Abstract
Sigma receptor 1 (σR1), a non-opiate transmembrane protein located on endoplasmic reticulum (ER) and mitochondrial membranes, is considered a molecular chaperone. Marked protection against cell death has been observed when ligands for σR1 have been used in in vitro and in vivo models of retinal cell death. Mice lacking σR1 (σR1−/−) manifest late onset loss of retinal ganglion cells and retinal electrophysiological changes (after many months). The role of σR1 in retina and the mechanisms by which its ligands afford neuroprotection are unclear. To explore this we used σR1−/− mice and investigated expression of ER stress genes (BiP/GRP78, Atf6, Atf4, Ire1α) and proteins involved in apoptosis (BCL2, BAX) and examined the retinal transcriptome at young ages. While there were no significant changes in expression of major ER stress genes (over a period of a year) in neural retina, there were marked changes in these genes especially Atf6 in isolated retinal Müller glial cells. BCL2 levels decreased in σR1−/− retina concomitant with decreases in NFkB and pERK1/2. We postulate that σR1 regulates ER stress in retinal Müller cells and that the role of σR1 in retinal neuroprotection likely involves BCL2 and some of the proteins that modify its expression (such as ERK, NFκB). Data from the analysis of the retinal transcriptome of σR1 null mice provides new avenues to understand the role of σR1 in retinal neuroprotection.
Keywords: retinal neuroprotection, mouse, Müller cells, endoplasmic reticulum stress, retinal disease
Introduction
Sigma receptor 1 (σR1) is a non-opioid transmembrane protein located at the ER, mitochondrial and plasma membranes (Hayashi and Su, 2007). It shares no homology with any other mammalian proteins (Hanner et al, 1996), but is expressed ubiquitously in numerous tissues including the central nervous system (Su et al, 1988). In retina, σR1 is expressed abundantly including in the ganglion cell and inner nuclear layers, in photoreceptor and RPE cells; it is detected in the optic nerve and optic nerve head (Ola et al, 2001; Liu et al, 2010).
There are several in vivo and in vitro studies reporting that over-expression of σR1 or activation of σR1 by high-affinity ligands protects against neuronal cell death (Martin et al, 2004; Dun et al, 2007; Bucolo et al, 2006; Techedre et al, 2008, Techedre and Yorio, 2008; Zhang et al, 2011; Smith et al, 2008). We previously studied the neuroprotective effects of σR1 in the Ins2Akita/+ mouse model, which has been used as a model for diabetic retinopathy. This mouse develops hyperglycemia, marked disruption of the inner nuclear layer and loss of ganglion cells (Barber et al, 2005). Treatment of Ins2Akita/+mice for a 22 week period with (+)-pentazocine ((+)-PTZ), a high affinity σR1 ligand afforded marked preservation of retinal structure (Smith et al, 2008). The mechanism underlying neuroprotection by σR1 ligands is not clear. Techedre and co-workers reported that in vitro σR1 ligands regulate intracellular Ca2+ levels concomitant with attenuated activation of pro-apoptotic genes (Techedre et al, 2008). Others reported that σR1 forms a complex at the mitochondrial associated membrane (MAM) with BiP/GRP78, a key regulator of ER stress. Upon ER Ca2+ depletion or via ligand stimulation, σR1s dissociate from BiP/GRP78, leading to prolonged Ca2+ signaling into mitochondria via IP3Rs. Increasing σR1 in vitro counteracts the ER stress response, whereas decreasing σR1 enhances apoptosis (Hayashi and Su, 2007). These studies suggested that σR1 has a role as a modulator for ER stress. Previously we performed in vitro studies exposing a retinal neuronal cell line to oxidative stress and observed increased expression of a broad array of ER stress genes, which was attenuated when the cells were pre-treated with (+)-PTZ (Ha et al, 2011a). We observed an increase in expression of several ER stress-related genes in retinas of Ins2Akita/+ mice, which decreased in (+)-PTZ-treated mice. Recent work from the Wormstone lab has shown that lens cells exposed to hydrogen peroxide to induce oxidative stress upregulated ER stress genes, the expression of which was attenuated upon treatment with (+)-PTZ (Wang et al, 2012).
In addition to ER stress, neuroprotection mediated by σR1 activation may involve BCL2-mediated pathways (Meunier and Hayashi, 2010) as ligands for σR1 increase BCL2 levels under various cellular stress conditions (Yang et al, 2007; Zhang et al, 2012). Bcl2 is a key anti-apoptotic gene overexpressed in B-cell lymphoma that promotes expression of neuroprotective factors such as αB crystallin (Yang et al, 1997; Zhan et al, 1999, Hockenbery et al, 1993). Studies in mice that overexpressed Bcl2 in neurons demonstrated an increased number of retinal ganglion cell somas (Bonfanti et al, 1996; Cenni, 1996). Previous studies suggest that σR1 regulates BCL2 via its action on nuclear factor κ-light-chain enhancer (NFκB) (Yang et al, 1997). BCL 2 also regulates IP3Rs, which regulate Ca2+-induced Ca2+ release (Monaco et al, 2012; Gerasimenko et al, 2010; Rong et al, 2008). These intriguing findings set the stage for the current study, which utilized the σR1−/− mouse as an in vivo tool to inform about the role of σR1 with respect to ER stress genes (BiP/GRP78 and its downstream effector proteins) as well as BCL2 and proteins that modulate its roles in survival (including NFκB, ERK, αB crystallin). σR1−/− mice do not exhibit a profound retinal phenotype in the early stages of development; retinas are similar to wildtype structurally and functionally for many months. By ~36 weeks of age, however, apoptotic cell death is evident in the σR1−/− optic nerve head and by ~1 year there is loss of ganglion cells and diminished electrophysiological function (Ha et al, 2011b). More rapid cellular and functional losses are observed, when σR1−/− mice are diabetic (Ha et al, 2012) or when they are subjected to optic nerve crush (Mavlyutov et al, 2011).
Our findings in the current study of σR1−/− mice indicate no alterations of the major ER stress effector genes or their proteins in the absence of σR1 in studies of the whole retina, yet significant alterations of ER stress genes in isolated σR1−/− retinal Müller glial cells, as well as significant alterations in BCL2 and some of its related proteins in retinas of mice lacking σR1.
Methods
Animals
Mice (wildtype (σR1+/+) and σR1 knockout (σR1−/−)) ranging in age from 4 days to 96 weeks were used in these studies (Table 1). σR1−/− mice were generated by gene trapping (Oprs1Gt(IRESBetageo)33Lex/Oprs1Gt(IRESBetageo)33Lex) conducted at Lexicon Genetics Corporation as described (Sabino et al, 2009). Heterozygote Oprs1 mutant (+/−) Oprs1Gt(IRESBetageo)33Lex embryos on a C57BL/6J × 129S/SvEv mixed background were obtained from Mutant Mouse Resource Regional Center and implanted into female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) at The Scripps Research Institute, LaJolla, CA. Founder heterozygous mice were transferred to the animal facility at Georgia Regents University and a colony of wildtype (σR1+/+), heterozygous (σR1+/−) and homozygous (σR1−/−) mice established. Genotyping of mice was performed as described (Ha 2011b). Maintenance of animals adhered to institutional guidelines for humane treatment of animals and to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research.
Table 1.
