Abstract
Decades of research have been undertaken towards the goal of tissue engineering using xenogeneic scaffolds. The primary advantages associated with use of xenogeneic tissue-derived scaffolds for in vitro development of replacement tissues and organs stem from the inherent extracellular matrix (ECM) composition and architecture. Native ECM possesses appropriate mechanical properties for physiological function of the biomaterial and signals for cell binding, growth, and differentiation. Additionally, xenogeneic tissue is readily available. However, translation of xenogeneic scaffold-derived engineered tissues or organs into clinical therapies requires xenoantigenicity of the material to be adequately addressed prior to implantation. Failure to achieve this goal will result in a graft-specific, host immune rejection response, jeopardizing in vivo survival of the resultant scaffold, tissue, or organ. This review explores (1) the appropriateness of scaffold acellularity as an outcome measure for assessing reduction of the immunological barriers to the use of xenogeneic scaffolds for tissue engineering applications and (2) the need for tissue engineers to strive for antigen removal during xenogeneic scaffold generation.
Key terms: Antigen removal, decellularization, xenogeneic scaffold, extracellular matrix, tissue engineering
1. INTRODUCTION
The extracellular matrix (ECM) of native tissues has the potential to be an ideal scaffold for use in tissue engineering and regenerative medicine applications. Indeed, clinical use of biologically-derived ECM scaffolds in non-critical sites for urethroplasty [1], fistula repair [2], and hernia repair [3] have been deemed successful. For a given tissue type, the inherent ECM composition and architecture confers the appropriate mechanical properties necessary for immediate physiological function of the resultant scaffold [4]. This is in contrast to synthetic scaffolds which generally require extended periods of time to undergo tissue morphogenesis and remodeling under relevant environmental conditions in order to gain acceptable mechanical properties [5–8]. The advantage of having an inherently mechanically-appropriate scaffold is particularly important for grafts that are required to function immediately upon implantation, such as heart valves which must maintain unidirectional blood flow in the heart. Moreover, the complex compositional and architectural elements that constitute ECM, organized at the levels of molecules, macromolecules, and complexes of macromolecules, provide both molecular and topographical cues which are capable of directing cellular phenotype and organization [9–12]. These multi-scale intricacies of native ECM are currently beyond the capabilities of even the most sophisticated synthetic approaches. Finally, as the composition and structure of ECM are not yet fully characterized, completely synthetic ECM cannot currently be generated in vitro [4]. However, additional research will be required for the potential of xenogeneic tissue-derived ECM scaffolds to be fully realized in both tissue engineering and basic science applications.
Recipient immune response to the antigenic components of xenogeneic tissues represents the critical barrier to the use of xenogeneic scaffolds in translational applications. Indeed, the National Heart, Lung, and Blood Institute (NHLBI): Heart and Lung Xenotransplantation Working Group has identified the antigenicity of xenogeneic tissues to be the major hurdle to increased utilization of these materials in clinical medicine [13]. In spite of the aforementioned early clinical success of ECM scaffolds [1–3], low levels of immune response to these products [14] will lead to loss of function or destruction in more critical sites of the body such as whole organs. For tissue engineering to reach its full potential, even residual antigenicity which elicits a low grade immune response may need to be resolved. Heart valve replacements exemplify the magnitude of the xenogeneic tissue antigenicity issue. Even a minor immune response to antigens that persist within a glutaraldehyde-fixed, bioprosthetic heart valve is capable of triggering a rejection cascade, resulting in graft calcification, destruction and loss of function [15, 16]. Owing to the crucial role of heart valves in the circulatory system, immune-mediated loss of valve function has catastrophic effects on the medical status of the patient. Therefore, heart valve bioprostheses serve as a rigorous model for the study of tissue antigenicity, which can inform attempts to utilize xenogeneic scaffolds in tissue engineering efforts for other functionally critical tissues and organs.
Initial attempts to overcome the antigenicity of xenogeneic tissues utilized chemical fixation processes, primarily glutaraldehyde cross-linking, to mask xenoantigens from being accessed and recognized by the host immune response [8, 15, 16]. Such chemical cross-linking methods have been successful in delaying hyperacute and acute recipient immune responses towards xenogeneic tissues [17–19]. However, chronic, low grade immune surveillance of antigens in chemically fixed biological heart valves eventually results in immune-mediated degeneration and subsequent calcification of the biomaterial [15, 16] limiting bioprosthesis lifespan to 5–10 years in adults [8, 16, 20]. The potent immune response of juvenile patients reduces the bioprosthesis lifespan to less than 5 years in children [16, 21, 22], with the need for replacement likely within 3 years [23]. Additionally, chemical fixation prevents both recellularization and ECM turnover of treated xenogeneic tissue, rendering the biomaterial incapable of adapting to changes in the microenvironment, integration with the host, and growth [24].
To overcome the issues associated with implanting chemically fixed, xenogeneic tissues in human patients, an intense research effort has been undertaken with the goal of developing immunologically acceptable, unfixed, xenogeneic tissue-derived scaffolds. An immunologically acceptable biomaterial would be capable of avoiding the destructive host immune response following implantation for a length of time sufficient to allow for complete matrix turnover to occur. A strategy that sufficiently reduces the antigenicity of unfixed, xenogeneic tissue without compromising the biomaterial structure-function properties and recellularization potential will be critical for expanding the use of xenogeneic scaffolds in tissue engineering and regenerative medicine. This review discusses the current state of decellularization and antigen removal approaches in the generation of immunologically acceptable, unfixed, xenogeneic scaffolds and highlights important considerations for future work. While it focuses primarily on the cellular and humoral adaptive immune responses that mediate rejection in the stringent heart valve model, examples from other tissues are included where relevant.
2. ANTIGENICITY IN XENOTRANSPLANTATION
Recipient immune response to transplantation of xenogeneic tissues and organs has been extensively described in immunology journals [25–35] and is therefore not the primary focus of the current review. However, a basal understanding of the recipient immune response towards xenoantigens is critical to inform the strategies necessary for generating immunologically acceptable, xenogeneic scaffolds for tissue engineering applications. The majority of our current understanding about xenograft rejection processes stems from characterization of the immune response towards whole organ xenotransplantation. Although the manifestation of immune rejection may differ for tissues as compared to organs which possess an extensive, pre-existing xenogeneic vascular network, the underlying mechanisms responsible for initiation of rejection are largely conserved.
2.1. Hyperacute rejection of xenogeneic organs
Hyperacute rejection (HAR) is an aggressive rejection response, occurring on the order of minutes to hours following implantation of a vascularized xenograft [25–35]. The primary effectors of HAR are pre-existing, xenoreactive natural antibodies, present within the host circulation prior to introduction of foreign material. Upon implantation, these xenoreactive natural antibodies bind to their corresponding antigens within the xenograft, activating the complement cascade. Complement activation along the endothelium of the vasculature results in endothelial damage and subsequent intravascular coagulation, edema formation, and bleeding into the implanted graft. Ultimately, HAR leads to organ destruction and rapid loss of graft function. The antigenic carbohydrate galactose-α(1,3)-galactose (α-gal) epitope is a post-translational modification on numerous glycoproteins and glycolipids expressed on the surface of all cells derived from non-primate mammals and New World monkeys [36]. In humans and Old World monkeys, constitutive loss of the α1,3-galactosyltransferase enzyme responsible for synthesizing the α-gal moiety results in the absence of α-gal expression on the cell surface. Due to continuous exposure to gut bacteria expressing the α-gal moiety on mucosal surfaces [37], naturally occurring xenoreactive antibodies against α-gal (anti-Gal) are generated by humans and Old World monkeys [36]. In humans, anti-Gal antibodies make up 1— 8% of immunoglobulin M (IgM) and 1—2.4% of immunoglobulin G (IgG) antibodies [38]. Therefore, the α-gal epitope is the dominant mediator of HAR in discordant xenotransplantations [26].
2.2. Acute immune rejection of xenogeneic organs
In the absence of α-gal-mediated immune rejection (through anti-Gal antibody depletion from recipient circulation, use of α-gal-deficient (Gal −/−) donor material, and allotransplantation), subsequent avoidance of HAR permits xenograft rejection through acute mechanisms [25–35]. Acute humoral xenograft rejection (AHXR) of transplanted grafts occurs days to weeks after introduction into the recipient due to the presence of non-Gal xenoreactive natural antibodies circulating at low levels. Antibody binding to xenoantigens on donor cells results in complement fixation and resultant cell lysis. Additionally, binding o f xenoreactive natural antibodies to Fc receptors on the surface of host innate cells allows these cells to behave like antigen presenting cells, activating T cells and promoting cellular infiltration into the graft, initiating cell-mediated rejection [31]. These processes culminate in graft dysfunction and ultimate destruction.
