8.3±3.4 | 8.0±3.4 | 7.6±3.1 | 7.5±2.9 | 7.5±3.1 | <0.001 | <0.001 |
| nPNA (g/kg/day) | 0.95±0.26 | 0.97±0.26 | 0.94±0.25 | 0.94±0.25 | 0.96±0.24 | 0.94±0.24 | <0.001 | 0.002 |
| BMI (kg/m2) | 26.9±7.0 | 26.8±7.0 | 27.0±7.1 | 26.6±6.5 | 26.4±6.6 | 26.1±5.9 | 0.578 | <0.001 |
| WBC (×103/ul) | 7.5±2.5 | 7.4±2.5 | 7.5±2.6 | 7.5±2.4 | 7.4±2.4 | 7.6±2.9 | <0.001 | 0.009 |
| lymphocyte (% WBC) | 20±8 | 21±8 | 20±8 | 21±8 | 20±8 | 20±8 | <0.001 | 0.017 |
| bicarbonate (mg/dL) | 22.6±3.1 | 22.5±3.2 | 22.7±3.1 | 22.3±3.2 | 21.4±2.9 | 22.0±3.0 | <0.001 | <0.001 |
Values are in percentage or mean ± SD, as appropriate.
Trend p-values were calculated using a nonparametric test for trend across ordered groups.
Comparisons between highest (≥6000 ft) and reference (<250 ft) strata were calculated using an unequal variance t-test.
Abbreviations: AHD: atherosclerotic heart disease, CHF: congestive heart failure, COPD: chronic obstructive pulmonary disease, CVD: cerebrovascular disease, PVD: peripheral vascular disease, TIBC: total iron binding capacity, nPNA: normalized protein nitrogen appearance, BMI: Body Mass Index, WBC: white blood cell count.
Results
The national cohort of the dialysis patients included 164,789 adult subjects. After excluding patients who had missing ages or were <18 years of age (n=13,900); those who did not maintain at least 45 days of thrice-weekly dialysis treatment during the base calendar quarter (n=3,695); and those with a missing zip code linked residential altitude (n=2,302), our final sample was comprised of 144,892 dialysis patients with a median follow-up time of 801 days. Table 1 shows the relevant demographic, clinical and laboratory data of these patients according to 5 altitude categories. Approximately 50% of patients resided below 250 ft, whereas 10% of patients lived at 1250 ft or above, and less than 1% at 6000 ft or above. Patients in the highest altitudinal strata were more likely to be white, married, on peritoneal dialysis and receive catheter dialysis access, as compared to patients in the lowest altitudinal strata (<250 ft). These patients also had comparably higher hemoglobin and creatinine levels, but lower parathyroid hormone (PTH) levels.
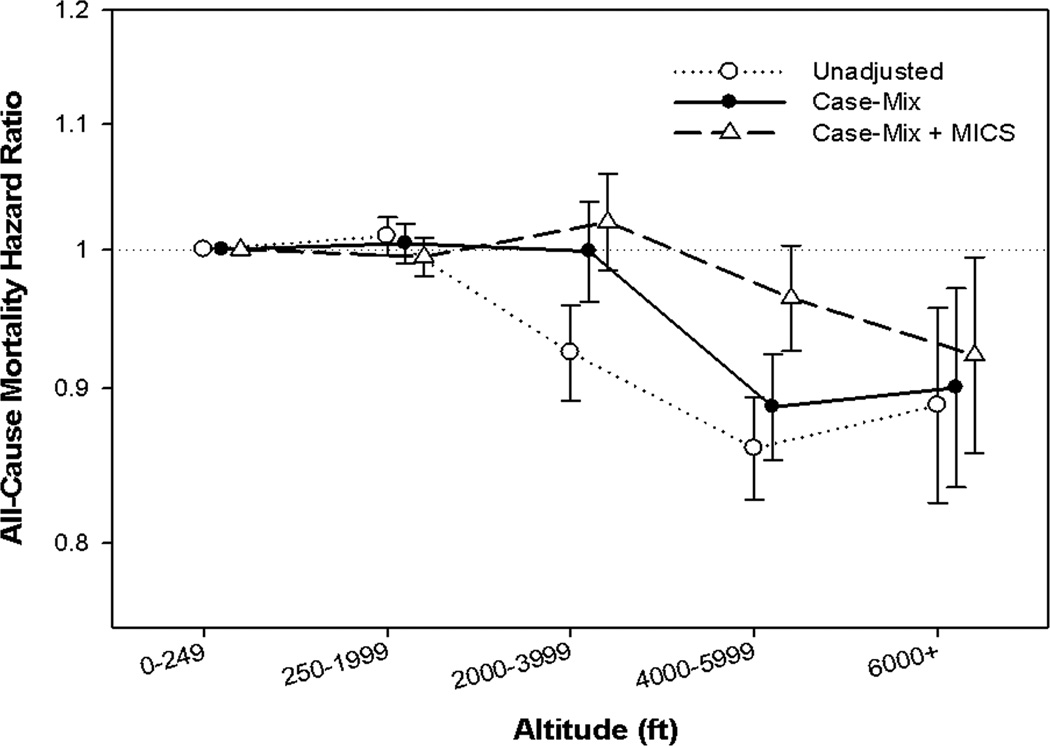