Summary of mice (age and body weights) used in the analyses
| Mouse genotype | n | Mouse Age | Mean body weight ± SEM (grams) |
|---|---|---|---|
| σR1 +/+ (wild-type) | 3 | 4 days | ~1.5 |
| σR1 −/− (homozygous, knockout) | 3 | 4 days | ~1.5 |
| σR1 +/+ (wild-type) | 12 | 5–7 days | ~3–4 |
| σR1 −/− (homozygous, knockout) | 12 | 5–7 days | ~3–4 |
| σR1 +/+ (wild-type) | 6 | 6 weeks | 17.2 ± 02 |
| σR1 −/− (homozygous, knockout) | 6 | 6 weeks | 17.3 ± 0.1 |
| σR1 +/+ (wild-type) | 3 | 24 weeks | 24.8 ± 0.2 |
| σR1 −/− (homozygous, knockout) | 3 | 24 weeks | 25.7 ± 0.2 |
| σR1 +/+ (wild-type) | 6 | 26–32 weeks | 30.3 ± 0.3 |
| σR1 −/− (homozygous, knockout) | 6 | 26–32 weeks | 29.4 ± 0.3 |
| σR1 +/+ (wild-type) | 3 | 52 weeks | 37.1 ±0.18 |
| σR1 −/− (homozygous, knockout) | 3 | 52 weeks | 34.1 ± 0.1 |
| σR1 +/+ (wild-type) | 8 | 96 weeks | 34.8 ± 0.7 |
| σR1 −/− (homozygous, knockout) | 8 | 96 weeks | 33.9 ± 1.43 |
Müller glial cells were isolated from 5 day old mice and cultured per our method (Jiang et al, 2006). Briefly, eyeballs were removed, placed in Dulbecco modified Eagle medium (DMEM) with gentamicin, and soaked for 3 hours at 25°C in the dark. Then they were rinsed in PBS and were incubated in buffer containing trypsin, EDTA, and collagenase. Retinas were removed from eyeballs (taking care to avoid contamination by pigmented RPE), placed in DMEM supplemented with glucose, FBS, and penicillin/streptomycin, and gently pipetted into small aggregates at a density of 10 to 16 retinas per dish. Isolated cells were detected within 1 to 3 days. By 3 to 5 days, substantial cell growth ensued. Cultures were washed vigorously with medium until only a strongly adherent flat cell population remained. Cells were passaged 1 to 3 days after washing and were seeded into culture flasks (50,000 cells/cm2); culture media was changed three times per week. The purity of cultures has been verified using antibodies that are known markers of Müller cells (CRALBP, vimentin, glutamine synthetase, GLAST) (Jiang et al, 2006). Immunocytochemical studies using markers for neurons (neurofilament-L, a major component of neuronal cytoskeleton) and RPE (RPE-65) show minimal detection.
Real time quantitative RT-PCR (RT-qPCR) analysis of genes in the ER stress pathways
Expression levels of mRNA transcripts specific for several key genes (BiP/GRP78, Perk, Atf6, Ire1α, Atf4, Chop) involved in ER stress pathways were examined in mouse retina and brain per our method (Ha et al, 2011a). Total RNA was isolated using TRIzol™ Reagent (Invitrogen, Carlsbad, CA) and quantified. 2 μg of RNA was reverse transcribed using iScript™ Synthesis kit (BioRad Laboratories, Hercules, CA). cDNAs were amplified for 45 cycles using Absolute SYBR Green Fluorescein (ABgene, Surrey, UK) and gene specific primers (Table 2) in an iCycler (Bio-Rad). Expression levels were calculated by comparison of Ct values (delta-delta Ct) (Ha et al, 2011a).
Table 2.
Sequences of primers used for qRT-PCR
| Gene | NCBI Accession No. | Primer Sequence | Predicted band size |
|---|---|---|---|
| BiP | NM_022310 | Forward: 5′-ACTTGGGGACCACCTATTCCT-3′ Reverse: 5′-ATCGCCAATCAGACGCTCC-3′ |
134 |
| PERK | NM_010121 | Forward: 5′-AGTCCCTGCTCGAATCTTCCT-3′ Reverse: 5′-TCCCAAGGCAGAACAGATATACC-3′ |
125 |
| ATF4 | NM_009716 | Forward: 5′-TCCTGAACAGCGAAGTGTTG-3′ Reverse: 5′-ACCCATGAGGTTTCAAGTGC-3′ |
129 |
| IRE1α | NM_023913 | Forward: 5′-ACACCGACCACCGTATCTCA-3′ Reverse: 5′-CTCAGGATAATGGTAGCCATGTC-3′ |
110 |
| ATF6 | NM_001107196 | Forward: 5′-TGCCTTGGGAGTCAGACCTAT-3′ Reverse: 5′-GCTGAGTTGAAGAACACGAGTC-3′ |
141 |
| CHOP | NM_007837 | Forward: 5′-CTGGAAGCCTGGTATGAGGAT-3′ Reverse: 5′-CAGGGTCAAGAGTAGTGAAGGT-3′ |
121 |
| IP3R3 | NM_080553 | Forward: 5′-AGACCCGCTGGCCTACTATGAGAA-3′ Reverse: 5′-GTCAGGAACTGGCAGATGGCAGGT-3′ |
111 |
| Bcl-2 | NM_009741 | Forward: 5′-AAGCCGGGAGAACAGGGTATGAT-3′ Reverse: 5′-TGCAGATGCCGGTTCAGGTACTCA-3′ |
541 |
| BAX | NM_007527.3 | Forward:5′-AGACAGGGGGCTTTTTGCTAC-3′ Reverse:5′-AAT TCG CCGGAGACACTCG-3 |
136 |
| 18S | NR_003278 | Forward: 5′-AGTGCGGGTCATAAGCTTGC-3′ Reverse: 5′-GGGCCTCACTAAACCATCCA-3′ |
90 |
| σR1 | NM_030996 | Forward: 5′-CATTCGGGACGATACTGGGC-3′ Reverse: 5′-CCTGGGTAGAAGACCTCACTTTT-3′ |
101 |
Western blot
Retinal proteins were isolated from mice and subjected to SDS-PAGE (Ha et al, 2011a,b). Immunoblotting was performed to assess levels of the following proteins: BiP/GRP78, IP3R3 (BD Bioscience, San Jose, CA), ATF6, BCL2, NF-κB (p50), BAX (Santa Cruz Corp., Santa Cruz, CA), PERK, IRE1α, total ERK and p-ERK (Cell Signaling, Danvers, MA), αB crystallin (Enzo Life Sciences, Farmingdale, NY). Nitrocellulose membranes, to which the proteins had been transferred, were incubated with primary antibodies at a concentration of 1:500. They were incubated with HRP-conjugated goat anti-rabbit (Santa Cruz Corp., 1:3000) or goat anti-mouse IgG antibody (Sigma-Aldrich, 1:3000). Proteins were visualized using the SuperSignal West Pico Chemiluminescent Substrate detection system (Pierce Biotechnology, Rockford, IL).
Analysis of retinal transcriptome in σR1−/− mice compared to wild-type mice
RNA was isolated from neural retinas of 5–6-week-old wild-type and σR1−/− mice. Three individual RNA preparations were made from each group to allow three independent samples to be analyzed per group. Total RNA was isolated using TRIzol and sense strand cDNA was generated using the Affymetrix GeneChip WT terminal labeling kit (Applied Biosystems, Foster City, CA) following the manufacturer’s guidelines. This kit is optimized for use with mouse Affymetrix GeneChip Sense Target (ST) gene arrays from the same vendor and these were used in the present study. The mouse gene ST array interrogates 28,853 genes with 770,317 distinct probes. Following hybridization, washing and staining, the array chips were imaged using the Affymetrix GeneChip Scanner 3000 7G Plus. Images were imported into the Partek Genomics Suite (Partek Inc, St. Louis, MO) to analyze probe intensity and to determine the differential gene expression between groups. The data obtained provided a p-value and fold change of gene expression determined from the three arrays for wild-type mice compared to three arrays for the σR1−/− mice. Genes for which fold changes were greater than 1.4 and the p-value was <0.05 were researched through NLM data bases to determine possible relevance to retinal function.
