Abstract
The use of stem cells in the treatment of various diseases and injuries has received increasing interest over the past decade. Injected stem cells, such as mesenchymal stem cells, stimulate tissue repair largely through the secretion of soluble factors that regulate various processes of tissue regeneration, including inflammatory responses, apoptosis, host cell proliferation, and angiogenesis. Recently, it has become apparent that stem cells also use membranous small vesicles, collectively called microvesicles (MVs), to repair damaged tissues. MVs are released by many types of cells and exist in almost all types of body fluids. MVs serve as a vehicle to transfer protein, mRNA, and miRNA to distant cells, altering the gene expression, proliferation, and differentiation of the recipient cells. Although animal models and in vitro studies have suggested promising applications for MV-based regeneration therapy, its effectiveness and feasibility in clinical medicine remain to be established. Further studies of the basic mechanisms responsible for MV-mediated tissue regeneration could lead to novel approaches in regenerative medicine.
Introduction
Tissue modeling and maintenance is a complex and dynamic process that requires constant input from many different cell types. Historically, the study of cell-cell communication has been primarily confined to the context of chemical and physical signals, in the form of soluble proteins, bioactive lipids, gases, and electrical impulses. More recently, a growing body of evidence has shown that membranous vesicles released from multiple cell types also contribute to cell-cell communication.1–6 Two such populations of membranous vesicles released from cells are exosomes and microparticles.
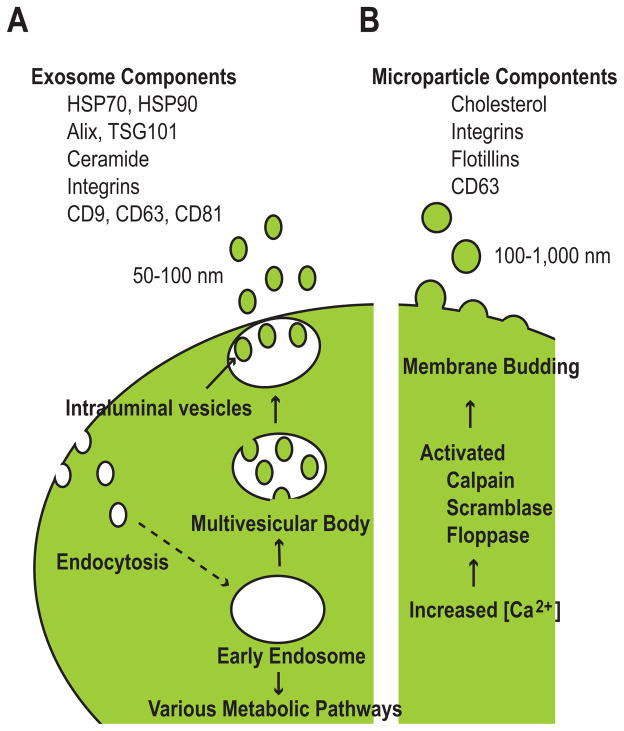
Exosomes are small (50–100 nm) membranous vesicles that arise in the endocytic pathway and are released by numerous cell types, including neurons, immune cells, cancer cells, and stem cells.3, 5, 6 As early endosomes mature, they become multivesicular bodies (MVBs) by accumulating intraluminal vesicles through the invagination of the limiting membrane of the endosome (Fig. 1A). MVBs destined for degradation fuse with lysosomes, but a subset of MVBs fuse with the plasma membrane and release intraluminal vesicles into the extracellular environment as exosomes. Exosomes are enriched in heat shock proteins (HSP70 and HSP90) as well as endosome-specific proteins, such as Alix and TSG101. In addition, exosomes contain cholesterol, ceramide, integrins, and tetraspanins (CD9, CD63, and CD81), all of which are typical components of microdomains in the plasma membrane called lipid rafts. Lipid rafts are rigid membrane domains involved in sorting lipids and proteins during endocytosis.7–9
Figure 1. Synthesis and release of microvesicles.