In Figure 1 and Table 2, we assessed the association between 5 categories of residential altitude and 8 year all-cause mortality across 3 levels of adjustment. In case-mix adjusted models, a protective effect was only observed in patients living at 4000 ft or greater, with patients living in the highest altitudinal stratum (≥6000 ft) having a 10% lower mortality risk relative to patients residing below 250 ft. Upon further adjustment for markers of malnutrition and inflammation, there was only a minimal increase in the relative mortality risk for patients living at ≥6000 ft (HR: 0.92 (95% CI, 0.86–0.99)); however, there was no longer a significant protective effect for patients living between 4000–<6000 ft.
Figure 1.
Association with residential altitude stratified into 5 levels (<250, 250–<2000, 2000–<4000, 4000–<6000, and ≥6000) with all-cause mortality in 144,892 dialysis patients.
Footnote: The Y-axis shows the logarithm of the hazard ratio of all-cause mortality over 8 years in. unadjusted, case-mix adjusted, and case-mix + MICS adjusted models. The unadjusted model adjusts only for calendar quarter covariate. The case-mix model adjusts additionally for age, sex, dialysis modality, race, diabetes, dialysis vintage, insurance, marital status, dialysis dose, erythropoietin-α dose, and the following comorbidities: alcohol dependence, smoking, atherosclerotic heart disease, congestive heart failure, chronic obstructive pulmonary disorder, cerebrovascular disease, peripheral vascular disease, and cancer. The case-mix + MICS model adjusts further for albumin, calcium, bicarbonate, creatinine, ferritin, hemoglobin, lymphocyte, normalized protein nitrogen appearance, phosphorus, white blood cell count, alkaline phosphatase, parathyroid hormone, and body mass index. The reference group is dialysis patients residing below 250 feet.
Table 2.
Hazard ratios for all-cause mortality across 5 altitude categories
| Altitude Categories (feet) |
Unadjusted* (n=144,892) |
Case-mix adjusted† (n=144,892) |
Fully adjusted†† (n=144,892) |
|||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | |
| <250 | 61,253 | 1.00 | 61,253 | 1.00 | 61,253 | 1.00 |
| 250–<2000 | 71,403 | 1.01 (1.00–1.02) | 71,403 | 1.00 (0.99–1.02) | 71,403 | 0.99 (0.98–1.01) |
| 2000–<4000 | 5,804 | 0.92 (0.89–0.96) | 5,804 | 1.00 (0.96–1.04) | 5,804 | 1.02 (0.98–1.06) |
| 4000–<6000 | 5,094 | 0.86 (0.83–0.89) | 5,094 | 0.89 (0.85–0.92) | 5,094 | 0.96 (0.93–1.00) |
| 6000+ | 1,338 | 0.89 (0.82–0.96) | 1,338 | 0.90 (0.83–0.97) | 1,338 | 0.92 (0.86–0.99) |
Includes adjustment for entry calendar quarter covariate
Adjusted for age, sex, dialysis modality, race, diabetes, dialysis vintage, insurance, marital status, dialysis dose, erythropoietin-α dose, and comorbidities
Adjusted for case-mix characteristics plus 13 malnutrition-inflammation complex syndrome (MICS) variables
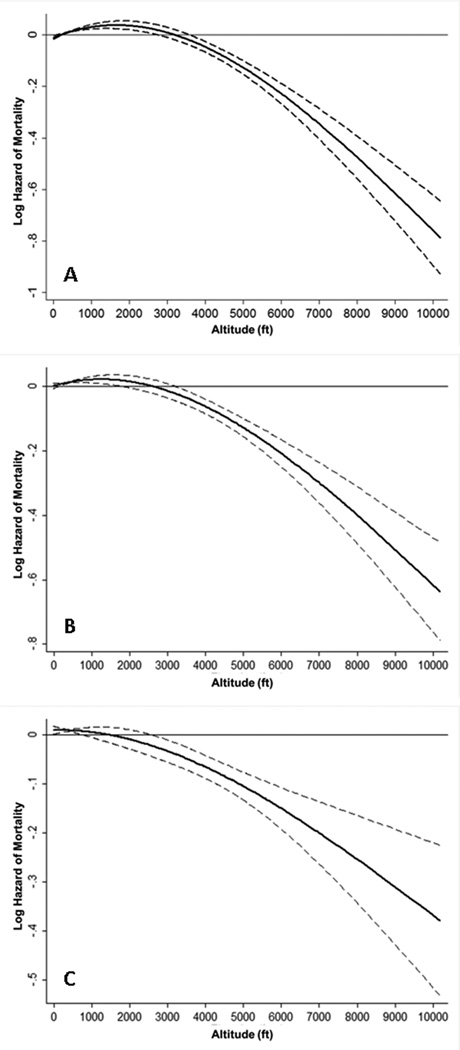