Statistical analysis
The data were analyzed by one- or two-way ANOVA as appropriate (post-hoc test: Tukey). Statistical analysis was conducted using the GraphPad Prism analytical program, (LaJolla, CA). A p value < 0.05 was considered significant.
Results
Analysis of regulators of ER stress gene/protein expression in retina and brain of σR1−/− mice
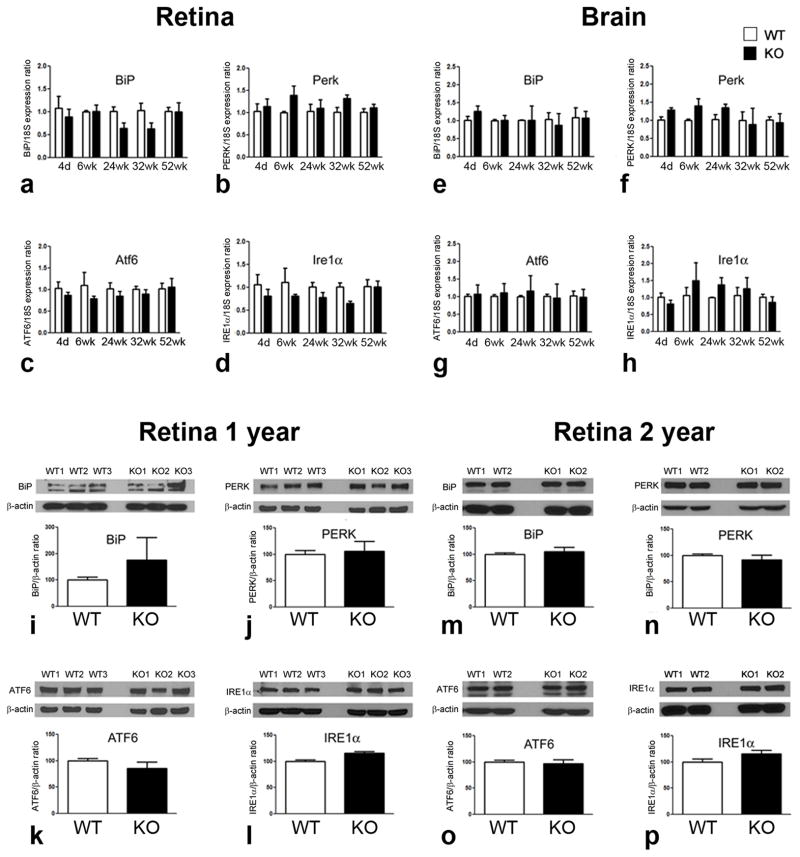
Previously published studies provided in vitro evidence that σR1 forms a complex at the mitochondrial associated membrane with another chaperone, BiP/GRP78 (Hayashi and Su, 2007). In those studies σR1 dissociated from BiP/GRP78 when cells were subjected to thapsigargin, but showed increased binding to BiP/GRP78 when cells were glucose deprived. Studies from our lab using retinal neuronal cells showed increased σR1 binding to BiP/GRP78 under oxidative stress (Ha et al, 2011a). When ER calcium levels are depleted or when σR1 is stimulated by a ligand in vitro, σR1 dissociates from BiP/GRP78, leading to prolonged calcium signaling into mitochondria via IP3R. We explored the in vivo role of σR1 in modulating expression of major ER stress genes, either BiP/GRP78 or its downstream effector genes (Perk, Atf6, Ire1α), by analyzing their expression in neural retina and brain of mice that lacked σR1. We anticipated that retinas of σR1−/− mice would manifest altered expression of these ER stress-regulating genes and/or the proteins they encode. Temporal analysis of the expression of these genes by qRT-PCR using retinas harvested from σR1+/+ and σR1−/− mice over a one year period (4 days–52 weeks), however, revealed no significant differences between groups in mRNA expression levels of BiP/GRP78 (Fig. 1a), Perk (Fig. 1b), Atf6 (Fig. 1c), or Ire1α (Fig. 1d) at any age examined. Similar findings were obtained in brain for BiP/GRP78 (Fig. 1e), Perk (Fig. 1f), Atf6 (Fig. 1g), or Ire1α (Fig. 1h). We analyzed the proteins encoded by these ER stress genes in 1 year σR1−/− mice owing to our recent observations that by this age there are phenotypical alterations in the retina accompanied by functional changes (Ha et al, 2011b). There were no statistically significant differences in BiP (Fig. 1i), PERK (Fig 1j), ATF6 (Fig. 1k), IRE1α (Fig. 1l) protein levels in neural retinas of σR1−/− compared to WT at 1 year or 2 years of age BiP (Fig. 1m), PERK (Fig 1n), ATF6 (Fig. 1o), IRE1α (Fig. 1p). We examined also expression of Atf4 and Chop, two ER stress-related genes whose expression increased in Ins2Akita/+ diabetic mice but returned to wildtype levels following (+)-PTZ treatment (Ha et al, 2011a). There was no change in expression of these genes in retinas of σR1−/− versus σR1+/+ mice (data not shown).
Fig. 1. Analysis of genes encoding BiP/GRP78 and its downstream effector proteins in neural retina and brain of σR1+/+ and σR1−/− mice.
mRNA of neural retina or brain was isolated from σR1+/+ (WT) and σR1−/− (KO) mice at 4 days, 6, 24, 32 and 52 weeks; qRT-PCR was performed to analyze the expression in retina of (a) BiP, (b) Perk, (c) Atf6, (d) Ire1α normalized to 18S and the expression in brain of (e) BiP, (f) Perk, (g) Atf6, (h) Ire1α. Proteins from neural retinas of 1 year mice were isolated and subjected to immunoblotting to detect major proteins implicated in the ER stress response (i) BiP, (j) Perk, (k) Atf6, (l) Ire1α. Proteins were isolated also from neural retinas of 2 year mice and subjected to immunoblotting to detect major (m) BiP, (n) Perk, (o) Atf6, (p) Ire1α. Band densities were normalized to β-actin and densitometric analysis of the bands are provided below each set of blots.
One interpretation of these data is that σR1 does not directly regulate expression of BiP/GRP78 or its three major downstream effector proteins. Another is that analysis of expression of ER stress genes using the entire neural retina (which is comprised of neurons, glial supportive cells, and blood vessels) may be masking ER stress gene changes within specific retinal cell types. For this reason, we isolated Müller cells, the major retinal glial cell type, from the retinas of σR1+/+ and σR1−/− mice. We evaluated ER stress gene expression and observed differences including a 0.6 fold increase in BiP/GRP78 expression and 0.2, 0.4 and 0.8 fold decreases, respectively, in expression of Perk, Ire1α and Atf4 in σR1+/+ versus σR1−/− mouse Müller cells (Fig. 2a). There was an increase in expression of Chop. Interestingly, there was a dramatic increase (130 fold) in expression of Atf6 in Müller cells harvested from the σR1+/+ and σR1−/− mice compared with σR1+/+ and σR1−/− mice (Fig. 2b).
Fig. 2. Analysis of genes encoding BiP/GRP78 and its downstream effector proteins in Müller glial cells isolated from retinas of σR1+/+ and σR1−/− mice.