(A) Synthesis of exosomes during the process of endocytosis. Exosomes arise from early endosomes through the invagination of the endosomal membrane to form intraluminal vesicles within multivesicular bodies. The intraluminal vesicles are then released as exosomes.
(B) Synthesis of microparticles independently of endocytosis. Increases in intracellular Ca2+ activate key enzymes, leading to the dynamic redistribution of phospholipids and membrane budding.
Microparticles (100–1000 nm) are shed vesicles that arise from the budding of the plasma membrane through the dynamic redistribution of phospholipids. Plasma membrane reorganization and microparticle budding are triggered by increased intracellular Ca2+ concentrations and subsequent activation of several key enzymes, notably calpain, scramblase, and floppase (Fig. 1B).1, 3, 10, 11 Microparticle secretion has been best characterized in platelets, red blood cells, and endothelial cells. Microparticles lack proteins of the endocytic pathway but are enriched in cholesterol and lipid raft-associated proteins, such as integrins and flotillins. Although tetraspanins are commonly used as unique markers for exosomes, they can be detected in microparticles in some cases.12 This overlap in molecular markers makes the distinction between exosomes and microparticles sometimes ambiguous and, therefore, both types of membranous vesicles will collectively be referred to as microvesicles (MVs) in the following discussion.
Functions of MVs
MVs have been isolated from many types of biological fluids, including serum, cerebrospinal fluid, and urine, by use of ultracentrifugation and size exclusion column chromatography.6, 13, 14 The physiological functions of MVs in many tissues remain largely unknown; however, their potential roles in pathological settings have been widely studied in oncology and immunology. A subset of molecules, including proteins and RNAs, have been identified in association with MVs, and their effects on recipient cells have been intensively studied in these fields, primarily in vitro. The ExoCarta website (http://exocarta.org/#) provides a comprehensive list of MV-associated proteins, RNAs, and lipids reported from more than 140 studies. Several examples of MV-mediated cell-cell communication are briefly discussed below.
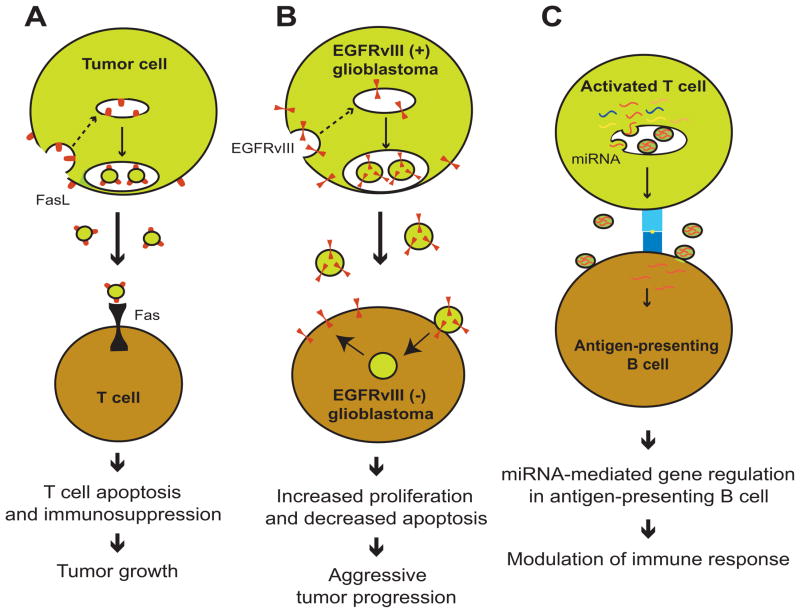
Several types of cancer cells secrete MVs containing the pro-apoptotic molecule Fas ligand (FasL) on the surface, which then binds to its receptor Fas on T cells, activating the apoptotic pathway and resulting in T cell death (Fig. 2A).15–17 When T cells are pretreated with a neutralizing antibody that inhibits Fas-FasL interactions, they become insensitive to MV-mediated apoptosis. In this way, the release of MVs by tumor cells creates an immunosuppressed microenvironment optimal for tumor progression. MVs can also transfer active cytokine receptors to recipient cells. For example, tumor-derived MVs isolated from the serum of glioblastoma patients contain mRNA encoding the truncated and oncogenic receptor epidermal growth factor receptor variant III (EGFRvIII).18 Cultured glioma cells that overexpress EGFRvIII secrete the oncogenic receptor protein in association with MVs. EGFRvIII-negative glioblastoma cells take up these MVs, which causes overexpression of anti-apoptotic genes and increased proliferation, thus conferring a more aggressive phenotype to the recipient glioblastoma cells (Fig. 2B).19 The transfer of oncogenic molecules via MVs could be a potential mechanism leading to tumor progression.