2.3. Chronic immune rejection as informed by allotransplantation
The inability to overcome HAR and AHXR associated with xenotransplantation has limited the current understanding of chronic immune response towards unfixed xenografts [25– 33]. Therefore, observations made from allotransplantation inform the potential onset of chronic rejection following xenotransplantation if HAR and AHXR can be ameliorated. Chronic allograft rejection has been induced through introduction of anti-donor antibodies [25, 39], suggesting that antibody-mediated (humoral) responses play an important role in mediating chronic rejection episodes. Proliferation of endothelial cells and smooth muscle cells has been observed after complement activation, suggesting the potential for chronic lesion formation in xenografts [25, 40]. The cell-mediated mechanism of chronic allograft rejection is primarily due to major histocompatibility complex (MHC) molecules expressed on the surface of donor cells. The high degree of polymorphism present in MHC molecules makes them potent mediators of both innate and adaptive immune responses in both allograft and xenograft transplantation [25, 26]. The MHC I molecule, equivalent to human leukocyte antigen (HLA) I in non-human species, is located in the cell membrane of all nucleated cells within a tissue. Thus, MHC I molecules serve as a marker of self versus non-self to circulating CD8+ cytotoxic T cells [31, 32] and natural killer cells [25]. Incompatibility of donor MHC I molecules with inhibitory receptors on natural killer cells initiates a cytotoxic response. The MHC II molecule is found on specialized antigen presenting cells of the immune system. Interaction of CD4+ helper T cells with donor MHC II molecules triggers allorecognition, activation, and amplification of both T and B cell responses [31, 32]. Therefore, MHC molecules represent the most critical mediator of chronic rejection. However, cross-matching of the MHC antigen does not guarantee absence of subsequent chronic rejection events. For example, foreign minor histocompatibility antigens are recognized by T cells between siblings with identical MHC molecules [31, 41, 42]. The allotransplantation literature indicates that recipients develop rising antibody titers against numerous non-MHC antigens, which correlates with subsequent chronic rejection episodes [43]. Genetic diversity provides the basis for a spectrum of non-MHC proteins which are capable of triggering immune rejection in the recipient. Presentation of donor peptides on recipient antigen presenting cells stimulates CD4+helper T cells to mount a rejection response. Therefore, both MHC and non-MHC antigenic components of donor tissues and organs are capable of mediating chronic rejection responses.
2.4. Tissue rejection as informed by organ transplantation
The body of work on immune rejection of tissue- and organ-derived biological scaffolds is growing, but still at a nascent stage. Initiating factors and resultant immune responses are still less well-characterized in relatively avascular grafts as compared to whole organs. Undoubtedly, the extensive vasculature in whole organs accelerates the interactions between donor antigenic epitopes and recipient humoral and cell-mediated effectors that initiate the immune rejection process [44]. Nonetheless, the initiators described above in the whole organ setting are still likely to be relevant in the tissue setting for facilitating immune rejection of the implanted graft. In particular, the common denominator in elicited rejection responses against an implanted graft is the persistence of antigens [25–35]. As mentioned above, the ubiquitous α-gal and MHC I molecules represent the most critical barriers to the acceptance of a xenograft. Additionally, increasing levels of elicited, graft-specific antibodies over time [45] and rising antibody titers [14, 46] against non-MHC protein antigens in xenogeneic tissues have been observed and are likely mediators of chronic rejection even if α-gal and MHC I responses are overcome. Finally, non-protein antigenic components are also known to exist, such as the non-Gal, carbohydrate Hanganutziu-Deicher and Forssman antigens [30, 34, 35]. While antibodies against these two carbohydrate antigens exist, it is not yet known whether they indeed translate to an immune response. Existence of lipid antigens has not yet been confirmed, but cannot be ruled out as a trigger of immune rejection. Therefore, as noted by the NHLBI: Heart and Lung Xenotransplantation Working Group, xenoantigens represent the major hurdle to expanding the use of xenogeneic tissues and organs in clinical practice [13].
3. DECELLULARIZATION
Decellularization is a process intended to eliminate the host immune response towards xenogeneic tissues and organs while leaving the ECM intact [47] to serve as a scaffold for recellularization. Many approaches for decellularization have been investigated [48, 49] and include osmotic [5, 47, 50–55], chemical [5, 7, 46, 51–77], enzymatic [5, 52, 55, 63, 69, 75, 78], and mechanical [55, 69, 79] methods for removal of the cellular component of a xenograft. The efficacy of various decellularization [75] strategies have been tested on relatively simple tissue types including skin [14, 46, 73], small intestine submucosa (SIS) [14, 70, 73], pericardium [5, 53, 75], heart valve [47, 50–52, 56–63, 72, 76, 78, 80, 81], blood vessel [55, 75, 79, 82], cartilage [68, 83], and bone [54] and applied to more complex organs such as liver [64, 69, 73], kidney [65, 66, 71, 77], and heart [67]. Application of decellularization methods to reduce the immunological potential of xenogeneic tissues and organs is grounded in the assumptions that (1) the cellular component of a xenograft represents the sole contributor to its antigenicity and (2) the absence of cells (by microscopic evaluation of residual nuclei, DNA quantification, and/or assessment of residual, cellular proteins of neither confirmed nor denied immunogenicity) correlates with removal of antigenic components. It should be noted that some groups have attempted to better define the measures with which they classify a decellularized scaffold as acellular [44, 73]. Approaches to evaluate scaffold acellularity following decellularization have primarily utilized histological assessment of residual nuclei [5, 44, 46, 47, 51, 52, 56–62, 70, 73–76]; however, recent attempts to more rigorously characterize r esultant scaffold acellularity have included DNA quantification [7, 44, 46, 60, 70, 73–75], analysis of residual DNA fragment length [44, 70, 73], and assessment of residual cell structural proteins (without demonstrated antigenicity) [7, 58, 74, 82]. That being said, no standard currently exists for success criteria of decellularization. Therefore, the validity of scaffold acellularity as an outcome measure for the assessment of residual antigenicity demands closer inspection.
3.1. In vivo immune response to decellularized allografts and xenografts
Evaluation of decellularization as a means of producing immunologically acceptable, unfixed, tissue-derived scaffolds has yielded conflicting results. Various decellularization methods have been shown to achieve acellularity [5, 44, 46, 47, 51, 52, 56–62, 73, 75–77]. These studies encouraged several subsequent in vivo studies to assess whether achievement of acellularity translated to avoidance of the host immune response. Unfortunately, implantation of decellularized allografts into human patients produced mixed results regarding compatibility of acellular scaffolds with the recipient immune response. Standard cryopreserved heart valve allografts resulted in elevated HLA class I and II panel reactive antibody levels; however, SynerGraft decellularized allografts exhibiting 99% reduction in staining for cellular components and notable reduction in staining for HLA class I and II molecules did not significantly increase HLA class I or II panel reactive antibody levels in children up to 12 months post-operatively compared to pre-operative levels [50]. Conversely, Sayk et al. [61] reported macrophage infiltration into a SynerGraft decellularized, acellular pulmonary valve allograft 5 weeks after implantation into and 5 weeks before death of a human patient. Increasing levels of donor-specific antibodies against HLA class I and II antigens were measured in adults implanted with heart valve allografts decellularized using the ionic detergent sodium dodecyl sulfate (SDS) [81]. Affonso da Costa et al. [57] reported mild to no humoral response towards HLA class I and II antigens in most cases following implantation of acellular, AutoTissue Ltd™ decellularized allografts into human patients up to 180 days post-implantation. However, even within the same study, some patients showed contradictory responses. In patients receiving acellular, AutoTissue Ltd™ decellularized allografts that developed antibodies against HLA class I and II antigens, the humoral response was detected as early as the 5th or 10th day post-implantation compared to patients receiving cryopreserved allografts developing a humoral response at day 15. Xenotransplantation of decellularized, animal-derived tissue into various animal and human hosts also failed to yield conclusive findings regarding host tolerance of acellular scaffolds. Acellular, porcine aortic root decellularized using SDS and octylphenoxy polyethoxyethanol (Triton X-100) was associated with minimal inflammatory cell infiltration and reduced calcification following subdermal implantation into rats for up to 60 days [56]. The same acellular porcine aortic root implanted into the pulmonary valve position of dogs for up to 6 months showed no signs of infiltration by immune cells or calcification. Similarly, porcine aortic valve leaflets rendered acellular using the SynerGraft decellularization process and reassembled into a composite trileaflet structure were implanted into the right ventricular outflow tract of sheep for 150 days [47]. In this model, the authors noted only a small population of monocytes and lymphocytes associated with these SynerGraft composite heart valves that otherwise largely resembled their allograft controls. However, implantation of acellular, peracetic acid decellularized porcine SIS into a rat partial thickness abdominal wall defect model was associated with marked cellular infiltration [70]. Although decreased following treatment with peracetic acid compared to PBS, the M1 pro-inflammatory macrophage response towards SIS was still observed following decellularization over 28 days of implantation. In the clinical setting, rapid failure of SynerGraft decellularized porcine pulmonary and aortic roots was observed following implantation into juvenile patients [84]. This outcome was attributed to severe inflammatory response and fibrous encapsulation of the prostheses, leading to the deaths of three out of four children and explantation of the valve from the remaining subject. Similarly, re-operation was required for 6 patients under 25 years of age due to obstruction of implanted AutoTissue Ltd™ Matrix P plus heart valves [80]. Graft failure was attributed to the severe inflammatory response towards the decellularized porcine pulmonary valve and glutaraldehyde-fixed equine pericardial-walled conduit in this short to mid-term study. Histological analysis of the stenotic explants revealed fibrosis at the peripheral end of the conduit, dense intimal peel and prominent narrowing, and “dissection-like” separation of the wall layers—concluded to stem from an “immunogenic-boost”-like reaction. The inconsistencies between these various in vivo results can be attributed to several experimental design parameters, including immunological environment of the transplantation model (small animal versus large animal, concordant versus discordant transplantation), graft implantation site (relatively vascular or avascular), and duration of the in vivo study. Collectively, these in vivo studies cast considerable doubt on the ability of decellularization techniques alone and the associated outcome measure of acellularity to generate immunologically acceptable, unfixed scaffolds and prompted closer inspection of the immune response towards decellularized, unfixed, tissue- and organ-derived scaffolds.