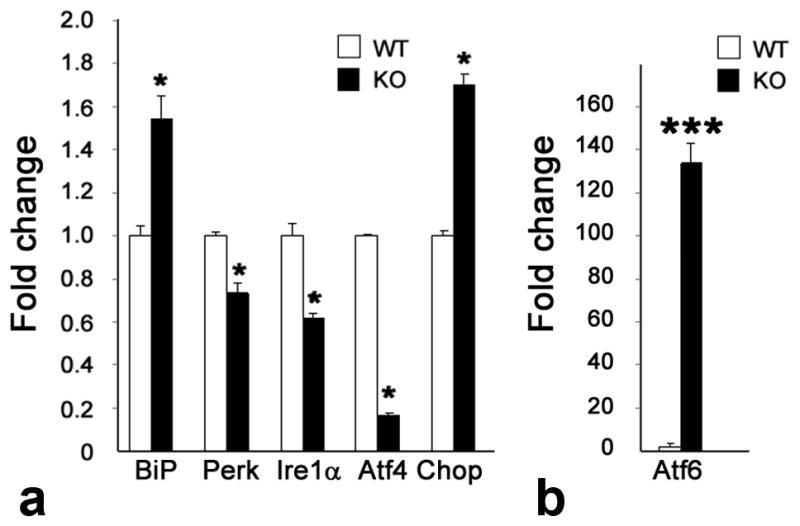
Müller cells were isolated from twelve 5–7 day σR1+/+ (WT) and σR1−/− (KO) mice, mRNA was prepared and qRT-PCR was performed to analyze the expression of (a) BiP, Perk, Ire1α, Atf4, Chop and (b) Atf6 normalized to GAPDH. Each experiment was performed in triplicate; *p<0.05,***p<0.001.
Analysis of IP3R3, Bcl2 and Bax in retinas of σR1−/− mice
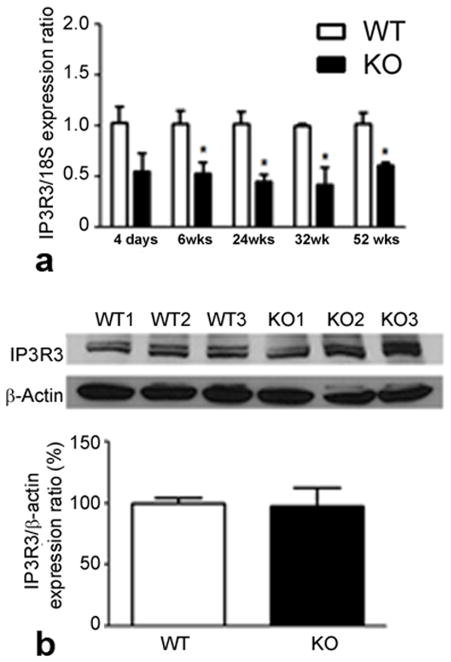
Evaluation of neural retinas showed a marked decrease in retinal IP3R3 expression in σR1−/− mice compared to age-matched wildtype mice at all ages examined (Fig. 3a), although protein levels were not different in year old mice (Fig. 3b). There was a significant decrease also in expression in Müller cells harvested from σR1−/− mice (data not shown). IP3R3 encodes inositol 1,4,5-triphosphate receptor type 3 (IP3Rs), which governs the release of Ca2+ stored within the ER lumen (Wojcikiewicz et al, 2009). σR1 has been shown to stabilize IP3Rs at the mitochondria-associated ER membrane (Hayashi and Su, 2007), thus these data support a role for σR1 in regulating IP3R gene expression. IP3Rs interact with BCL2 (Monaco et al, 2012; Rong et al, 2008) prompting investigations of this antiapoptotic protein.
Fig. 3. Analysis of IP3R3 in retina σR1+/+ and σR1−/− mice.
(a) mRNA was isolated from neural retina of σR1+/+ (WT) and σR1−/− (KO) mice at 4 days, 6, 24, 32 and 52 weeks; qRT-PCR was performed to analyze the expression of IP3R3 normalized to 18S. (b) Protein of neural retinas from 1 year mice were isolated and subjected to immunoblotting to detect IP3R3. Band densities were normalized to β-actin and densitometric analysis of the bands are provided below blots. (n = 3 mice per group; *p<0.05)
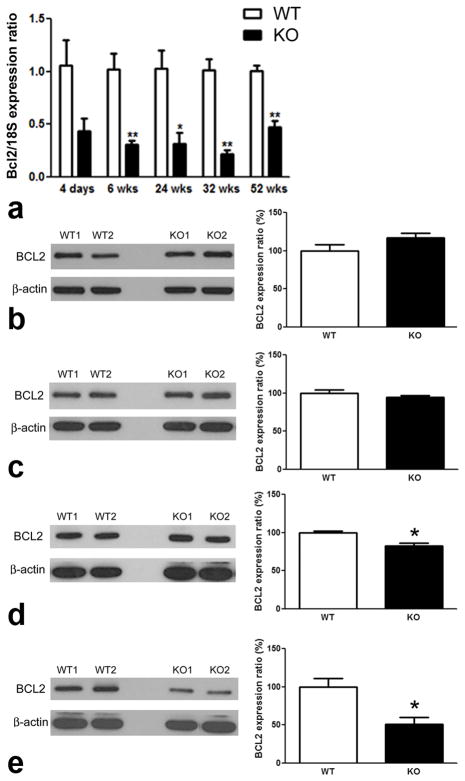
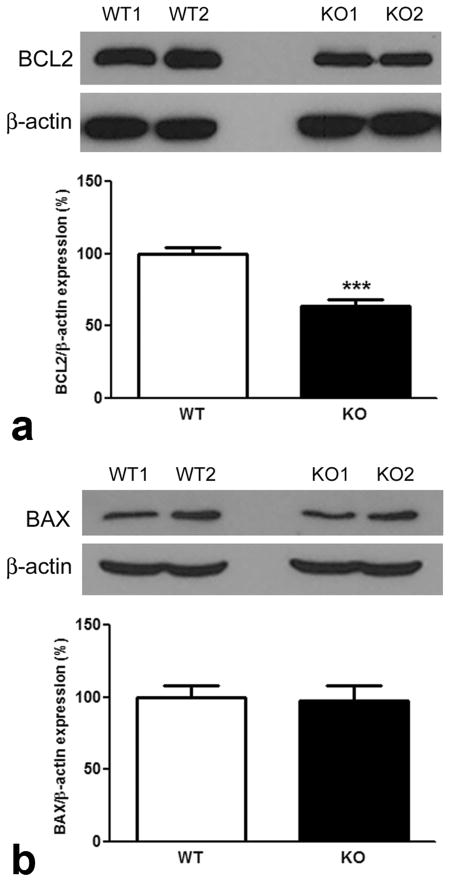
Previous in vitro studies using siRNA technology to knockdown σR1 levels in CHO cells showed a decrease in BCL2 protein levels (Meunier and Hayashi, 2010). Here, we used qRT-PCR in retinas of σR1−/− mice to analyze Bcl2 expression over an age range of 4 days to 1 year. Bcl2 expression decreased significantly in σR1−/− versus σR1+/+ mice (Fig. 4a). As early as 4 days, Bcl2 expression in σR1−/− mice was less than that of age-matched wildtype mice; by 6 weeks this difference had reached statistical significance. BCL2 protein levels were analyzed in σR1−/− mouse retinas at 4 days, 6 wks, 24 wks, 52 wks (Fig. 4b–e) and were decreased significantly at 24 and 52 wks (Fig. 4d–e). Indeed, by 1 year the BCL2 levels were only half that observed in age-matched wildtype animals. We examined BCL2 in retinas of σR1−/− mice at 2 years and found that the levels were similar to those of σR1−/− mice at 1 year (Fig. 5a). In addition to examining BCL2 levels, we also examined levels of BAX. BAX is a BCL2-interacting protein that is pro-apoptotic (Raisova et al, 2001). Some reports suggest that balance of BAX/BCL2 is important for cell survival (Raisova et al, 2001), while others caution that other newly discovered proteins in the BCL2 family also affect this balance (Nickells, 2010). Our investigations of BAX showed no alterations in protein levels in retinas of σR1−/− compared to σR1+/+ mice (Fig. 5b). This is consistent with the in vitro studies using siRNA toward σR1 in which BAX expression was not altered despite the effects on BCL2 expression (Meunier and Hayashi, 2010).