Figure 2 displays the association between continuous residential altitude and all-cause mortality using restricted cubic spline models. In our unadjusted (Figure 2A) model, we found a modest rise in mortality risk as residential altitude reached 2000 ft. Beyond this point, mortality risk decreased substantially as altitude increased. After case-mix adjustment (Figure 2B), the rise in mortality risk was only seen up to an altitude of 750 ft; thereafter, mortality was also inversely associated with altitude. However, as noted with the decrease in slope, the association of mortality with rising altitude was modestly attenuated after case-mix adjustment. After further adjustment for MICS, (Figure 2C), an inverse trend for mortality was observed throughout all levels of residential altitude, but was further attenuated compared to the previous models and was only statistically significant between 2500 ft and 7500 ft.
Figure 2.
Association of average residential altitude with mortality in 144,892 dialysis patients over 8 years (7/2001–6/2009). Figure 2A represents unadjusted analyses, while figures 2B and 2C represent case-mix adjusted analyses, and case-mix + MICS adjusted analyses, respectively.
Footnote: The Y-axis shows the logarithm of the hazard ratio of all-cause mortality over 8 years in. unadjusted, case-mix adjusted, and case-mix + MICS adjusted models. The unadjusted model adjusts only for calendar quarter covariate. The case-mix model adjusts additionally for age, sex, dialysis modality, race, diabetes, dialysis vintage, insurance, marital status, dialysis dose, erythropoietin-α dose, and the following comorbidities: alcohol dependence, smoking, atherosclerotic heart disease, congestive heart failure, chronic obstructive pulmonary disorder, cerebrovascular disease, peripheral vascular disease, and cancer. The case-mix + MICS model adjusts further for albumin, calcium, bicarbonate, creatinine, ferritin, hemoglobin, lymphocyte, normalized protein nitrogen appearance, phosphorus, white blood cell count, alkaline phosphatase, parathyroid hormone, and body mass index. Dashed lines represent 95% pointwise confidence bands.
Discussion
In a large cohort of dialysis patients within a U.S. based dialysis organization followed for up to 8 years, we found that high residential altitude (≥6000 ft) is associated with a significant reduction in all-cause mortality risk in dialysis patients. These results support previous findings by Winkelmayer et. al. (HR: 0.85, 95% CI, 0.79–0.92 in dialysis patients ≥6000 ft relative to <250 ft)2, though the effect size we observed was smaller in comparison.