3.2. In vitro immune potential of decellularized scaffolds
Unfortunately, numerous studies have demonstrated the ability of acellular scaffolds to elicit inflammatory and immune responses in animal and human in vitro models. Platelet activation [59], classical complement system pathway activation [62], monocyte recruitment [58–60], granulocyte recruitment [60], granulocyte activation [62], granulocyte binding [62], lymphocyte recruitment [60], T cell proliferation [78], and T cell activation [78] have all been associated with decellularized scaffolds. For the studies in which only inflammatory response towards an ECM scaffold was mentioned, no observations of the immune response were reported. It is unknown whether no immune response towards the biomaterial occurred or no assessment of potential immune response was performed. Therefore, no comment on the role of the immune response in the reported outcomes can be made. Kasimir et al. [59] reported both adhesion of activated platelets and migration of human monocytes to acellular, porcine pulmonary valve conduits and SynerGraft model 700 decellularized pulmonary valves. Rieder et al. [60] observed reduced, but not abolished, monocyte and lymphocyte migration following decellularization, associated with removal of proteins promoting migration. However, granulocyte migration towards acellular porcine and human pulmonary roots was maintained compared to native tissue and attributed to inability to remove proteins promoting granulocyte migration via decellularization. Bastian et al. [62] demonstrated adsorption of human IgG proteins onto acellular porcine pulmonary heart valves, which in turn activated the classical complement pathway, and promoted adhesion of activated granulocytes. Zhou et al. [52] observed no difference in complement activation or granulocyte activity of acellular, porcine pulmonary valve following decellularization using sodium deoxycholate (SDC), SDS, trypsin-EDTA, or trypsin-EDTA with Triton X-100 compared to untreated tissue. Finally, Bayrak et al. [78] found that decellularized porcine pulmonary heart valves promote T cell proliferation and activation. Recent work in macrophage polarization has described two macrophage phenotypes in host response: M1 macrophages which are effectors of the pro-inflammatory degenerative response and M2 macrophages which have been linked to a regenerative response following tissue damage [44, 85–87]. Identification of M2 macrophages suggests the possibility of a benefit to the inflammatory response in tissue regeneration, an area under much investigation. Nonetheless, a significant body of work indicates that the sole goal of scaffold acellularity does not guarantee the elimination of inflammatory and immune responses towards decellularized biomaterials.
3.3. Persistence of antigens in decellularized scaffolds
Persistence of the host immune response towards decellularized tissues implies that scaffold acellularity alone is an insufficient outcome measure of adequate elimination of antigenicity and the corresponding graft-specific host immune response. Indeed, both in vitro and in vivo studies have demonstrated that known xenoantigens remain in acellular tissues subjected only to decellularization processes. Goncalves et al. [5] reported persistence of one of the most critical barriers to xenotransplantation, α-gal, on acellular bovine pericardium following a hypotonic lysis-mediated decellularization approach similar to that used for generation of SynerGraft porcine aortic valves coupled with SDC. Even more critically, Kasimir et al. [59, 88] identified α-gal epitopes on an actual SynerGraft decellularized porcine valve. Residual MHC I antigens have also been detected following hypotonic lysis [5] or SDS decellularization [89] of bovine pericardium and osmotic lysis or Triton X-100 decellularization of rat aortic valve [90]. Consequently, persistence of either α-gal or MHC antigens has substantial and potentially catastrophic implications on the longevity of xenogeneic scaffold implants subjected to the human host immune response. No significant increase in α-gal antibody titers was reported for seventy patients (59.2±9.3 years old) 12 months after implantation with AutoTissue Ltd™ Matrix P plus decellularized porcine heart valves [72]. Similarly, Sandor et al. [14] observed a minimal increase in α-gal titers from Old World monkeys—a discordant xenotransplantation model—during the 6 month period following abdominal wall resection and repair using decellularized porcine SIS (Surgisis® Gold) or acellular dermal matrix (Permacol® or CollaMend). However, the dramatic rise of graft-specific antibodies (directed at other unknown donor tissue antigens) in the serum indicates that the processes employed to render these porcine tissues acellular failed to eliminate unknown xenoantigens from the biomaterial, resulting in the development of an immune reaction towards the implant. Therefore, it is clear that both known (α-gal and MHC I) and unknown (against which graft-specific antibodies were produced) xenoantigens persist in apparently acellular tissue scaffolds, demonstrating the deficiency of utilizing scaffold acellularity as the sole outcome measure for assessment of tissue scaffold antigen content.
3.4. Flawed fundamental assumptions of scaffold acellularity as a sole outcome measure
The failure of decellularization strategies alone to adequately reduce the immunological barriers associated with xenotransplantation questions the validity of using scaffold acellularity as the sole outcome measure for assessment of xenogeneic ECM scaffold production methods. This insufficiency is rooted in the fundamental assumptions of decellularization. First, decellularization assumes that xenoantigens are solely cellular in origin. As a result, decellularization approaches focus on compromising the cell membrane and eliminating the nucleus and other structural elements, as indicated by assessment of residual nuclei, DNA, or cell components without demonstrated antigenicity. However, ECM components themselves have been identified to elicit the host immune response. Bovine collagen has been observed to induce an allergic reaction in 3% of the population, resulting in foreign body granuloma formation [91] and necessitating employment of a skin test for sensitivity to bovine collagen prior to administration of collagen-based products. Antibodies against linear and conformational epitopes in bovine collagen type I, II, and III have been found in sera of these hypersensitive individuals [91]. Furthermore, Griffiths et al. [45] discovered several non-collagenous, matrix-associated xenoantigens in bovine pericardium. The second assumption commonly made in decellularization work is that assessment of residual cellularity (via residual nuclei counts under light or fluorescence microscopy, residual DNA content, or persistence of structural cellular proteins without confirmed antigenicity) serves as an appropriate indicator of residual antigenicity. However, scanning electron microscopy [84] and near infrared multiphoton laser scanning microscopy [63] of apparently acellular material have revealed residual cells and cellular debris, illustrating that absence of nuclei does not equate to absence of cellular material. Cell debris [8] and associated specific humoral [15, 16] and cell-mediated [16] xenograft rejection responses have been implicated in tissue calcification. Thus, microcalcifications on SDS-decellularized porcine aortic valve conduits 3 weeks after implantation into sheep further supports the presence of residual cell debris on microscopically acellular tissue [51]. Additionally, persistence of the known α-gal [5, 59, 82, 88] and MHC I [5, 82, 89, 90] xenoantigens, and development of graft-specific antibodies [14, 46, 82] following decellularization treatments as discussed above underscores the concern that acellularity alone does not accurately reflect residual antigenicity of tissue scaffolds. Finally, no correlation was found between residual nuclei counts and either residual hydrophilic antigenicity [92] or residual lipophilic antigenicity [89] of bovine pericardium. Highlighted by the absence of a link between the nuclei within a tissue and remaining cell debris, known antigens (α-gal or MHC I), or global antigenicity (graft-specific antibodies), tissue acellularity alone is an inappropriate goal in determining the degree to which the antigenic burden of a tissue or organ has been reduced. Concerns over the rigor of histological analysis of scaffold acellularity, have prompted additional scaffold evaluation methods including quantification of residual DNA content [7, 44, 46, 60, 70, 73–75] and residual cellular proteins (not necessarily antigenic in nature) [7, 58, 74, 82]. Unfortunately, such scaffold assessment methods still only inform the presence of cellular components with neither confirmed nor denied antigenicity and thus do not actually ensure measurement of persistent antigenic determinants. Assessment of DNA content is quantitative means to determine presence of nuclear remnants and still merely a surrogate for immunogenicity. Similarly, evaluation of residual proteins—not determined to be antigenic—also represent a means to infer immunogenicity without actually measuring persistence of antigens. Collectively, these studies indicate that fundamental assumptions of decellularization and subsequent use of acellularity as the only outcome measure of residual antigenicity are inaccurate. That is, antigenic determinants are not merely associated with the cellular fraction of a tissue and apparent elimination of nuclei, DNA, or cellular proteins without confirmed antigenicity does not closely correlate with removal of antigenic components.
4. ANTIGEN REMOVAL
The shortcomings associated with use of decellularization and scaffold acellularity as the only outcome measure for the generation of immunologically acceptable, unfixed, xenogeneic scaffolds have reinforced the immediate need for improved strategies to remove antigenic components of xenogeneic tissues and organs, and use of resultant scaffold residual antigenicity as a more specific outcome measure. The antigen removal paradigm avoids erroneous oversimplification of the immunogenicity problem as observed with decellularization methods that merely target cell removal as a surrogate for removal of antigens. Instead, antigen removal approaches (1) recognize that cellular and non-cellular antigens within a tissue or organ both represent immunological barriers, (2) strive to eliminate these sources of antigenicity, and (3) appreciate the need to assess residual antigenicity and determine how this outcome measure translates to the ultimate goal of generating immunologically acceptable, unfixed, tissue- and organ-derived scaffolds.