Fig. 4. Temporal expression of Bcl2 mRNA and protein (4 days – 1 year).
qRT-PCR was performed to analyze the expression of (a) bcl2 normalized to 18S. Protein was isolated from neural retinas of σR1+/+ (WT) and σR1−/− (KO) mice at (b) 4 days; (c) 6 wks; (d) 24 wks and (e) 52 wks. Band densities were normalized to β-actin. Densitometric analysis of the bands normalized by β-actin are provided beside each set of blots. (n = 3 mice per group; *p<0.05, **p<0.01)
Fig. 5. Western blot analysis of Bcl-2 and BAX (2 years).
Neural retinas were harvested from σR1+/+ (WT) and σR1−/− (KO) mice at 96 weeks, protein isolated, subjected to SDS-PAGE followed by immunoblotting to detect (a) BCL2 and (b) BAX. Band densities were normalized to β-actin. Densitometric analysis of the bands normalized by β-actin are provided below each set of blots. (n = 4–5 mice per group; ***p<0.001)
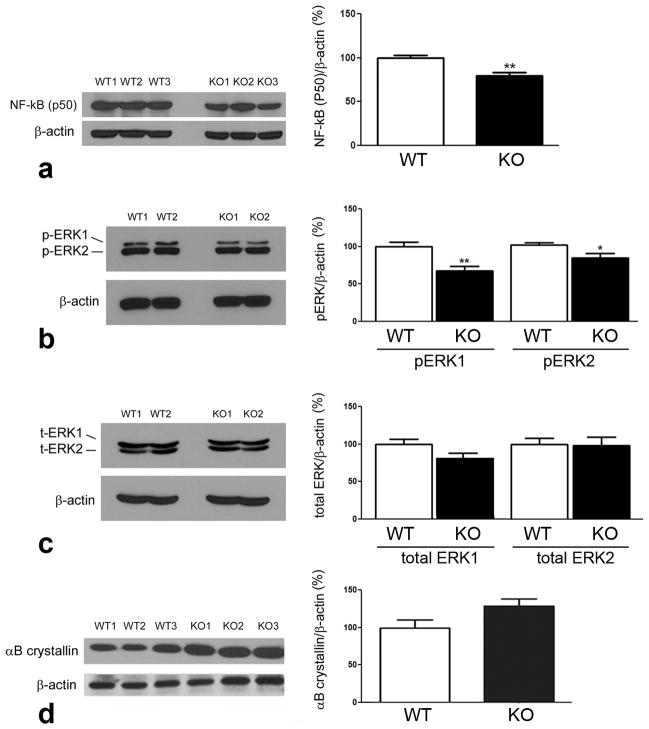
Analysis of NFκB, ERK and αB crystallin
Bcl2 expression is under the control of transcription factors including the NFκB family of proteins, specifically the p50 form (Kurland et al, 2001; Kurland et al, 2003, Tamatani et al, 1999; Galante et al, 2009). In in vitro studies, in which σR1 was knocked down using siRNA methods in CHO cells, protein levels of p50 increased substantially (Meunier and Hayashi, 2010). However, in the current study when we evaluated the protein level of NFκB (p50) in retinas of σR1−/− mice compared to σR1+/+ mice, we did not observe a marked increase of p50, rather levels of p50 decreased slightly (Fig. 6a). Thus, endogenous absence of σR1 does not appear to increase p50 as occurs when σR1 levels are altered experimentally under cell culture conditions. Besides NFκB signaling, BCL2 expression can be increased by activated ERK signaling (Galanate et al, 2009). Consistent with decreased BCL2 protein levels, we observed decreased levels of phosphorylated ERK-1 and a slight decrease in the level of phosphorylated ERK-2 in retinas of σR1−/− mice (Fig. 6b and 6c). Taken collectively, it appears that absence of σR1 in retina is associated with a decrease in the anti-apoptotic protein BCL2 and its regulators NFκB and ERK.
Fig. 6. Western blot analysis of NFκB (p50), ERK and αB crystallin proteins.
Neural retinas were harvested from σR1+/+ (WT) and σR1−/− (KO) mice, protein isolated, subjected to SDS-PAGE followed by immunoblotting to detect (a) NFκB (p50), (b) phosphorylated ERK1 and ERK2, (c) total ERK1 and ERK2, (d) αB crystallin. Band densities were normalized to β-actin. Densitometric analysis of the bands normalized by β-actin are provided adjacent to each set of blots. (n = 4–5 mice per group; *p<0.05,**p<0.01)
Several years ago, Feng and co-workers showed that BCL2 regulates αB crystallin levels via ERK signaling (Feng et al, 2004). αB crystallin is a molecular chaperone belonging to the small heat shock protein superfamily. It is present in the retinal ganglion cell layer, the inner plexiform layer, in photoreceptor cells and the pigment epithelium. Just as σR1 normally resides at the ER-mitochondrial interface, so also does αB crystallin. In vitro, when cells are stimulated by ligands or undergo prolonged stress, σR1 translocates from the MAM to the ER network and plasmalemma/plasma membrane and is thought to regulate a variety of proteins including ion channels, receptors and kinases. Crystallins also translocate to the nucleus under stress. αB crystallins have a role in neuroprotection (Mercatelli et al, 2010) including in retina (Kannan et al, 2012; Munemasa et al, 2009). When the retina sustains insult, for example in the form of light-induced toxicity or trauma, αB crystallin expression increases. αB crystallins can inhibit stress-induced apoptosis by interacting with members of the BCL2 family (including sequestering proapoptotic molecules (Mao et al, 2004)). We examined the levels of αB crystallin in retinas of σR1−/− mice compared to σR1+/+ mice and observed a trend toward increased levels of αB crystallin in the σR1−/− retinas (Fig. 6d), although the data did not reach statistical significance.
Examination of the retinal transcriptome of in σR1−/− mice
We examined the retinal transcriptome of young σR1−/− mice compared to wild-type mice to provide additional clues as to expression of which genes might be altered early that could account for the preservation of retinal structure in young σR1−/− mice. Of more than 20,000 genes examined by microarray, 76 were altered whose function might be related to retinal structure/function by a value greater than 1.4 fold (Table 3). Several genes related to eye development were also increased slightly (Cryba1, Rgr, Elovl2). It is noteworthy that genes related to antioxidant functions (Gpx3, Gstm6 and Gstm3) were altered in retinas of σR1−/− mice as were genes related to regulation of VEGF (Ctsg and Nrarp). Interestingly, Slc7a11, the gene encoding the cystine-glutamate transporter (System Xc-) is downregulated. Whether alterations of these genes translates to protein changes, which preserves retinal structure in σR1−/− mice, remains to be determined.
Table 3.