Notably, our fully-adjusted restricted cubic spline analysis (Figure 2c) depicted a fairly different visualization of the altitude-mortality relationship as compared to the fully-adjusted categorical hazard analyses (Figure 1). The spline model indicated statistically significant reductions in mortality risk at approximately 2000 ft, while the categorical hazard model indicated a statistically significant survival benefit only for patients residing at or above 6000 ft relative to those below 250 ft, although patients one stratum lower (4000–<6000 ft) also tended to have improved survival. We might account for these differences by recognizing that when altitude is rendered as a continuous variable, low frequencies of patients at various points of its domain may result in unstable instantaneous estimates of mortality. The grouping effects of categorical hazard analysis, on the other hand, while protective of unstable estimates, provide a weaker resolution of the altitude-mortality relationship. That both analytical methods demonstrate a survival benefit associated with high residential altitude (≥6000 ft), then, is reassuring, though spline model mortality estimates were less stable beyond 7500 ft where patient sample size was low.
Previous reports attribute improved survival at high altitudes in dialysis patients to the regulatory effects of hypoxia-induced factors on enzymes associated with cardiovascular (CV) risk2, 17 such as heme oxygenase-1, inducible nitric oxide synthase, and VEGF.4–6 However, the activation of hypoxia-induced factors may be substantially influenced by protein-energy malnutrition and chronic inflammation18, 19 conditions which are highly prevalent in maintenance dialysis patients.20, 21 Moreover, chronic malnutrition and inflammation may attenuate responsiveness to EPO administration in dialysis patients.11, 12 We found that most markers of malnutrition and inflammation (MICS) significantly according to altitudinal strata. These differences might be accounted for, at least in part, by 1) variations in dietary habits according to patients’ geographical environments22 or 2) the tendency of patients to migrate to lower altitudes during their lifetime according to their health status.23 Contrary to our expectations, we observed that higher altitudes were associated with lower creatinine levels, which may indicate reduced muscle mass and poorer nutritional status in dialysis patients,24, 25 and hence would not explain the improved survival in these altitudinal groups. While further studies are needed to explore the paradoxical association between altitude and creatinine levels, adjustment for MICS characteristics did not markedly influence survival trends among our cohort, suggesting the patients’ composite inflammation and malnutrition status may not have varied meaningfully as to differentially influence mortality.
Furthermore, while hypoxia-induced factor activation stimulates endogenous erythropoietin in normal persons, this process is nearly absent in end-stage renal disease. Moreover, dialysis patients are treated with exogenous erythropoietin to maintain serum hemoglobin levels within KDOQI guidelines (≥11.0 g/dL but no greater than 13.0 g/dL26). Indeed, serum hemoglobin levels varied only slightly (approximately 3%) by altitudinal strata in our cohort. Because hypoxia-induced factors regulate a number of important biological pathways beyond erythropoiesis that may influence mortality, including angiogenesis, vascular remodeling, redox homeostasis, and energy metabolism,27–30 this lack of variation in hemoglobin does not rule out hypoxia as a plausible mechanism for our observed results.
We also noted a “threshold” effect of altitude on mortality in our categorical hazard analyses. Specifically, reductions in mortality became apparent only when residential altitude reached 4000 ft or greater. One rationale for this observation pertains to hypoxia—HIF-1α is an important oxygen-threshold sensor in humans,31, 32 but little is known about the nature of this threshold. A number of studies cite 1500 meters (4921 ft) as the lower limit for the onset of acute physiological hypoxic adaptations,31, 33–35 including the HIF-1α dependent hypoxic ventilatory response31, 33, 36 hypoxic pulmonary vasoconstriction,33 and stimulated erythropoiesis.34, 35 Moreover, in a study exploring the effects of acute hypobaric hypoxia on endogenous EPO production, a simulated altitude of 2100m (~6890 foot) was a threshold for sustained EPO release in healthy persons.31 While endogenous EPO production is virtually absent in CKD patients, these findings may indicate a HIF-activation threshold that may explain the reduced mortality we observed only at higher altitudes.