4.1. Targeted antigen removal
Targeted removal of specific, known xenoantigens has been greatly informative to the field of antigen removal (Figure 1). Application of α-galactosidase for enzymatic removal of α-gal epitopes has been shown to eliminate this specific, known xenoantigen from xenogeneic tissues [5, 46, 53, 93]. Goncalves et al. [5] demonstrated the ability to target the removal of α-gal from bovine pericardium using the enzyme α-galactosidase. Park et al. [93] showed that human recombinant α-galactosidase could eliminate α-gal from porcine aortic valve and pericardium in a tissue-specific and enzyme concentration-dependent manner. Nam et al. [53] was able to avoid the use of potentially damaging acidic conditions required for optimal human recombinant α-galactosidase activity through the use of Bacteroides thetaiotaomicron-derived α-galactosidase for removal of α-gal from bovine pericardium. At pH 7.2 and 4°C, the activity of bacterial-derived enzyme was 100 times greater than its human recombinant analog. Xu et al. [46] subjected decellularized porcine dermis (porcine acellular dermal matrix, PADM) to α-galactosidase treatment to generate Gal-reduced PADM. This targeted removal of α-gal resulted in a reduction of the extensive α-gal staining observed on PADM to resemble that of decellularized porcine dermis acquired from Gal −/− pigs (Gal −/− PADM). Furthermore, the application of α-galactosidase to PADM reduced anti-α-gal titers following subcutaneous implantation into the backs of vervet monkeys, from a 32—64-fold increase with wild-type PADM over Gal −/− PADM to a minimal <4-fold increase with Gal-reduced PADM over Gal −/− PADM. Indeed, the benefit that stems from specific elimination of the α-gal xenoantigen from donor tissue has been translated into the in vivo setting. For example, hearts [94] and kidneys [95] harvested from Gal −/− pigs and implanted into baboons did not elicit hyperacute rejection, an α-gal-mediated immune response normally associated with xenotransplantation. Additionally, incidence of acute humoral xenograft rejection was reduced, occurring in only two out of eight baboons. Graft survival was increased, to a median of 78 days and maximum of 179 days, compared to hearts from low α-gal expressing pigs which were rejected in 20 min by the hyperacute response [94]. Kidneys derived from Gal −/− pigs survived beyond any other life-sustaining xenografts reported at the time of publication, up to 83 days, while maintaining normal graft function and histology until death [95]. Although these studies validate the ability to effectively and specifically target the removal of α-gal antigens from xenogeneic tissues, and thereby avoid hyperacute rejection, this strategy alone is insufficient for xenogeneic scaffold generation, particularly when expected to function in a critical site such as a heart valve or whole organ. Organs derived from Gal −/− pigs are still unable to survive acute humoral xenograft rejection [30, 34, 46, 94, 96]. Chen et al. [96] observed increased levels of graft-specific, non-Gal antibodies following implantation of Gal −/− porcine kidneys into baboons. Induction of these non-Gal antibodies elicited severe acute humoral xenograft rejection, ultimately resulting in graft dysfunction and renal failure. Similarly, a vervet monkey model of abdominal wall resection and repair revealed elevated titers of graft-specific antibodies upon implantation of Gal −/− PADM over 6 months post-implantation [46]. Thus, presence of non-Gal antigens in xenogeneic grafts elicits a graft-specific, acute humoral immune response in non-human primate hosts. Identification of non-Gal antigens on xenogeneic tissue is critical for methods to achieve targeted removal of these xenoantigens to be developed. Incubation of a porcine endothelial cell lysate with baboon sera against Gal −/− porcine kidney has implicated a 47 kDa protein in non-Gal mediated immune rejection [96]. Griffiths et al. [45] identified 31 xenoantigens in bovine pericardium resulting in IgG production in a rabbit host. However, attempts to determine whether non-Gal xenoreactive antibodies exist in baboon and human sera towards a subset of saccharides were inconclusive [97]. Unfortunately, such antigen identification studies are time consuming to perform, and it is unclear whether targeted removal will be possible in all cases. Thus, employing targeted removal of known antigens is still in a nascent stage and requires greater understanding of tissue and organ antigenic components for expanded implementation of this strategy.
Figure 1. Antigenicity, antigen removal strategies, and scaffold assessment.
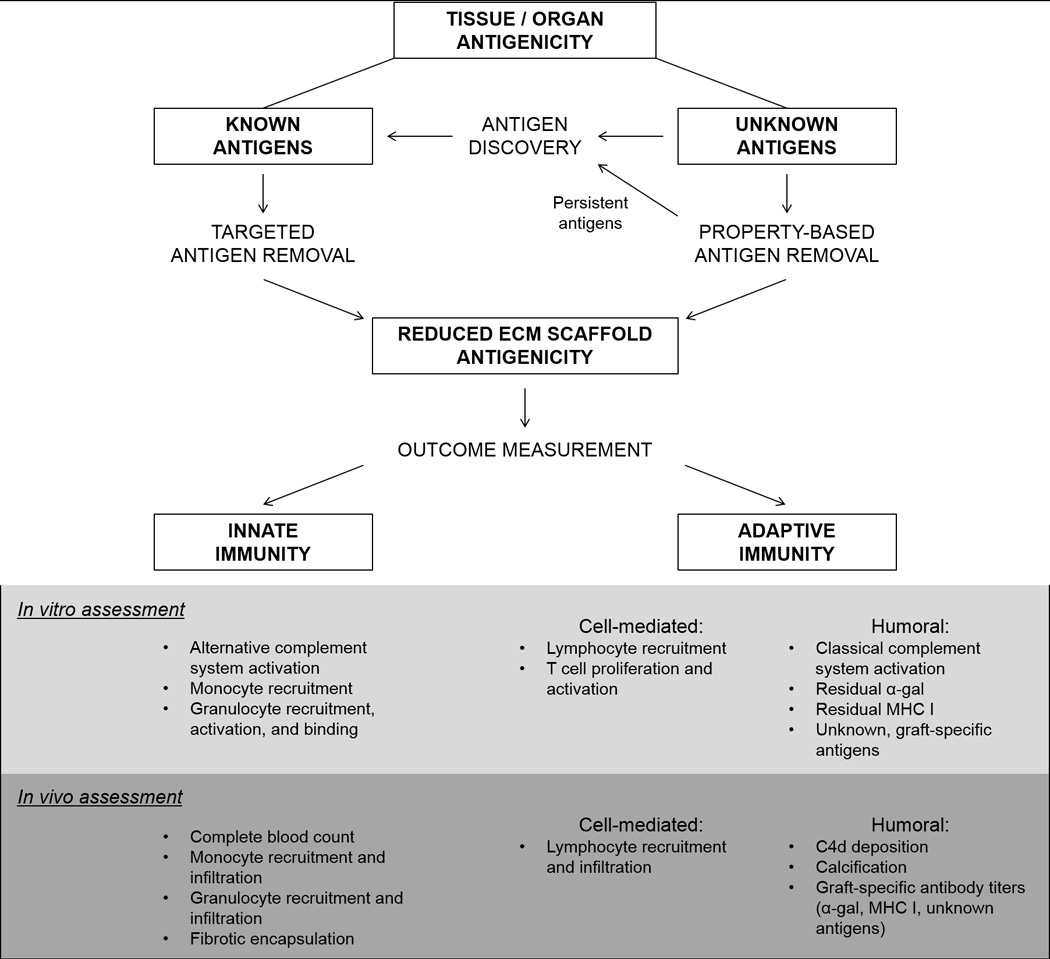
Antigens within a tissue or organ can be categorized into those with known and currently unknown identities. Known antigens may be removed using targeted approaches, such as removal of α-gal antigens using α-galactosidase. Unknown antigens may be removed in groups on the basis of shared physiochemical properties. Antigens which persist following application of property-based removal strategies can be identified using discovery techniques and then may be specifically targeted for removal. Both innate and adaptive immune potential of scaffolds can then be assessed in vitro utilizing a variety of approaches which allow for individual components of the immune system to be evaluated. Similarly, in vivo methods to assess immunogenicity are also available which allow for evaluation of various immune components. The extent to which each in vitro assessment method correlates with recipient graft-specific in vivo response has yet to be determined. Clarification of such correlations between in vitro assessments and in vivo outcome will be critical in determining which of the available in vitro assessment method(s) most accurately predict in vivo response and ultimately become a standard for measuring scaffold immunogenicity.