Expression changes of 76 selected genes in retinas of σR1−/− mice compared to wild-type mice
| Gene | Accession number | p-value | Function | Fold |
|---|---|---|---|---|
| Genes related to retina & eye development | ||||
| Cryba1 | NM_009965 | 0.074 | eye development; structural constituent of lens | 1.80 |
| Rgr | NM_021340 | 0.280 | retinal G protein | 1.54 |
| Shox2 | NM_013665 | 0.068 | expressed in CNS | 1.51 |
| Cryaa | NM_013501 | 0.159 | expressed neuronal differentiation, eye development | 1.49 |
| Crygs | NM_009967 | 0.074 | structural constituent of lens | 1.47 |
| Pin1l | NM_001033768 | 0.004 | pin1 isoform, AMD neurodegeneration | 1.46 |
| Tcn2 | NM_015749 | 0.064 | retinal expression | 1.45 |
| Grp | NM_175012 | 0.049 | expressed in CNS | 1.45 |
| Tlr7 | NM_133211 | 0.005 | AMD neurodegeneration | 1.45 |
| Crygd | NM_007776 | 0.332 | structural constituent of lens | 1.45 |
| Bcas1 | NM_029815 | 0.005 | rat retina maturation, oncogene | 1.45 |
| Crygb | NM_144761 | 0.082 | structural constituent of lens | 1.44 |
| Elovl2 | NM_019423 | 0.057 | expressed in retina, decreased in diabetes | 1.43 |
| Maob | NM_172778 | 0.006 | neuroprotective in retina | 1.43 |
| Tirap | NM_054096 | 0.034 | induced CNS glial activation | 1.42 |
| Gfap | NM_010277 | 0.006 | Glial cell marker, increased during retinal stress | 1.26 |
| Mybl1 | NM_008651 | 0.034 | reactive gliosis | −1.41 |
| Btrc | NM_001037758 | 0.004 | E3 ubiquitin ligase family; absence leads to amacrine cell loss | −1.45 |
| Capn7 | NM_009796 | 0.024 | role in Huntington’s disease | −1.48 |
| Lnp | NM_001110209 | 0.014 | expressed in CNS | −1.56 |
| Tox4 | NM_023434 | 0.008 | critical for certain pathological processes | −1.64 |
| Rb1 | NM_009029 | 0.028 | retinoblastoma protein (pRB) | −1.64 |
| Hint1 | NM_008248 | 0.012 | pronounced expression in neuronal ganglia | −1.81 |
| Skp1a | NM_011543 | 0.013 | modifier of Parkinson’s disease neurodegeneration | −2.14 |
| Duxbl | NM_183389 | 0.003 | Double homeobox gene, highly expressed in eye | −2.78 |
| Apoptosis | ||||
| Ckap2l | NM_181589 | 0.023 | increases in cell death | 1.54 |
| Foxh1 | NM_007989 | 0.022 | component of fas mediated apoptosis; axon transporter | 1.53 |
| Hspa1a | NM_010479 | 0.015 | stress response, anti-proliferative | 1.41 |
| Peg3 | NM_008817 | 0.004 | mediator between p53-Bax in DNA damage-induced neuronal death | −1.40 |
| Fancm | NM_178912 | 0.011 | prevent tumorigenesis | −1.41 |
| Rbm17 | NM_152824 | 0.007 | regulate apoptosis genes | −1.42 |
| Atxn2l | NM_183020 | 0.006 | stress related apoptosis genes | −1.43 |
| H1f0 | NM_008197 | 0.004 | apoptotic pathway | −1.69 |
| Anti-apoptosis | ||||
| Adipoq | NM_009605 | 0.032 | anti-apoptosis | 1.52 |
| Mycs | NM_010850 | 0.005 | oncogene | 1.51 |
| Dnd1 | NM_173383 | 0.034 | tumorigenesis | 1.48 |
| Gpr182 | NM_007412 | 0.051 | stimulate cell proliferation | 1.48 |
| Lcn2 | NM_008491 | 0.005 | carcinogenesis | 1.45 |
| Gml | ENSMUST00000096400 | 0.033 | p53 pathway | 1.45 |
| Bcl2 | NM_009741 | 0.009 | anti-apoptosis | 1.43 |
| Ccl27a | NM_011336 | 0.027 | enhance primary tumor | −1.40 |
| Ddhd2 | BC046229 | 0.021 | oncogenesis | −1.41 |
| Son | NM_178880 | 0.006 | protects cells from apoptosis | −1.42 |
| Rhoa | NM_016802 | 0.006 | protects cell from death under during stress | −1.43 |
| Angiogenesis | ||||
| Ctsg | NM_007800 | 0.003 | upregulate VEGF, angiogenesis | 1.43 |
| Nrarp | NM_025980 | 0.003 | VEGF, angiogenesis in retina | 1.42 |
| Rock2 | NM_009072 | 0.014 | retinal neovascularization, neuritogenesis | −1.42 |
| Axon | ||||
| Ermn | NM_029972 | 0.011 | myelinating oligodendrocyte specific protein | 1.51 |
| Prl8a6 | NM_011167 | 0.003 | permeabilized oligodendrocyte marker | 1.50 |
| Qk | U44941 | 0.052 | myelin basic protein mRNA homeostasis | 1.45 |
| Ptprz1 | NM_001081306 | 0.006 | neuritogenesis, anti-apoptotic | −1.49 |
| Oxidative stress/ER stress | ||||
| Ndufb5 | NM_025316 | 0.022 | increases under oxidative stress | 1.46 |
| Anti-oxidant | ||||
| Gpx3 | NM_001083929 | 0.007 | glutathione peroxidase family, detoxification of hydrogen peroxide | 2.34 |
| Gstm6 | NM_008184 | 0.049 | regulated by NRF2, oxidative stress | 1.47 |
| Gstm3 | NM_010359 | 0.055 | Related to Alzheimers disease | 1.45 |
| Cul3 | NM_016716 | 0.030 | regulate NRF2 level, oxidative stress | −1.43 |
| Calcium signaling | ||||
| Fstl5 | NM_178673 | 0.009 | calcium binding motif; diverse superfamily of calcium sensors/signal modulators | −1.56 |
| Neuropeptide | ||||
| Npvf | NM_021892 | 0.057 | FF1 receptor endogenous ligand, anti-opioid effect | 1.75 |
| Penk | NM_001002927 | 0.025 | mimic the effect of opiate drug, increase glutamate release | 1.67 |
| Galr1 | NM_008082 | 0.037 | neuropeptide galranin, expressed in brain | 1.61 |
| Immune response | ||||
| Igh-6 | BC053409 | 0.050 | antigen binding, protein binding | 1.95 |
| Bst1 | NM_009763 | 0.001 | immune response | 1.95 |
| Cd59a | NM_001111060 | 0.014 | immune response | 1.63 |
| Klk1 | NM_010639 | 0.050 | immune response | 1.69 |
| Ptgdr | NM_008962 | 0.009 | immune response | 1.50 |
| Pou2af1 | NM_011136 | 0.012 | immune response | 1.49 |
| Defb35 | NM_139224 | 0.012 | immune response | 1.49 |
| Ccl24 | NM_019577 | 0.003 | immune response | 1.48 |
| Cd2 | NM_013486 | 0.017 | immune response | 1.48 |
| Il23a | NM_031252 | 0.046 | immune response | 1.47 |
| C3 | NM_009778 | 0.005 | immune response | 1.40 |
| Defa24 | NM_001024225 | 0.035 | immune response | −1.42 |
| Transporter | ||||
| Slc6a20a | NM_139142 | 0.041 | transporter express in brain, glycine and proline | 1.49 |
| Mmgt2 | NM_175002 | 0.013 | transporter, upregulated in low Mg2+ | 1.48 |
| Nipal1 | NM_001081205 | 0.045 | Mg2+ transporter | 1.41 |
| Slc7a11 | NM_011990 | 0.011 | cystine/glutamate antiporter (system Xc-) | −1.94 |
Discussion
Numerous studies have demonstrated the profound neuroprotective effects of ligands for σR1 (Martin et al, 2004, Dun et al, 2007; Bucolo et al, 2006; Tchedre et al, 2008, Tchedre and Yorio, 2008; Smith et al, 2008; Ha et al, 2011a), however the mechanism(s) underlying this protection have been elusive. Some investigators have speculated that σR1 has a role in modulating ER stress pathway, especially because of its location at the ER-MAM. The conclusions have been that decreased levels of σR1 lead to upregulation of ER stress genes (Hayashi and Su, 2007) and decreased levels of the anti-apoptotic protein BCL2 concomitant with increased NFκB levels (Meunier and Hayashi, 2010). Indeed, such conclusions have been drawn from elegant studies using in vitro systems (immortalized cell lines) and molecular tools to knockdown σR1 expression (Meunier and Hayashi, 2010).