The roles of hypoxia and anemia-independent pathways in the inverse altitude-mortality association also merit consideration. For example, UV-B radiation exposure, which increases 15% per 1000 meter (~3300 foot) rise in altitude, is an important source of vitamin D,37 the latter of which has been associated with improved CV risk and all-cause mortality in both healthy populations and incident dialysis patients.14, 38, 39 Although vitamin D data was unavailable in this study, we found that patients residing in higher altitudinal strata tended to have lower parathyroid hormone (PTH) levels, which may indicate improved vitamin D status resulting from greater UV-B exposure at higher altitudes.40
Key strengths of our study include the use of a large contemporary and nationally representative database with 8-years follow-up and the comprehensive availability of clinical data allowing for adjustment of several important confounders such as nutritional status, inflammation, preexisting co-morbidities, laboratory data, and erythropoietin dose. However, several limitations of our study bear mention. Because our study is observational, we are unable to definitively determine whether residential altitude itself influences all-cause mortality. Furthermore, although we adjusted for a large number of covariates there is always the possibility of residual confounding. We also lacked information on measurements of Vitamin D levels and cause of death for this study, which may be important in delineating the confounding effects of UV, and the contribution of cardiovascular mortality, respectively, in future studies.
Conclusion
Our data confirm previous findings that high altitudes, in excess of 6000 ft, are associated with a significant reduction in mortality risk. Further studies are needed to confirm our findings, and to more clearly delineate the pathways by which high altitude confers a reduction in mortality risk.
Acknowledgements
Funding Source:
The study was supported by Dr. Kalantar-Zadeh's research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01-DK078106), a research grant from DaVita Clinical Research (DCR), and a philanthropic grant from Mr. Harold Simmons.
Footnotes
Financial Disclosure Declaration:
Relevant Potential Conflict of Interest:
Dr. Kalantar-Zadeh was medical director of the DaVita Harbor-UCLA Long Beach during 2007-2012. Other authors have not declared any conflicts of interest.
Some of the data have been presented in an abstract published in the Journal of the American Society of Nephrology and in a poster presentation at the American Society of Nephrology (ASN) annual conferences, October 27–31, 2012, San Diego, CA.
References
- 1.Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299(1):F1–F13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkelmayer WC, Liu J, Brookhart MA. Altitude and all-cause mortality in incident dialysis patients. JAMA. 2009;301(5):508–512. doi: 10.1001/jama.2009.84. [DOI] [PubMed] [Google Scholar]
- 3.Brookhart MA, Schneeweiss S, Avorn J, Bradbury BD, Rothman KJ, Fischer M, et al. The effect of altitude on dosing and response to erythropoietin in ESRD. Journal of the American Society of Nephrology : JASN. 2008;19(7):1389–1395. doi: 10.1681/ASN.2007111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24(8):449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33(11):1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88(4):1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 7.el-Nawawy A, Barakat S, Elwalily T, Abdel-Moneim Deghady A, Hussein M. Evaluation of erythropoiesis in protein energy malnutrition. East Mediterr Health J. 2002;8(2–3):281–289. [PubMed] [Google Scholar]
- 8.Means RT., Jr Advances in the anemia of chronic disease. Int J Hematol. 1999;70(1):7–12. [PubMed] [Google Scholar]
- 9.Trey JE, Kushner I. The acute phase response and the hematopoietic system: the role of cytokines. Crit Rev Oncol Hematol. 1995;21(1–3):1–18. doi: 10.1016/1040-8428(94)00141-3. [DOI] [PubMed] [Google Scholar]
- 10.Borelli P, Blatt S, Pereira J, de Maurino BB, Tsujita M, de Souza AC, et al. Reduction of erythroid progenitors in protein-energy malnutrition. Br J Nutr. 2007;97(2):307–314. doi: 10.1017/S0007114507172731. [DOI] [PubMed] [Google Scholar]
- 11.Rattanasompattikul M, Molnar MZ, Zaritsky JJ, Hatamizadeh P, Jing J, Norris KC, et al. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gfs368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locatelli F, Andrulli S, Memoli B, Maffei C, Del Vecchio L, Aterini S, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21(4):991–998. doi: 10.1093/ndt/gfk011. [DOI] [PubMed] [Google Scholar]