4.2. Stepwise, solubilization-based antigen removal
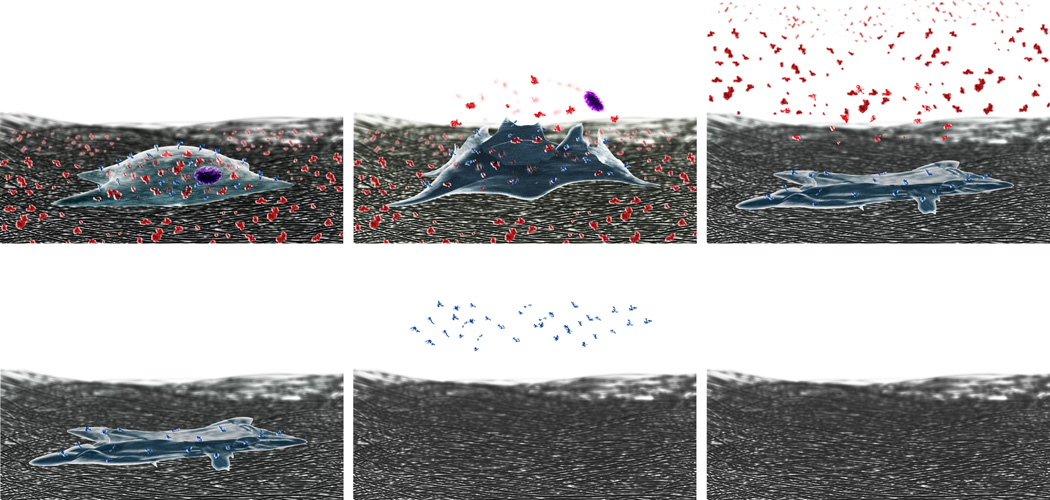
Strategies to remove unknown antigenic components target groups of antigens by exploiting their shared physiochemical properties (Figure 1). We have developed a solubility-based antigen removal strategy grounded in the underlying principle that solubilization of tissue components is necessary for their removal from the ECM. Solubilization-based antigen removal is an adaptation of protein extraction techniques from proteomics wherein proteins of interest are solubilized from cell lysate or homogenized tissue or organ into an extraction buffer for downstream analysis. However, instead of processing lysate or homogenate, solubilization-based antigen removal is performed on intact tissue for the generation of immunologically acceptable, unfixed, tissue engineering scaffolds. The ultimate advantage of a solubilization-based strategy for antigen removal is independence from identification of individual antigens within a graft to accomplish their removal. Instead, subsets of antigens can be removed based on shared physiochemical properties—solubility in antigen removal solution—using principles of protein chemistry. The extraction of proteins from a tissue into an aqueous solution during antigen removal is dependent upon its solubility in the antigen removal buffer as a protein can only be extracted into a solution in which it is soluble [98, 99]. We tested the ability of reducing agent and salt, used to promote hydrophilic protein solubility for extraction of proteins from homogenized bovine pericardium [45, 100], to enhance the removal of antigens. Intracellular thioredoxin and glutathione systems maintain a reducing environment within the cell, facilitating solubilization of hydrophilic, cytoplasmic proteins [101]. During decellularization, cell lysis compromises maintenance of hydrophilic proteins in a reduced state. In an oxidizing environment, the increased propensity for disulfide bond formation between proteins leads to intermolecular aggregation and precipitation from solution [102]. By preventing intermolecular disulfide bridge formation [102–104], use of a reducing agent mitigates protein aggregation and precipitation from solution [105]. Indeed, we demonstrated that inclusion of a reducing agent in antigen removal for hydrophile solubilization enhanced removal of hydrophilic antigens in bovine pericardium [92]. Similarly, we also showed that use of salt further promoted hydrophile solubilization and removal when incorporated into the antigen removal buffer. Protein solubility is known to increase with the initial addition of salt to a solution (salting in) and decrease as more salt is added (salting out) [106]. Maintenance of the environment between the points of salting in and salting out disrupts intermolecular protein-protein interactions, which in turn prevents intermolecular protein aggregation [106] and facilitates protein solubilization for removal. Through incorporation of both a reducing agent and salt, we were able to reduce residual hydrophilic antigenicity of bovine pericardium by an additional 80% compared to decellularization by hypotonic solution or 60% compared to decellularization by 0.1% SDS, without compromising scaffold structure-function properties [92] (see 4.3 below for antigenicity assessment method). Thus, promoting hydrophile solubilization represents a means to remove a subset of antigens based on a common property without first determining their individual identities.
The ability to remove hydrophilic antigens by promoting hydrophile solubilization validates the importance of solubilization-based antigen removal and underscores the need for a multi-step antigen removal process. Hydrophilic antigens only represent one subset of antigenic determinants within a tissue or organ. The unique primary amino acid sequences of antigenic proteins within a tissue or organ result in a wide spectrum of solubilities and inability of all proteins to be simultaneously solubilized by a single solution [107–109]. Thus, a stepwise approach is likely to be required for removal of protein subsets based on differential solubility, with each step utilizing a different solution to facilitate removal of a particular fraction of antigens (Figure 2). We subjected bovine pericardium to hydrophile solubilization (promoted using a reducing agent and salt and represented in the illustration as red molecules), followed by lipophilic protein solubilization (promoted using an amphiphile and represented in the illustration as blue molecules), reducing residual lipophilic antigenicity by an additional 60% and eliminating detection of the two most critical barriers to xenotransplantation (α-gal and MHC I) while maintaining biomaterial structure-function properties [89] (see 4.3 below for antigenicity assessment method). Ongoing work seeks to determine whether these promising, in vitro findings can be translated into the in vivo setting. However, early results following implantation of bovine pericardium following stepwise, solubilization-based antigen removal in a small animal model indicate that incorporation of such an approach is critical to reducing the recipient immune response to an implanted biomaterial (data not yet published).
Figure 2. Stepwise, solubilization-based antigen removal.
Untreated, native tissue is represented by a cell on the surrounding ECM. Antigenic components of tissues have been simplified in this illustration to be either hydrophilic (red) or lipophilic (blue). Following hypotonic lysis of the cell membrane, hydrophiles—both intra- and extracellular—are maintained in solution by presence of reducing agent and salt for subsequent removal. Then, lipophile solubilization is promoted by ASB-14 for subsequent removal. Removal of antigens in this sequential and differential manner has been shown to significantly reduce antigens without compromising the structure-function properties of bovine pericardium.
4.3. Assessment of residual antigenicity
Assessments of residual antigenicity have been employed to evaluate the antigen removal efficiency of various techniques. The simplest method of determining persistence of known antigens is immunohistochemical staining of tissue sections [5, 46, 59, 88–90]. However, the presence of known antigens does not inform the presence of unknown antigens. Generation of graft-specific antibodies towards persistent antigens post-implantation has been quantified using an ELISA-based approach [14, 46]. Recipient sera collected pre-implantation and at several time points post-implantation is used to probe immobilized graft homogenate to determine fold change in graft-specific antibody titers compared to baseline over time. We have developed a Western blot-based method for evaluating the persistence of antigens [89, 92]. Hydrophilic and lipophilic proteins sequentially and differentially extracted from homogenized tissue are separated using gel electrophoresis and blotted onto a membrane. Rabbit serum generated against untreated tissue—containing antibodies against all antigens recognized within the native tissue—is used to probe proteins immobilized to the membrane and IgG positivity is assessed. Intensity of the resultant banding pattern, an indication of residual antigenicity, was determined using densitometry. Although these in vitro means of assessing residual antigenicity represent a more specific and rigorous approach than evaluating residual cellular components to determine the efficacy of antigen removal, in vivo assessment of host immune response will be essential to identify how these antigenicity-based outcome measures correlate with in vivo recipient immune response towards and longevity of the graft, and therefore serve as a success criterion of antigen removal.
5. FUTURE WORK
The initial intention behind decellularization was to reduce the antigenicity of a tissue or organ to provide clinical therapies for human patients. Consideration of this goal is fundamental in designing strategies to achieve this result, establishing appropriate outcome measures, and determining success criteria. Tissue and organ antigenicity stems from the presence of antigenic determinants. Therefore, persistence of antigens in tissue following utilization of decellularization alone without consideration of additional antigen removal will ultimately elicit recipient immune response following implantation. Research to date suggests the following directions for future work: (1) aim for removal of antigens, (2) utilize evaluation of scaffold antigenicity as a specific outcome measure of persistent antigens, and (3) increase understanding of how the level of residual antigens translates to in vivo outcomes.
The early success associated with stepwise, solubilization-based antigen removal is encouraging, but still requires further investigation to fully characterize the benefits and potential limitations of this technique. For instance, it is unknown whether such strategies, in effectively eliminating antigenic tissue components, potentially reveal or create new epitopes not accessible in native tissue. Also, it has yet to be determined if antigens that persist following stepwise, solubilization-based antigen removal are sufficient to elicit in vivo recipient immune response. It is possible that identification of persistent antigenic determinants [45] may permit future development of targeted antigen removal approaches. Ultimately, a combination of stepwise, solubilization-based removal of unknown antigens with targeted removal of known antigens may provide sufficient reduction in scaffold antigenicity to achieve the goal of an immunologically acceptable, unfixed, tissue- or organ-derived scaffold (Figure 1). Furthermore, stepwise, solubilization-based antigen removal is effective for molecules which can be solubilized. For antigens which may be associated with insoluble components of the ECM, methods to specifically remove, modulate, or mask epitopes may need to be applied. Additionally, targeted removal of other antigen classes, e.g., carbohydrates [34], may also need to be considered. Finally, the ability to adapt these antigen removal strategies to generation of whole organ scaffolds will need to be determined. Due to the limit of diffusion, the size scale of whole organs will demand utilization of a perfusion-based approach, much like those used for heart [67] or kidney [71] decellularization. Nonetheless, antigen removal strategies, applied with or without decellularization techniques, appear to be better aligned than decellularization alone towards the ultimate goal of translating unfixed, tissue- or organ-derived scaffolds into clinical applications.
Another critical take-away point from the literature is the need to actually evaluate antigenic components of the tissue or organ as an outcome measure (Figure 1). Since the antigens within a tissue are the targets of recipient immune system recognition and response, it is vital that their presence in the scaffold and/or their ability to elicit the immune response is assessed. While the cellular component of a tissue or organ is a source of antigens, it is not the sole origin of the antigens. Therefore, graft cellularity alone without additional evaluation of scaffold antigenicity is not an appropriate indicator of antigen removal. Methods that assess binding of graft-specific antibodies; binding of antibodies against known antigens; or migration, proliferation, or activation of inflammatory cells or lymphocytes to the scaffold or scaffold extract in vitro are likely to more closely approximate the potential of a biomaterial to elicit the in vivo recipient immune response than methods that evaluate scaffold acellularity alone. However, in vivo studies to assess the host immune response to scaffolds subjected to antigen removal will be required (1) to determine which in vitro graft-specific antigenicity assessment(s) best correlate with the actual in vivo response towards tissue-and organ-derived scaffolds, (2) to define a standard for acceptable scaffold immunogenicity, and (3) to fully demonstrate the merit in this new antigen removal paradigm.