The present study used a different approach to address the role of σR1. First, our studies focused on a tissue that has demonstrated profound response to σR1 ligands, namely the retina. Second, rather than using cell lines and gene knockdown methods, we have exploited the σR1−/− mouse as the experimental model system. Our laboratory has had a keen interest in the mechanism of σR1 in retinal neuroprotection owing to profound neuroprotection observed in vivo (Smith et al, 2008) and in vitro (Martin et al, 2004; Dun et al, 2007). It is clear that σR1 is not required for survival since the σR1−/− mice have a normal lifespan. It is equally evident that σR1, while not essential for retinal development, may play a role in maintaining the retina especially under conditions of stress as has been reported by our lab (Ha et al, 2011b, Ha et al, 2012) and others (Bucolo et al, 2006; Zhang et al, 2011; Tchedre and Yorio, 2008). The availability of the σR1−/− mouse allowed comprehensive evaluation of genes and proteins whose expression might be altered in retina that might provide clues as to its neuroprotective roles.
We first evaluated genes involved in the ER stress pathway focusing on those that play a major role including BiP/GRP78 and its downstream effectors. ER stress has been implicated in retinal degenerations (Kroeger et al, 2012). Previous work has shown that BiP/GRP78 levels increase under certain stress conditions when σR1 expression is reduced. These studies were performed in vitro and insults were generally acute (e.g. within 24 h) (Hayashi and Su, 2007; Ha et al, 2011a). Indeed, when we induced oxidative stress in a retinal neuronal cell line, we observed robust increase in all of the major ER stress genes, which was reduced when the σR1 ligand (+)-PTZ was used in pre-treatment experiments (Dun et al, 2007). The current in vivo studies using neural retinas from mice over a two-year age range, however, showed no change in BiP/GRP78 in σR1−/− null mice compared to wildtype mice. Moreover expression of the downstream effector genes (Perk, Atf6, Ire1α) was not altered compared to retinas of age-matched wildtype mice. There were no differences in protein levels either. We also examined brains of these mice since σR1 is detected at high levels in brain. Again, absence of σR1 was not associated with an alteration of ER stress genes/proteins. These findings were unexpected and prompted analysis within a subset of retinal cells, namely the Müller glial cells. The retina is a network of connections between various neuronal cell types supported by the radially oriented Müller glial cells that serve numerous maintenance roles. We reasoned that in its supportive role, Müller cells might alter gene expression of stress modulators more readily than some of the other retinal cell types. Previously, we demonstrated that σR1 is localized on the ER membrane in Müller cells (Jiang et al, 2006) and so we isolated these cells from wildtype and σR1−/− mice and analyzed major ER stress genes. There were significant differences in ER stress gene expression in the Müller cells. BiP/GRP78 levels were elevated, while Perk and Ire1α expression decreased. The most profound ER stress gene expression change was observed in Atf6. Levels of this gene were increased over 100 fold in the Müller cells isolated from σR1−/− mice. ATF6 is tethered to the ER membrane by BiP/GRP78. When unfolded proteins accumulate, it is released and translocates to the Golgi apparatus by vesicular transport (Yoshida, 2007; Kaufman, 2004). Unlike Perk and Ire1α, whose expression was not altered significantly in the Müller cells of σR1−/− mice, ATF6 does not undergo oligomerization, rather it is cleaved by proteases in the Golgi and the resultant cytoplasmic portion translocates to the nucleus, where it binds to an ER stress response element to activate transcription of ER chaperone genes such as BiP/GRP78, GRP94 and calreticulin. Thus, ATF6 activation can increase ER chaperone activity. At least within Müller cells, there are significant alterations in ER stress genes that occur in the absence of σR1. These observations were made in Müller cells isolated from very young mice (~5 days). Ideally, we would want to monitor ER stress gene changes over a period of many months in Müller cells isolated from σR1−/− mice compared to wildtype, however efforts to isolate Müller cells from retinas at older ages are hampered by significant technical difficulties and have not been feasible. Relevant to the visual system as a whole, it has been reported that cells isolated from human lens show an increased expression of ER stress genes (BiP, Atf6,Eif2α) when subjected to oxidative stress and expression is attenuated upon treatment with (+)-PTZ (Wang et al, 2012).
In addition to studying whether ER stress gene expression was altered when σR1 was absent, we also investigated levels of BCL2. In earlier σR1−/− knockdown experiments using CHO cells, BCL2 levels decreased (Meunier and Hayashi, 2010). These are very important findings because of the major role BCL2 plays as an anti-apoptotic protein. When we examined Bcl2 in retinas of σR1−/− mice, expression was similar between null and wildtype mice initially, however by 6 weeks of age there was a significant decrease in expression. A decrease in BCL2 protein levels was observed in retinas of σR1−/− mice by 24 weeks (~6 months) of age. The BCL2 levels remained significantly lower than wildtype through two years of age. These studies of the in vivo model strongly support the studies using the σR1 knockdown in cell lines that σR1 mediates its neuroprotective effects by modulating BCL2 (Meunier and Hayashi, 2010). Thus, σR1 appears to modulate Bcl2 expression, though it does not appear to modulate Bax expression.
We next investigated genes that regulate Bcl2 expression in σR1−/− mouse retina. There are many transcription factors that regulate Bcl2 expression in various tissues. Among these, NF-κB, which is comprised of several subunits such as p105, p50 and p65, has been reported to control Bcl2 expression (Kurland et al, 2001). Just as BCL2 protein levels were lower in retinas of σR1−/− mice compared to age-matched controls, so also were NF-κB (p50) levels reduced in σR1−/− retinas compared to normal mice. ERK signaling is well known to regulate Bcl2 expression (Feng et al, 2004). Our studies showed that levels of phosphorylated ERK1/2 (but not total ERK1/2) were reduced in retinas of σR1−/− mice compared to wildtype. Taken collectively, the data suggest that σR1 modulates Bcl2 levels. The age-related decrease in levels of the anti-apoptotic protein BCL2 and proteins that regulate it may account for the late onset inner retinal degeneration observed in σR1−/− mice (Ha et al, 2011b).
An important protein that is regulated by BCL2 via ERK signaling is αB crystallin. It has been reported that BCL2 negatively regulates expression of the gene encoding αB crystallin through ERK signaling. We found a trend toward an increase in the levels of αB crystallin protein in σR1−/− retina compared to wildtype mice. These data raise the possibility that σR1 may modulate αB crystallin expression, an area of research that deserves further investigation. This is noteworthy given that αB crystallin deficient mice have an increase in ER stress gene expression in retina (Dou et al, 2012).
The outcome of these studies underscores the complexity of σR1 and its role in neuroprotection. It appears that σR1 may regulate ER stress, especially in Müller cells, whether this involves αB crystallin remains to be investigated. The role of σR1 in neuroprotection likely involves BCL2 and some of the proteins that modify its expression (such as ERK, NFκB). Finally, our data from the analysis of the retinal transcriptome of σR1 null mice provides new avenues to understand the role of σR1 in neuroprotection including investigation of genes involved in antioxidant functions and VEGF regulation.