- 13.Ricks J, Molnar MZ, Kovesdy CP, Shah A, Nissenson AR, Williams M, Kalantar-Zadeh K. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61(3):708–715. doi: 10.2337/db11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JE, Molnar MZ, Kovesdy CP, Zaritsky JJ, Streja E, Salusky I, et al. Administered paricalcitol dose and survival in hemodialysis patients: a marginal structural model analysis. Pharmacoepidemiol Drug Saf. 2012;21(11):1232–1239. doi: 10.1002/pds.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55(1):100–112. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar MZ, Kovesdy CP, Bunnapradist S, Streja E, Mehrotra R, Krishnan M, et al. Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant. 2011;11(5):1006–1015. doi: 10.1111/j.1600-6143.2011.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkelmayer WC, Hurley MP, Liu J, Brookhart MA. Altitude and the risk of cardiovascular events in incident US dialysis patients. Nephrol Dial Transplant. 2012;27(6):2411–2417. doi: 10.1093/ndt/gfr681. [DOI] [PubMed] [Google Scholar]
- 18.Stasinopoulos I, O'Brien DR, Bhujwalla ZM. Inflammation, but not hypoxia, mediated HIF-1alpha activation depends on COX-2. Cancer Biol Ther. 2009;8(1):31–35. doi: 10.4161/cbt.8.1.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. The New England journal of medicine. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42(5):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney international. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 22.Cupisti A, D'Alessandro C, Valeri A, Capitanini A, Meola M, Betti G, Barsotti G. Food intake and nutritional status in stable hemodialysis patients. Ren Fail. 2010;32(1):47–54. doi: 10.3109/08860220903391234. [DOI] [PubMed] [Google Scholar]
- 23.Regensteiner JG, Moore LG. Migration of the elderly from high altitudes in Colorado. JAMA. 1985;253(21):3124–3128. [PubMed] [Google Scholar]
- 24.Keshaviah PR, Nolph KD, Moore HL, Prowant B, Emerson PF, Meyer M, et al. Lean body mass estimation by creatinine kinetics. Journal of the American Society of Nephrology : JASN. 1994;4(7):1475–1485. doi: 10.1681/ASN.V471475. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B, Bergstrom J. Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney international. 1998;53(3):773–782. doi: 10.1046/j.1523-1755.1998.00812.x. [DOI] [PubMed] [Google Scholar]
- 26.KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;50(3):471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. Journal of applied physiology. 2000;88(4):1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL. Hypoxia-Inducible Factor 1 and Cardiovascular Disease. Annual review of physiology. 2013 doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16(10):1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 30.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. Journal of the American College of Cardiology. 2005;46(11):2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 31.Ge RL, Witkowski S, Zhang Y, Alfrey C, Sivieri M, Karlsen T, et al. Determinants of erythropoietin release in response to short-term hypobaric hypoxia. J Appl Physiol. 2002;92(6):2361–2367. doi: 10.1152/japplphysiol.00684.2001. [DOI] [PubMed] [Google Scholar]
- 32.Millonig G, Hegedusch S, Becker L, Seitz HK, Schuppan D, Mueller S. Hypoxia-inducible factor 1 alpha under rapid enzymatic hypoxia: cells sense decrements of oxygen but not hypoxia per se. Free Radic Biol Med. 2009;46(2):182–191. doi: 10.1016/j.freeradbiomed.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Paralikar SJ, Paralikar JH. High-altitude medicine. Indian J Occup Environ Med. 2010;14(1):6–12. doi: 10.4103/0019-5278.64608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartsch P, Saltin B. General introduction to altitude adaptation and mountain sickness. Scand J Med Sci Sports. 2008;18 Suppl 1:1–10. doi: 10.1111/j.1600-0838.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 35.Schobersberger W, Greie S, Humpeler E, Mittermayr M, Fries D, Schobersberger B, et al. Austrian Moderate Altitude Study (AMAS 2000): erythropoietic activity and Hb-O(2) affinity during a 3-week hiking holiday at moderate altitude in persons with metabolic syndrome. High Alt Med Biol. 2005;6(2):167–177. doi: 10.1089/ham.2005.6.167. [DOI] [PubMed] [Google Scholar]
- 36.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(2):821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? Journal of the American College of Cardiology. 2008;52(24):1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 39.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney international. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 40.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney international. 2007;71(1):31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]