One of the essential questions to be answered is what constitutes an acceptable amount of residual antigenicity to render an unfixed, tissue- or organ-derived scaffold immunologically acceptable following implantation. The success criterion for residual antigenicity is likely to be tissue-/organ- and function-dependent. For instance, the use of an unfixed, tissue-derived scaffold for hernia repairs is simply intended to close a defect in the wall of a body cavity. Thus, incomplete removal of antigens, resulting in immune-mediated fibrosis and scarring, may be considered an acceptable outcome in a hernia repair application. Indeed, favorable outcomes with clinical use of decellularized scaffolds for hernia repair have been reported [3]. In contrast, heart valves are expected to be elastic and pliable in order to maintain unidirectional blood flow through the heart. Persistence of antigenicity leading to subsequent fibrosis and stiffening of the valve leaflets (valvular stenosis) could drastically reduce blood flow through the valve. Additionally, residual antigens and the associated immune response have been shown to stimulate calcification of the biomaterial. These localized regions of calcification prevent complete leaflet coaptation, resulting in retrograde leaking of blood back through the valve (valvular insufficiency). The resultant compromise in function is not an acceptable outcome of heart valve replacement. Therefore, the success criterion for reduction of antigenicity is closely linked to graft function, and may be less stringent for hernia repair than it is for heart valves. Tissues with more rigorous function requirements, such as heart valves and whole organs (where scaffold-associated inflammatory and immune responses are likely to impair graft function), will be more sensitive to residual antigenicity of the graft. Another consideration to be made regarding persistent antigenic components in the tissue is ECM turnover time. Whether cell-mediated ECM remodeling through elimination of antigenic scaffold molecules decreases scaffold antigenicity remains to be determined in future studies [73, 77]. However, if the time for complete ECM turnover outpaces the time over which immune rejection occurs, then the antigenic components can potentially be eliminated before a significant immune response is mounted and the graft is destroyed. As ECM turnover has been shown to begin approximately 1 month post-implantation and be completed by approximately 6 months post-implantation [78], reduction of residual antigenicity to permit graft survival past 6 months may be sufficient to confer immunological-acceptance upon tissue- and organ-derived scaffolds. Cell type will presumably be a key determinant of this critical ECM turnover period. Quiescent cells may require more time to remodel ECM in comparison to more active cells. It can be reasoned that the longer the period of time required for ECM turnover, the further the scaffolds antigenicity will need to be reduced. In vivo models which take into account the graft site and function will therefore be necessary to identify an acceptable antigen burden for a graft—the threshold of residual antigenic material which permits in vivo success [73]. Additionally, these models will need to include a relevant cell population so that ECM turnover time in the context of adequately reduced scaffold antigenicity can be better characterized. These studies will be instrumental in further understanding the role of M2 macrophages recently attributed to the regenerative response observed during scaffold remodeling [44]. Thus, the success criteria for amount of residual antigens that can be tolerated by the recipient following implantation are likely to be largely dependent on the ultimate function of the scaffold and ability of the graft to avoid immune rejection until the ECM can be fully replaced by recipient matrix components.
In addition to appropriately reduced antigenicity for maintained function in vivo, other important considerations associated with antigen removal include compatibility of the antigen removal treatment with scaffold structure-function properties, toxicity, and recellularization potential. One of the main motivators for use of unfixed, tissue- or organ-derived scaffolds, warranting attempts to overcome the immunological barriers, is the inherent structure-function properties of the biomaterial. The ability to acquire a starting material that does not require alteration of ECM structure to attain the necessary mechanical properties is a substantial advantage for tissue- and organ-derived scaffolds. Indeed, an immunologically acceptable, tissue- or organ-derived scaffold lacking the desired structure-function properties is an inadequate result when a functional scaffold for tissue engineering is the goal. Therefore, the ideal antigen removal strategy should not compromise ECM structure or the resultant in vivo function. The disparate functions of the various tissues and organs of the body will demand that all scaffolds be rigorously evaluated for maintained biomaterial properties which are critical to the necessary physiological function. In the same vein, a scaffold should neither be toxic to surrounding tissues with which it is in contact nor distant tissues which may be adversely affected if a toxic chemical is retained in the scaffold initially but later leached into the bloodstream. Consequently, chemical components of an antigen removal protocol should be neither cytotoxic nor retained in the scaffold. Assessment of scaffold toxicity may be performed through measurement of residual chemicals in the scaffolds or maintenance of cell viability. As different cell types likely have varying degrees of sensitivity to chemicals, all relevant cell types will need to be assessed. Finally, the in vivo elimination of any residual antigenic components within a scaffold via ECM turnover is dependent upon cellular repopulation of the graft. As mentioned above, cell-secreted matrix metalloproteinases degrade ECM proteins, which may result in liberation of previously insoluble antigens for clearance from the scaffold. Thus, cell-mediated matrix turnover permits replacement of donor scaffold with recipient ECM, ultimately promoting graft avoidance of immune rejection and increasing graft longevity. Furthermore, cells are required to recapitulate biological function to the scaffold. Inability to recellularize the graft represents an inability for the graft to adapt to the local environment and to fully restore tissue functionality. Taken together, an ideal antigen removal strategy renders a tissue- or organ-derived scaffold immunologically acceptable without compromising structure-function properties, introducing scaffold toxicity, or eliminating the recellularization capacity of the biomaterial.
In conclusion, the primary obstacle to the use of xenogeneic tissues and organs in clinical applications is the presence of xenoantigens. Therefore, to overcome this hurdle, efforts must focus on the antigens and their persistence in the xenograft. Firstly, strategies to generate xenogeneic scaffolds must be aimed at removing the antigenic content of tissues and organs. While decellularization strategies can be incorporated into such an approach, relying on decellularization as the sole means to eliminate antigens is short-sighted. Secondly, scaffold immunogenicity must be specifically evaluated via antigen content and/or antigenic potential. Employment of only a surrogate for antigen content like residual nuclei, residual DNA, or residual proteins (not known to be immunogenic) is problematic as it relies on critical assumptions that have been demonstrated to be invalid. As scaffold acellularity may still be an important goal, albeit secondary to reduced antigenicity, a combinatorial approach utilizing both antigen removal and decellularization may represent a promising xenogeneic scaffold generation strategy. Finally, for translation of an antigen removal strategy into a therapy, in vivo assessment of the resultant scaffold will be essential. Success criteria for the adequacy of antigen removal required to achieve an immunologically acceptable outcome will be dependent upon the site of ultimate implantation and demand of physiological function. Furthermore, the antigen removal strategy will be required to maintain biomaterial structure-function properties and recellularization capacity. Successful production of a functional tissue- or organ-derived scaffold for tissue engineering and regenerative medicine will be a significant step towards addressing the shortage of replacement tissues and organs for patients worldwide.
ACKNOWLEDGEMENTS
The authors thank Dr. Christopher W. Simmons for illustrations of stepwise, solubilization-based antigen removal (Figure 2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors are co-founders of ViVita Technologies, Inc. and hold shares in the company. Dr. Griffiths serves as Chairman and Dr. Wong serves as CEO and CTO.