Acknowledgments
Supported by a grant from the National Institutes of Health, R01 EY014560
References
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou JC, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16:4186–4194. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Drago F, Lin LR, Reddy VN. Sigma receptor ligands protect human retinal cells against oxidative stress. Neuroreport. 2006;17:287–291. doi: 10.1097/01.wnr.0000199469.21734.e1. [DOI] [PubMed] [Google Scholar]
- Cenni MC, Bonfanti L, Martinou JC, Ratto GM, Strettoi E, Maffei L. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur J Neurosci. 1996;8:1735–1745. doi: 10.1111/j.1460-9568.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R, Hinton DR. Deficiency of αB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med. 2012;53:1111–1122. doi: 10.1016/j.freeradbiomed.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci. 2007;48:4785–4794. doi: 10.1167/iovs.07-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Xiang H, Mao YW, Wang J, Liu JP, Huang XQ, Liu Y, Liu SJ, Luo C, Zhang XJ, Liu Y, Li DW. Human Bcl-2 activates ERK signaling pathway to regulate activating protein-1, lens epithelium-derived growth factor and downstream genes. Oncogene. 2004;23:7310–7321. doi: 10.1038/sj.onc.1208041. [DOI] [PubMed] [Google Scholar]
- Galante JM, Mortenson MM, Bowles TL, Virudachalam S, Bold RJ. ERK/BCL-2 pathway in the resistance of pancreatic cancer to anoikis. J Surg Res. 2009;152:18–25. doi: 10.1016/j.jss.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Gerasimenko J, Ferdek P, Fischer L, Gukovskaya AS, Pandol SJ. Inhibitors of Bcl-2 protein family deplete ER Ca2+ stores in pancreatic acinar cells. Pflugers Arch. 2010;460:891–900. doi: 10.1007/s00424-010-0859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Dun Y, Thangaraju M, Duplantier J, Dong Z, Liu K, Ganapathy V, Smith SB. Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Invest Ophthalmol Vis Sci. 2011a;52:527–540. doi: 10.1167/iovs.10-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Saul A, Tawfik A, Williams C, Bollinger K, Smith R, Tachikawa M, Zorrilla E, Ganapathy V, Smith SB. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (σR1) Invest Ophthalmol Vis Sci. 2011b;52:7749–7760. doi: 10.1167/iovs.11-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Saul A, Tawfik A, Zorrilla E, Ganapathy V, Smith SB. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1 (σR1) Mol Vision. 2012;18:2860–2870. [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma 1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, GanapathyVSmith SB. Expression, subcellular localization and regulation of sigma receptor in retinal Müller cells. Invest Ophthamol Vis Sci. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Sreekumar PG, Hinton DR. Novel roles for α-crystallins in retinal function and disease. Prog Retin Eye Res. 2012;31:576–604. doi: 10.1016/j.preteyeres.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Regulation of mRNA translation by protein folding the endoplasmic reticulum. Trends Biochem Sci. 2004;29:152–158. doi: 10.1016/j.tibs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kroeger H, Messah C, Ahern K, Gee J, Joseph V, Matthes MT, Yasumura D, Gorbatyuk MS, Chiang WC, LaVail MM, Lin JH. Induction of endoplasmic reticulum stress genes, BiP and chop, in genetic and environmental models of retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:7590–7599. doi: 10.1167/iovs.12-10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland JF, Kodym R, Story MD, Spurgers KB, Mcdonnell TJ, Meyn RE. Nf-kB1 (p50) homodimers contribute to transcription of the bcl-2 oncogene. J Biol Chem. 2001;276:45380–45386. doi: 10.1074/jbc.M108294200. [DOI] [PubMed] [Google Scholar]
- Kurland JF, Voehringer DW, Meyn RE. The MEK/ERK pathway acts upstream of NF-kB1 (p50) homodimer activity and bcl-2 expression in a murine B-cell lymphoma cell line. J Biol Chem. 2003;278:32465–32470. doi: 10.1074/jbc.M212919200. [DOI] [PubMed] [Google Scholar]
- Liu LL, Wang L, Zhong YM, Yang XL. Expression of sigma receptor 1 mRNA and protein in rat retina. Neuroscience. 2010;167:1151–1119. doi: 10.1016/j.neuroscience.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- Martin PM, Ola MS, Agarwal N, Ganapathy V, Smith SB. The sigma receptor ligand (+)-pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Mol Brain Res. 2004;123:66–75. doi: 10.1016/j.molbrainres.2003.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Nickells RW, Guo LW. Accelerated retinal ganglion cell death in mice deficient in the sigma-1 receptor. Mol Vis. 2011;17:1034–1043. Mavlyutov et al, 2011. [PMC free article] [PubMed] [Google Scholar]
- Mercatelli N, Dimauro I, Ciafré SA, Farace MG, Caporossi D. AlphaB-crystallin is involved in oxidative stress protection determined by VEGF in skeletal myoblasts. Free Radic Biol Med. 2010;49:374–382. doi: 10.1016/j.freeradbiomed.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Meunier J, Hayashi T. Sigma receptors regulate bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kB. J Pharmacol Exp Ther. 2010;2:388–397. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, Parys JB, Leybaert L, Bultynck G. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa Y, Kwong JM, Caprioli J, Piri N. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci. 2009;50:3869–3875. doi: 10.1167/iovs.08-3138. [DOI] [PubMed] [Google Scholar]
- Nickells RW. Variations in the rheostat model of apoptosis: what studies of retinal ganglion cell death tell us about the functions of the Bcl2 family proteins. Exp Eye Res. 2010;91:2–8. doi: 10.1016/j.exer.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Moore P, El-Sherbeny A, Roon P, Agarwal N, Sarthy VP, Casellas P, Ganapathy V, Smith SB. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Mol Brain Res. 2001;95:86–95. doi: 10.1016/s0169-328x(01)00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333–340. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, Mignery GA, Parys JB, De Smedt H, Distelhorst CW. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Duplantier J, Dun Y, Mysona B, Roon P, Martin PM, Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces bcl-2 and bcl-x expression through NFkB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- Tchedre KT, Huang RQ, Dibas A, Krishnamoorthy RR, Dillon GH, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci. 2008;49:4993–5002. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- Tchedre KT, Yorio T. Sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest Ophthalmol Vis Sci. 2008;49:2577–2588. doi: 10.1167/iovs.07-1101. [DOI] [PubMed] [Google Scholar]
- Wang L, Eldred JA, Sidaway P, Sanderson J, Smith AJ, Bowater RP, Reddan JR, Wormstone IM. Sigma 1 receptor stimulation protects against oxidative damage through suppression of the ER stress responses in the human lens. Mech Ageing Dev. 2012;133:665–674. doi: 10.1016/j.mad.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJH, Pearce MMP, Sliter DA, Wang Y. When worlds collide: IP3 receptors and the ERAD pathway. Cell Calcium. 2009;46:147–153. doi: 10.1016/j.ceca.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T, Jones DP, Wang X. Prevention of apoptosis by bcl2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yang S, Bhardwaj A, Cheng J, Alkayed N, Hurn PD, Kirsch JR. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth Analg. 2007;104:1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–58. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- Zhan Y, van de Water B, Wang Y, Stevens JL. The roles of caspase-3 and bcl-2 in chemically-induced apoptosis but not necrosis of renal epithelial cells. Oncogene. 1999;18:6505–6512. doi: 10.1038/sj.onc.1203060. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Liu LL, Jiang SX, Zhong YM, Yang XL. Activation of the σ receptor 1 suppresses NMDA responses in rat retinal ganglion cells. Neuroscience. 2011;177:12–22. doi: 10.1016/j.neuroscience.2010.12.064. [DOI] [PubMed] [Google Scholar]