Figure 2. Examples of microvesicle-mediated transfer of protein and RNA.
(A) FasL on microvesicles from tumor cells binds to Fas on T cells, causing T cell apoptosis and resulting in tumor progression.
(B) Transfer of the oncogenic receptor EGFRvIII into glioblastoma cells causes more aggressive growth of the cells.
(C) Activated T cells transfer miRNAs into antigen-presenting B cells, modulating their immune response.
In addition to the transportation of proteins, MVs also transfer miRNA between cells (Fig. 2C).20 It was recently demonstrated that T cells release miRNA-enriched MVs following stimulation by antigen-presenting B cells.21 Some miRNAs are specifically enriched in MVs compared with the parent cells, while other miRNAs are more abundant in parent cells than in MVs.21 This skewed miRNA content indicates a selective uptake of miRNAs into MVs, which has been reported in many other cases, although the mechanism is unknown.22–25 Furthermore, MV-associated miRNAs are transferred from activated T cells to antigen-presenting B cells, where they effectively regulate target gene expression. This was demonstrated by the ability of transferred miR-335 to knock down expression of a luciferase reporter gene containing the seed sequence of miR-335 at the 3′-UTR.21 These data provide evidence for a novel form of communication between T cells and antigen-presenting cells.
Mesenchymal stem cells and regenerative medicine
Recent progress in basic stem cell biology has greatly contributed to the realization of regenerative medicine. Differentiated cells derived from various types of stem cells are expected to facilitate regeneration of damaged tissues. In particular, the establishment of human embryonic stem cells (ESCs)26 and more recent creation of induced pluripotent stem cells (iPSCs)27 provided a major driving force for the medical and public enthusiasm toward regenerative medicine. While ESCs are pluripotent stem cells established from the inner cell mass of the blastocyst28, iPSCs are commonly established from human skin fibroblasts by overexpressing four transcription factor genes—Oct4, Sox2, Klf4, and c-Myc. Patient-derived iPSCs provided hitherto unimaginable opportunities for autologous transplantation, disease modeling, and drug screening. Despite their clinical appeal, however, no ongoing human trials have been undertaken with iPSCs primarily due to technological restrains. The most commonly used type of stem cells in regenerative medicine is autologous mesenchymal stem cells (MSCs), which will be discussed currently.
MSCs are generally defined as a heterogeneous population of non-hematopoietic stem cells with the capability to differentiate into cells of endoderm, mesoderm, and ectoderm origin. However, because of their heterogeneous nature, MSCs were defined by the International Society of Cellular Therapy more stringently for use in clinical trials, based on adhesion to plastic, the presence or absence of specific cell surface markers, and their ability to differentiate into mesodermal lineages.29 MSCs can be readily isolated from many sources, including bone marrow and adipose tissue, and expanded ex vivo for transplantation. Transplanted MSCs stimulate angiogenesis, cell survival, and proliferation and regulate the inflammatory process in host tissues. As of August 2013, 340 clinical trials using MSCs to treat various diseases had been registered with the U.S. National Institutes of Health (clinicaltrials.gov).