REFERENCES
- 1.Mantovani F, Trinchieri A, Castelnuovo C, Romanò AL, Pisani E. Reconstructive urethroplasty using porcine acellular matrix. Eur Urol. 2003;44:600–602. doi: 10.1016/s0302-2838(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Hooks VH, Jenkins B. Patch repair of ileoanal pouch-vaginal fistula with Permacol™ collagen implant. The American Surgeon. 2007;73:514–515. [PubMed] [Google Scholar]
- 3.Ansaloni L, Cambrini P, Catena F, Di Saverio S, Gagliardi S, Gazzotti F, et al. Immune response to small intestinal submucosa (Surgisis) implant in humans: Preliminary observations. J Invest Surg. 2007;20:237–241. doi: 10.1080/08941930701481296. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves A, Griffiths LG, Anthony R, Orton EC. Decellularization of bovine pericardium for tissue-engineering by targeted removal of xenoantigens. J Heart Valve Dis. 2005;14:212–217. [PubMed] [Google Scholar]
- 6.Ramaswamy S, Gottlieb D, Engelmayr GC, Jr, Aikawa E, Schmidt DE, Gaitan-Leon DM, et al. The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials. 2010;31:1114–1125. doi: 10.1016/j.biomaterials.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syedain ZH, Bradee AR, Kren S, Taylor DA, Tranquillo RT. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Engineering Part A. 2012;19:759–769. doi: 10.1089/ten.tea.2012.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 9.Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526–532. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 11.Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comp Biol. 2009;5:e1000445. doi: 10.1371/journal.pcbi.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platt J, DiSesa V, Gail D, Massicot-Fisher J. Recommendations of the National Heart, Lung, and Blood Institute Heart and Lung Xenotransplantation Working Group. Circulation. 2002;106:1043–1047. doi: 10.1161/01.cir.0000031064.67525.28. [DOI] [PubMed] [Google Scholar]
- 14.Sandor M, Xu H, Connor J, Lombardi J, Harper JR, Silverman RP, et al. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng. 2008;14:2021–2031. doi: 10.1089/ten.tea.2007.0317. [DOI] [PubMed] [Google Scholar]
- 15.Human P, Zilla P. Characterization of the immune response to valve bioprostheses and its role in primary tissue failure. Ann Thorac Surg. 2001;71:S385–S388. doi: 10.1016/s0003-4975(01)02492-4. [DOI] [PubMed] [Google Scholar]
- 16.Manji RA, Zhu LF, Nijjar NK, Rayner DC, Korbutt GS, Churchill TA, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–327. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien TK, Gabbay S, Parkes AC, Knight RA, Zalesky PJ. Immunological reactivity to a new glutaraldehyde tanned bovine pericardial heart valve. Trans Am Soc Artif Intern Organs. 1984;30:440–444. [PubMed] [Google Scholar]
- 18.Magilligan D, Jr, Lewis J, Jr, Tilley B, Peterson E. The porcine bioprosthetic valve. Twelve years later. J Thorac Cardiovasc Surg. 1985;89:499–507. [PubMed] [Google Scholar]
- 19.Cohn L, Allred E, Cohn L, Austin J, Sabik J, DiSesa V, et al. Early and late risk of mitral valve replacement. A 12 year concomitant comparison of the porcine bioprosthetic and prosthetic disc mitral valves. J Thorac Cardiovasc Surg. 1985;90:872–881. [PubMed] [Google Scholar]
- 20.Ueyama K, Kamiya H, Kanamori T, Ohashi H, Yasushi T, Kawai T, et al. Long-term follow-up of cardiac valve replacement using bioprosthesis in patients 70 years old and older. Artif Organs. 2002;26:1059–1062. doi: 10.1046/j.1525-1594.2002.07005_4.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams DB, Danielson GK, McGoon DC, Puga FJ, Mair DD, Edwards WD. Porcine heterograft valve replacement in children. J Thorac Cardiovasc Surg. 1982;84:446–450. [PubMed] [Google Scholar]
- 22.Rocchini A, Weesner K, Heidelberger K, Keren D, Behrendt D, Rosenthal A. Porcine xenograft valve failure in children: an immunologic response. Circulation. 1981;64:II162–II171. [PubMed] [Google Scholar]
- 23.Hawkins JA, Bailey WW, Dillon T, Schwartz DC. Midterm results with cryopreserved allograft valved conduits from the right ventricle to the pulmonary arteries. J Thorac Cardiovasc Surg. 1992;104:910–916. [PubMed] [Google Scholar]
- 24.Shinoka T, Breuer C, Tanel R, Zund G, Miura T, Ma P, et al. Tissue engineering heart valves: Valve leaflet replacement study in a lamb model. Ann Thorac Surg. 1995;60:S513–S516. doi: 10.1016/0003-4975(95)00733-4. [DOI] [PubMed] [Google Scholar]
- 25.Cascalho M, Platt JL. The immunological barrier to xenotransplantation. Immunity. 2001;14:437–446. doi: 10.1016/s1074-7613(01)00124-8. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y-G, Sykes M. Xenotransplantation: Current status and a perspective on the future. Nat Rev Immunol. 2007;07:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 27.Cozzi E, Bosio E, Seveso M, Vadori M, Ancona E. Xenotransplantation—Current status and future perspectives. Br Med Bull. 2005;75–76:99–114. doi: 10.1093/bmb/ldh061. [DOI] [PubMed] [Google Scholar]
- 28.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 29.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 30.Cooper DKC. Xenoantigens and xenoantibodies. Xenotransplantation. 1998;5:6–17. doi: 10.1111/j.1399-3089.1998.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 31.Wood KJ, Goto R. Mechanisms of rejection: Current perspectives. Transplantation. 2012;93:1–10. doi: 10.1097/TP.0b013e31823cab44. [DOI] [PubMed] [Google Scholar]
- 32.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012;19:23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouard S, Gagne K, Blancho G, Soulillou JP. T cell response in xenorecognition and xenografts: A review. Hum Immunol. 1999;60:455–468. doi: 10.1016/s0198-8859(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 34.Ezzelarab M, Ayares D, Cooper DKC. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005;83:396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 35.Cooper DKC, Koren E, Oriol R. Oligosaccharides and discordant xenotransplantation. Immunol Rev. 1994;141:31–58. doi: 10.1111/j.1600-065x.1994.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 36.Galili U. The [alpha]-gal epitope (Gal[alpha]1–3Gal[beta]1–4GlcNAc-R) in xenotransplantation. Biochimie. 2001;83:557–563. doi: 10.1016/s0300-9084(01)01294-9. [DOI] [PubMed] [Google Scholar]
- 37.Naso F, Gandaglia A, Iop L, Spina M, Gerosa G. Alpha-Gal detectors in xenotransplantation research: a word of caution. Xenotransplantation. 2012;19:215–220. doi: 10.1111/j.1399-3089.2012.00714.x. [DOI] [PubMed] [Google Scholar]
- 38.McMorrow IM, Comrack CA, Sachs DH, DerSimonian H. Heterogeneity of human anti-pig antibodies cross-reactive with the Gal(alpha1,3)Galactose epitope. Transplantation. 1997;64:501–510. doi: 10.1097/00007890-199708150-00021. [DOI] [PubMed] [Google Scholar]
- 39.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 40.Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. The Journal of Experimental Medicine. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peugh WN, Superina RA, Wood KJ, Morris PJ. The role of H-2 and non-H-2 antigens and genes in the rejection of murine cardiac allografts. Immunogenetics. 1986;23:30–37. doi: 10.1007/BF00376519. [DOI] [PubMed] [Google Scholar]
- 42.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Current Opinion in Organ Transplantation. 2009;14:419–425. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 43.Pompilio G, Polvani G, Piccolo G, Guarino A, Nocco A, Innocente A, et al. Six-year monitoring of the donor-specific immune response to cryopreserved aortic allograft valves: Implications with valve dysfunction. Ann Thorac Surg. 2004;78:557–563. doi: 10.1016/j.athoracsur.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 44.Daly KA, Stewart-Akers AM, Hara H, Ezzelarab M, Long C, Cordero K, et al. Effect of the αGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Engineering Part A. 2009;15:3877–3888. doi: 10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
- 45.Griffiths LG, Choe LH, Reardon KF, Dow SW, Christopher Orton E. Immunoproteomic identification of bovine pericardium xenoantigens. Biomaterials. 2008;29:3514–3520. doi: 10.1016/j.biomaterials.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu H, Wan H, Zuo W, Sun W, Owens RT, Harper JR, et al. A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: Removal of terminal galactose-α-(1, 3)-galactose and retention of matrix structure. Tissue Eng Part A. 2009;15:1807–1819. doi: 10.1089/ten.tea.2008.0384. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein S, Clarke DR, Walsh SP, Black KS, O'Brien MF. Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann Thorac Surg. 2000;70:1962–1969. doi: 10.1016/s0003-4975(00)01812-9. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hawkins JA, Hillman ND, Lambert LM, Jones J, Di Russo GB, Profaizer T, et al. Immunogenicity of decellularized cryopreserved allografts in pediatric cardiac surgery: comparison with standard cryopreserved allografts. J Thorac Cardiovasc Surg. 2003;126:247–252. doi: 10.1016/s0022-5223(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 51.Juthier F, Vincentelli A, Gaudric J, Corseaux D, Fouquet O, Calet C, et al. Decellularized heart valve as a scaffold for in vivo recellularization: Deleterious effects of granulocyte colony-stimulating factor. J Thorac Cardiovasc Surg. 2006;131:843–852. doi: 10.1016/j.jtcvs.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Fritze O, Schleicher M, Wendel H-P, Schenke-Layland K, Harasztosi C, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31:2549–2554. doi: 10.1016/j.biomaterials.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 53.Nam J, Choi S-Y, Sung S-C, Lim H-G, Park S-s, Kim S-H, et al. Changes of the Structural and Biomechanical Properties of the Bovine Pericardium after the Removal of α-Gal Epitopes by Decellularization and α-Galactosidase Treatment. Korean J Thorac Cardiovasc Surg. 2012;45:380–389. doi: 10.5090/kjtcs.2012.45.6.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339–7349. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 55.Conklin B, Richter E, Kreutziger K, Zhong D-S, Chen C. Development and evaluation of a novel decellularized vascular xenograft. Med Eng Phys. 2002;24:173–183. doi: 10.1016/s1350-4533(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 56.Iwai S, Torikai K, Coppin CM, Sawa Y. Minimally immunogenic decellularized porcine valve provides in situ recellularization as a stentless bioprosthetic valve. J Artif Organs. 2007;10:29–35. doi: 10.1007/s10047-006-0360-1. [DOI] [PubMed] [Google Scholar]