Although MSCs have been shown to facilitate regeneration of diseased tissue in various animal models, the exact mechanism underlying this benefit is not fully understood. MSCs are generally thought to achieve this function through paracrine mechanisms, rather than engraftment and subsequent repopulation of the tissues.30–32 Although engraftment of transplanted MSCs has been documented in some cases,33–35 only a small percentage of injected MSCs successfully engraft in various models of myocardial infarction and acute kidney injury (AKI).36–39 Consistent with these findings, injection of mice with cell-free, MSC-conditioned medium is sufficient to stimulate the structural and functional regeneration of cardiac and renal tissues in these models.36,40,41 This indicates an integral role for MSC-secreted signaling molecules in stimulating tissue repair. Indeed, MSCs are known to promote tissue regeneration through the secretion of cytokines, such as transforming growth factor β (TGF-β), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF), to name a few.30 Recent findings also indicate a pro-regenerative role for MVs released by MSCs (MSC-MVs) in several models of tissue regeneration, including regeneration of kidney, heart, liver, and nervous tissues, as discussed below.
MSC-MVs in kidney regeneration
Kidney regeneration mediated by MSC-MVs is commonly studied in AKI models induced by cisplatin, ischemia and subsequent reperfusion, or glycerol.42–46 Specifically, cisplatin-induced injury of cultured renal tubular epithelial cells is mitigated after the addition of purified MSC-MVs. MSC-MVs increase the amount of cellular incorporation of BrdU, indicative of proliferation, while decreasing the number of TUNEL-positive apoptotic cells (Fig. 3A).46 Similarly, the use of purified MSC-MVs to treat AKI in mice recapitulates the in vitro data, leading to an increase of BrdU-positive cells and a decrease of TUNEL-positive cells. Consequently, functional impairment is similarly reduced as indicated by decreased serum levels of blood urea nitrogen, creatinine, and renal cellular necrosis.46 Additionally, microarray analysis of the MV mRNA detected 239 species of mRNA molecules, with specific enrichment of mRNAs involved in cell proliferation, transcription, and immune regulation, providing another example of selective uptake of RNA species into MVs.
Figure 3. Kidney regeneration by the microvesicles released by mesenchymal stem cells and endothelial progenitor cells.
(A) Microvesicles derived from mesenchymal stem cells (MSCs) inhibit apoptosis and stimulate proliferation of renal tubular epithelial cells. Microarray analysis of microvesicle RNA detected 239 mRNA species, including species with the functions described at the right.
(B) Microvesicles released from endothelial progenitor cells (EPCs) induce quiescent endothelial cells to reenter the cell cycle and form blood vessels. In addition to mRNA species, miRNAs are also transferred by the microvesicles.
These processes appear to be mediated, at least in part, by the transfer of RNA by MSC-MVs, as indicated by the loss of regenerative effects after RNase treatment of the MVs.46 However, these results are contradictory to other studies that show MV-associated RNA is resistant to RNase treatment.18,47,48 Notably, these studies did not perform functional experiments with the RNase-treated MVs but instead isolated RNA from the MVs and either quantified total RNA or amplified specific targets using RT-PCR to demonstrate RNase insensitivity. These results are consistent with recent observations that RNA can be isolated from biologic fluids with high intrinsic RNase activity, such as bovine milk or human plasma, indicating some protective mechanism conferring RNase resistance.47,49 One possible explanation for these conflicting reports could stem from residual RNase that remained attached to the surface of the MVs and were subsequently incorporated into the cell with the MVs. The RNase could then degrade nearby MV-derived RNAs, leading to abrogation of the regenerative response. To address this possibility, MVs could be treated with RNase-coated beads and thereby eliminating the possibility of soluble RNase being incorporated into cells.
Recent reports have begun deciphering the molecular pathways modulated by MSC-MVs in the context of renal regeneration. Specifically, MSC-MVs induce the expression of several anti-apoptotic genes, including Bcl-xL, Bcl2, and BIRC8, in renal tubular epithelial cells while simultaneously downregulating pro-apoptotic genes, such as Casp1, Casp8, and LTA.50 In this manner, MSC-MVs confer an anti-apoptotic phenotype necessary for tissue repair. Additionally, MSC-MVs stimulate renal cell proliferation by inducing the phosphorylation and subsequent activation of extracellular regulated kinase (ERK) 1/2.51 Blockade of ERK activation with a chemical inhibitor significantly reduces cell proliferation after MSC-MV treatment.51 Although the exact molecules in the MVs that mediate the anti-apoptotic and pro-proliferative effects have not been identified, these data demonstrate the ability of MSC-MVs to simultaneously modulate several different pathways to stimulate renal regeneration.