- 57.Affonso da Costa FD, Dohmen PM, Duarte D, von Glenn C, Lopes SV, Filho HH, et al. Immunological and echocardiographic evaluation of decellularized versus cryopreserved allografts during the Ross operation. Eur J Cardiothorac Surg. 2005;27:572–578. doi: 10.1016/j.ejcts.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 58.Rieder E, Seebacher G, Kasimir M-T, Eichmair E, Winter B, Dekan B, et al. Tissue engineering of heart valves: Decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation. 2005;111:2792–2797. doi: 10.1161/CIRCULATIONAHA.104.473629. [DOI] [PubMed] [Google Scholar]
- 59.Kasimir M, Rieder E, Seebacher G, Nigisch A, Dekan B, Wolner E, et al. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Heart Valve Dis. 2006;15:278–286. [PubMed] [Google Scholar]
- 60.Rieder E, Nigisch A, Dekan B, Kasimir M-T, Mühlbacher F, Wolner E, et al. Granulocyte-based immune response against decellularized or glutaraldehyde cross-linked vascular tissue. Biomaterials. 2006;27:5634–5642. doi: 10.1016/j.biomaterials.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Sayk F, Bos I, Schubert U, Wedel T, Sievers H-H. Histopathologic findings in a novel decellularized pulmonary homograft: An autopsy study. Ann Thorac Surg. 2005;79:1755–1758. doi: 10.1016/j.athoracsur.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 62.Bastian F, Stelzmüller M-E, Kratochwill K, Kasimir M-T, Simon P, Weigel G. IgG deposition and activation of the classical complement pathway involvement in the activation of human granulocytes by decellularized porcine heart valve tissue. Biomaterials. 2008;29:1824–1832. doi: 10.1016/j.biomaterials.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Schenke-Layland K, Vasilevski O, Opitz F, König K, Riemann I, Halbhuber KJ, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 2003;143:201–208. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Zhou P, Lessa N, Estrada DC, Severson EB, Lingala S, Zern MA, et al. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl. 2011;17:418–427. doi: 10.1002/lt.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Engineering Part A. 2010;16:2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Renal tissue engineering with decellularized rhesus monkey kidneys: Age-related differences. Tissue Engineering Part A. 2011;17:2891–2901. doi: 10.1089/ten.tea.2010.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 68.Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–2387. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 69.Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2009;16:1075–1082. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 71.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bloch O, Golde P, Dohmen PM, Posner S, Konertz W, Erdbrügger W. Immune response in patients receiving a bioprosthetic heart valve: lack of response with decellularized valves. Tissue Engineering Part A. 2011;17:2399–2405. doi: 10.1089/ten.TEA.2011.0046. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. The Journal of Surgical Research. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gillies AR, Smith LR, Lieber RL, Varghese S. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Engineering Part C: Methods. 2010;17:383–389. doi: 10.1089/ten.tec.2010.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tedder ME, Simionescu A, Chen J, Liao J, Simionescu DT. Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering. Tissue Engineering Part A. 2010;17:25–36. doi: 10.1089/ten.tea.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iop L, Renier V, Naso F, Piccoli M, Bonetti A, Gandaglia A, et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials. 2009;30:4104–4116. doi: 10.1016/j.biomaterials.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 77.Ross EA, Abrahamson DR, St. John P, Clapp WL, Williams MJ, Terada N, et al. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis. 2012;8:49–55. doi: 10.4161/org.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bayrak A, Tyralla M, Ladhoff J, Schleicher M, Stock UA, Volk H-D, et al. Human immune responses to porcine xenogeneic matrices and their extracellular matrix constituents in vitro. Biomaterials. 2010;31:3793–3803. doi: 10.1016/j.biomaterials.2010.01.120. [DOI] [PubMed] [Google Scholar]
- 79.Funamoto S, Nam K, Kimura T, Murakoshi A, Hashimoto Y, Niwaya K, et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials. 2010;31:3590–3595. doi: 10.1016/j.biomaterials.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 80.Rüffer A, Purbojo A, Cicha I, Glöckler M, Potapov S, Dittrich S, et al. Early failure of xenogenous de-cellularised pulmonary valve conduits - a word of caution! Eur J Cardiothorac Surg. 2010;38:78–85. doi: 10.1016/j.ejcts.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 81.Kneib C, von Glehn C, Costa F, Costa M, Susin M. Evaluation of humoral immune response to donor HLA after implantation of cellularized versus decellularized human heart valve allografts. Tissue Antigens. 2012;80:165–174. doi: 10.1111/j.1399-0039.2012.01885.x. [DOI] [PubMed] [Google Scholar]
- 82.Böer U, Lohrenz A, Klingenberg M, Pich A, Haverich A, Wilhelmi M. The effect of detergent-based decellularization procedures on cellular proteins and immunogenicity in equine carotid artery grafts. Biomaterials. 2011;32:9730–9737. doi: 10.1016/j.biomaterials.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30:3749–3756. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT(TM) in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–1006. doi: 10.1016/s1010-7940(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 85.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Engineering Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 86.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of Pathology. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 87.Umashankar PR, Arun T, Kumary TV. Effect of chronic inflammation and immune response on regeneration induced by decellularized bovine pericardium. Journal of Biomedical Materials Research Part A. 2013;101A:2202–2209. doi: 10.1002/jbm.a.34535. [DOI] [PubMed] [Google Scholar]
- 88.Kasimir MT, Rieder E, Seebacher G, Wolner E, Weigel G, Simon P. Presence and elimination of the xenoantigen gal (alpha1, 3) gal in tissue-engineered heart valves. Tissue Eng. 2005;11:1274–1280. doi: 10.1089/ten.2005.11.1274. [DOI] [PubMed] [Google Scholar]
- 89.Wong ML, Wong JL, Athanasiou KA, Griffiths LG. Stepwise solubilization-based antigen removal for xenogeneic scaffold generation in tissue engineering. Acta Biomater. 2013;9:6492–6501. doi: 10.1016/j.actbio.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 90.Meyer SR, Chiu B, Churchill TA, Zhu L, Lakey JRT, Ross DB. Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res A. 2006;79A:254–262. doi: 10.1002/jbm.a.30777. [DOI] [PubMed] [Google Scholar]
- 91.Ellingsworth LR, DeLustro F, Brennan JE, Sawamura S, McPherson J. The human immune response to reconstituted bovine collagen. J Immunol. 1986;136:877–882. [PubMed] [Google Scholar]
- 92.Wong ML, Leach JK, Athanasiou KA, Griffiths LG. The role of protein solubilization in antigen removal from xenogeneic tissue for heart valve tissue engineering. Biomaterials. 2011;32:8129–8138. doi: 10.1016/j.biomaterials.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 93.Park S, Kim W-H, Choi S-Y, Kim Y-J. Removal of alpha-Gal epitopes from porcine aortic valve and pericardium using recombinant human alpha galactosidase A. J Korean Med Sci. 2009;24:1126–1131. doi: 10.3346/jkms.2009.24.6.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuwaki K, Tseng Y-L, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using α1, 3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat Med. 2004;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 95.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1, 3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2004;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 96.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeh P, Ezzelarab M, Bovin N, Hara H, Long C, Tomiyama K, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010;17:197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 98.Molloy MP, Herbert BR, Walsh BJ, Tyler MI, Traini M, Sanchez J-C, et al. Extraction of membrane proteins by differential solubilization for separation using two-dimensional gel electrophoresis. Electrophoresis. 1998;19:837–844. doi: 10.1002/elps.1150190539. [DOI] [PubMed] [Google Scholar]
- 99.Cordwell SJ. Sequential extraction of proteins by chemical reagents. Methods Mol Biol. 2008;424:139–146. doi: 10.1007/978-1-60327-064-9_12. [DOI] [PubMed] [Google Scholar]
- 100.Griffiths LG, Choe L, Lee KH, Reardon KF, Orton EC. Protein ex traction and 2-DE of water- and lipid-soluble proteins from bovine pericardium, a low-cellularity tissue. Electrophoresis. 2008;29:4508–4515. doi: 10.1002/elps.200800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masutani H, Ueda S, Yodoi J. The thioredoxin system in retroviral infection and apoptosis. Cell Death Differ. 2005;12:991–998. doi: 10.1038/sj.cdd.4401625. [DOI] [PubMed] [Google Scholar]
- 102.De Bernardez Clark E. Refolding of recombinant proteins. Curr Opin Biotechnol. 1998;9:157–163. doi: 10.1016/s0958-1669(98)80109-2. [DOI] [PubMed] [Google Scholar]
- 103.Shaw MM, Riederer BM. Sample preparation for two-dimensional gel electrophoresis. Proteomics. 2003;3:1408–1417. doi: 10.1002/pmic.200300471. [DOI] [PubMed] [Google Scholar]
- 104.Rabilloud T. Protein solubility in two-dimensional electrophoresis. In: Walker JM, editor. The Protein Protocols Handbook. Totowa: Humana Press; 2002. pp. 131–140. [Google Scholar]
- 105.Weiss W, Görg A. Protein solubilization. In: von Hagen J, editor. Proteomics Sample Preparation. Weinheim: Wiley-VCH; 2008. pp. 135–137. [Google Scholar]
- 106.Koolman J, Roehm K-H. Color Atlas of Biochemistry. 2nd ed. Stuttgart and New York: Thieme; 2005. Isolation and analysis of proteins; pp. 78–79. [Google Scholar]
- 107.Cedano J, Aloy P, Pérez-Pons JA, Querol E. Relation between amino acid composition and cellular location of proteins. J Mol Biol. 1997;266:594–600. doi: 10.1006/jmbi.1996.0804. [DOI] [PubMed] [Google Scholar]
- 108.Nozaki Y, Tanford C. The solubility of amino acids and related compounds in aqueous urea solutions. J Biol Chem. 1963;238:4074–4081. [PubMed] [Google Scholar]
- 109.Nozaki Y, Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. J Biol Chem. 1971;246:2211–2217. [PubMed] [Google Scholar]