While the ability of MSC-MVs to stimulate renal regeneration has been intensively studied, it is becoming clear that MVs from diverse cell types could potentially aid in the regenerative process. For example, endothelial progenitor cells (EPCs) may also contribute to tissue regeneration through released MVs.52, 53 EPCs are a subpopulation of non-hematopoietic bone marrow cell with a differentiation potential restricted to the endothelial lineage. EPC-derived MVs (EPC-MVs) activate an angiogenic program in quiescent endothelial cells by stimulating reentry into the cell cycle and promoting blood vessel formation (Fig. 3B).54 This process is dependent on the transfer of mRNAs by incorporation of EPC-MVs into endothelial cells. If MV incorporation is blocked using α4- or β1-integrin neutralizing antibodies, EPC-MVs do not activate quiescent endothelial cells. A microarray analysis of the mRNA in EPC-MVs detected 298 species of mRNA, including those involved in the phosphatidylinositol 3-kinase/AKT signaling pathway, which is critical for the activation of angiogenesis. Similarly, MVs released by injured renal epithelial cells promote proliferation of fibroblasts and secretion of type I collagen from fibroblasts, initiating a tissue repair process. This process is dependent on the presence and transfer of TGF-β1 mRNA by MVs.55 When TGF-β1 is knocked down using siRNA, renal epithelial cell-derived MVs could no longer stimulate tissue repair.
In addition to mRNAs, the transfer of miRNAs by MVs is important in the context of kidney regeneration. In a rat model of AKI, intravenously injected EPC-MVs mainly localize in the peritubular capillaries and renal tubular cells, and subsequently promote tissue repair and reduce functional impairment.22 Their protective effects can be inhibited by the knockdown of Dicer, which is an RNase essential for processing of pre-miRNA to miRNA, or by simultaneous inhibition of miR-126 and miR-296 in the parental EPCs. These two miRNAs are known to be essential for angiogenesis in many contexts.56 These data indicate an important and potentially underappreciated role of miRNA transfer by MVs in stimulating tissue regeneration.
MSC-MVs in cardiac regeneration
In addition to contributing to kidney regeneration, MSCs mediate functional and structural regeneration of cardiac tissue in a largely paracrine manner.57, 58 For instance, injection of rats with MSCs engineered to express the pro-survival gene Akt1 stimulates cardiac regeneration after ischemia.40, 59 Recovery of left ventricular function occurs as early as 72 hours after the addition of MSCs, suggesting that the functional recovery is not dependent on cellular engraftment and subsequent differentiation.40 Supporting this interpretation, injection of mice with MSC-Akt–conditioned medium significantly reduces the infarct size and increases left ventricular systolic pressure as compared with controls.40, 59 Similarly, intravenous injection of medium conditioned by wild-type MSCs also reduces the infarct size caused by ischemia and reperfusion.41 In this model of myocardial infarction, the cardioprotective effect of MSC-conditioned medium could be observed as early as 4 hours after reperfusion. Subsequent ultracentrifugation and biochemical characterization showed that MVs were the key components in the conditioned medium mediating myocardial protection.60 Administration of purified MSC-MVs 5 minutes before reperfusion significantly reduces infarct size and drastically improves left ventricular function. Furthermore, MSCs derived from fetal tissue are highly proliferative, with the potential of producing up to 1019 cells, and are therefore capable of producing substantial quantities of cardioprotective MVs for therapeutic use.61
In addition to MSCs, MVs isolated from cardiac progenitor cells (CPCs) may also contribute to cardiac regeneration.62 CPCs have been isolated from embryonic and adult hearts based on the expression of several specific marker proteins.63,64 For example, the first purified CPCs from adult rat hearts were negative for the expression of blood lineage markers (Lin−) and positive for the expression of the stem cell-related surface antigen c-kit.65 These cells are multipotent, being able to differentiate into cardiac muscle, smooth muscle, and endothelial cells, and promote cardiac regeneration after myocardial infarction. MVs isolated from the supernatant of the culture of CPCs stimulate endothelial cell migration through the extracellular matrix metalloproteinase inducer (EMMPRIN) expressed on the MV surface. EMMPRIN signaling subsequently activates the ERK1/2 pathway and induces expression of matrix metalloproteinases and secretion of VEGF, thus promoting angiogenesis.66,67 The ability of MVs to activate an angiogenic program in endothelial cells could provide, in part, a mechanism for their ability to promote cardiac regeneration.
MVs in regeneration of other tissues
Although the pro-regenerative role of MSC-MVs has been most intensely studied in the context of kidney and cardiac regeneration, recent reports indicate a protective role for MVs in other tissues as well. For example, MSC-MVs reduce tetrachloride-induced liver fibrosis, potentially by inhibiting the epithelial-to-mesenchymal transition of hepatocytes and collagen production.68 Human liver stem cells (HLSCs) have also been used as a source for MVs to stimulate liver regeneration after physical and chemical injury. HLSCs were first established after culture of primary hepatocytes for 2 weeks in selective media as a population of actively proliferating cells.69 These cells can differentiate into bone, adipose cells, and endothelial cells in addition to liver cells. MVs isolated from HLSCs can protect cultured hepatocytes from D-galactosamine-induced apoptosis when simultaneously added as determined by TUNEL assay. In parallel with the in vitro data, administration of HLSC-MVs to 70% hepatectomized rats significantly contributed to structural and functional hepatic recovery. Specifically, the amounts of the hepatic enzymes aspartate aminotransferase and alanine aminotransferase in the serum diminished and the production of albumin increased. At the histological level, more hepatic cells incorporated 5-bromo-2′-deoxyuridine (BrdU), indicating proliferation, and fewer cells were apoptotic than in control liver. HLSC-MVs were internalized into hepatocytes via a specific α4 integrin-dependent process and horizontally transferred a specific subset of mRNAs to the hepatocytes. A majority of the transcripts present in HLSC-MVs are involved in regulation of transcription, translation, and cell proliferation.
A role for MSC-MVs has also been proposed in an ischemic model of neural regeneration. Exposure of MSCs to ischemic rat brain extract for 72 hours induces the expression of miR-133b in MSCs and its subsequent presence in MSC-MVs.70 miR133b is essential for the functional regeneration of motor neuron axons after spinal cord injury in zebrafish.71 Treatment of healthy primary rat cortical neurons with MVs derived from the brain extract-treated MSCs increases the total number of neurites and neurite length after 48 hours. Furthermore, neurons previously transfected with a miR-133b inhibitor do not exhibit changes in neurite morphology after MSC-MV treatment. The changes in neurite number and length seem to result from the targeting and inhibition of the small GTPase RhoA, a known inhibitor of neurite growth, by miR-133b.70
MVs and embryonic stem cells
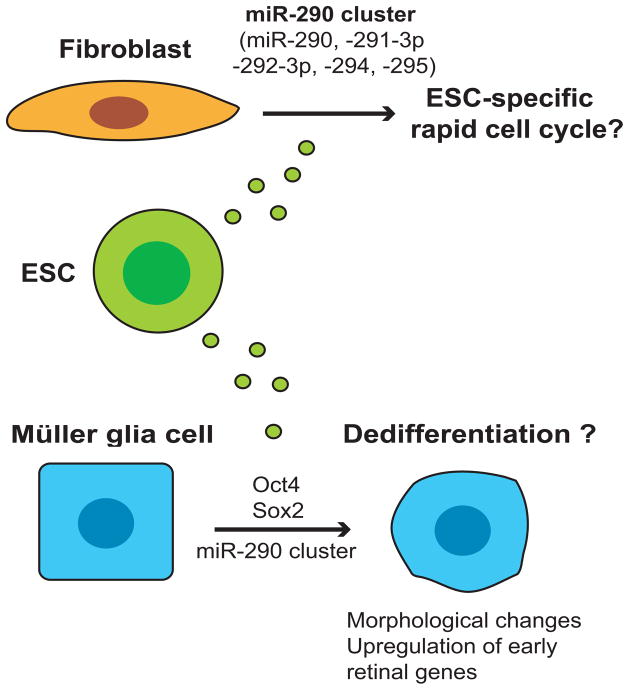
Because of their potential use in medical applications, ESCs have been extensively studied in the past decade, although MVs released from ESCs (ESC-MVs) have not attracted wide interest. ESC-MVs contain mRNA encoding Oct4, Sox2, and Rex1, which are key genes for pluripotency, as well as the Oct4 protein.72 After incorporation of ESC-MVs, hematopoietic progenitor cells translate mRNA encoding Oct4, the master transcription factor for pluripotency, although this has not been shown to result in pluripotency of the recipient cells. A subset of miRNAs can be transferred to irradiated fibroblasts via ESC-MVs (Fig. 4); specifically, several miRNAs of the miR-290 cluster, which are the most abundant miRNAs in ESCs73 and are involved in ESC-specific rapid cell cycle regulation, were found to have transferred to the fibroblasts 1 hour after addition of the MVs.25 The ability of MVs to transfer miRNA and mRNA has potential implications for iPSC formation from fibroblasts. For example, MVs from partially reprogrammed iPSCs might function in an autocrine manner to facilitate reprogramming nearby cells within a single colony. On the other hand, fibroblasts might secret inhibitory MVs for iPSC formation and therefore, inhibition of fibroblast-derived MVs might promote iPSC formation. Given the recent report on the creation of iPSCs from fibroblasts with miRNAs alone,74 albeit different miRNAs than those found in ESC-MVs, MV-mediated delivery of miRNAs warrants further investigation as a possible unrecognized communication process during iPSC development.
Figure 4. Potential dedifferentiation induced by microvesicles derived from embryonic stem cells.
Embryonic stem cell (ESC)-specific components of microvesicles, including the abundant miR-290 cluster, potentially affect the cell cycle regulation of fibroblasts and induce dedifferentiation of Müller glia cells.
Furthermore, ESC-MVs can reprogram Müller glia cells to less-differentiated, multipotent retinal progenitor cells.75 Through the selective transfer of members of the miR-290 cluster and mRNA encoding Oct4 and Sox2 to Müller cells, ESC-MVs induce drastic morphological changes and upregulation of early retinal genes and pluripotency genes, indicative of dedifferentiation. Although the long-term effects of the transfer and the stability of the newly acquired phenotype were not reported, this work indicates a potential application for the use of ESC-MVs to reprogram differentiated cells. Specifically, the reprogramming of Müller glia cells into retinal progenitor cells by ESC-MVs could prove to be a novel treatment for retinal diseases such as macular degeneration or cone-rod dystrophy. Moreover, in the context of tissue regeneration, this strategy of reprogramming quiescent tissues with ESC-MVs could be broadly applied to neurodegenerative, heart, lung, and kidney disease.
Future perspectives
The functional significance of MVs in regenerative medicine has yet to be firmly established. Although many studies have demonstrated that MVs isolated from various cells are capable of supporting tissue regeneration, it is not clear whether MVs are indeed necessary components of the regeneration process. To prove this, release or incorporation of MVs needs to be specifically blocked in vivo, which requires deeper understanding of their synthetic pathways, selective uptake of their contents, secretion, and incorporation by target cells. Fortunately, possible molecular targets involved in MV biogenesis and secretion have recently been elucidated in vitro. For instance, the GTPases Rab27a and Rab27b are necessary for the docking of MVBs to the plasma membrane before release to the extracellular space in several types of cancer cells.76, 77 Similarly, a role for the guanine nucleotide exchange factor BIG2 has been demonstrated for the constitutive release of MVs from human vascular endothelial cells.78 However, the necessity of these molecules for MV synthesis and secretion in vivo has not yet been demonstrated.
Regardless of our understanding of MV biogenesis, the clinical benefits of MVs are already being exploited. As of August 2013, there were 8 registered clinical trials using exosomes, primarily in the field of cancer diagnostics and therapeutics (clinicaltrials.gov). Given the promising results of MSC-MVs in animal models of tissue regeneration described above, human clinical trials with MVs are also likely to be conducted in the near future. However, several factors must be considered before the use of MVs in human clinical trials can be realized.79, 80
First, the type of effector cargo molecule (i.e. protein, mRNA, or miRNA) and the ability of that molecule to localize in MVs need to be determined. Proteins can be specifically targeted to MVs through fusion to MV-enriched molecules, such as lactadherin, Lamp2b, and the transmembrane domain of platelet-derived growth factor receptor.81–83 Additionally, exogenous molecules, such as short interfering RNA (siRNA), can be inserted into MVs via electroporation.81
Second, the source of membranous particles needs to be considered.75 Injection of MSCs would ensure the long-term, continuous release of pro-regenerative MVs. However, the large-scale production of a clinically acceptable population of MSCs could prove expensive. Therefore, the use of purified MVs or synthetic liposomes could be preferable in a clinical setting. One benefit of using purified MVs is that they are empirically known to contain the necessary components to dock with and be incorporated into injured tissues to promote regeneration. The specific molecules responsible for these effects are largely unknown; therefore, at this time using cell-derived MVs would be superior to synthetic liposomes. However, once these molecules are uncovered, large-scale production of synthetic liposomes enriched with these molecules would be more cost effective and safer than injection of living cells.
Third, targeting of MVs to specific tissues is a critical determinant for therapeutic efficacy. Tissue-specific differences in MV incorporation have been reported. For example, MSC-MVs are incorporated into renal epithelial cells via a CD44- and CD29-dependent pathway, whereas incorporation of EPC-MVs into human microvascular endothelial cells is dependent on the presence of α4 integrin and CD29.46, 54 Target-specific molecules have been used in delivering MVs to neural tissues for therapeutic use. For example, dendritic cells were genetically engineered to express the rabies virus glycoprotein, which specifically targets neural tissues, fused to the MV-enriched molecule Lamp2b.81 MVs were isolated from these genetically manipulated dendritic cells and loaded with an siRNA targeting BACE2, a common therapeutic target in Alzheimer’s disease. Subsequent injection of these targeted MVs into mice facilitated the knockdown of BACE2 expression only in neural tissues. Depending on the target tissue, parent cells would need to be chosen based on the endogenous levels of specific targeting molecules or would need to be genetically manipulated to express the targeting molecules.
Lastly, genetic manipulation of the parent cells could be used to enrich MVs with therapeutic cargo. A potential novel application of this technology would be the MV-mediated delivery of a defined set of mRNAs to damaged tissues, with the aim of regenerating the tissues by reprogramming resident cells, such as fibroblasts. A cocktail of several transcription factor genes can reprogram fibroblasts into various types of differentiated cells in vivo.84 For example, injection of a specific combination of 3 or 4 cardiac transcription factor genes into an infarct area can convert cardiac fibroblasts into cardiomyocytes in situ, improving cardiac function after infarction.85–87 Similarly, pancreatic exocrine cells can be converted to β cells with 3 β-cell–specific transcription factor genes in only 3 days, ameliorating hyperglycemia in a mouse model of diabetes.88 Therefore, MVs purified from the culture medium of MSCs that overexpress these genes could act as a novel delivery vector for fragile mRNAs protected from physical and chemical damage. Another application would be to inject the engineered MSCs, expecting that these cells would deliver the MVs to the diseased tissues. The in situ reprogramming approach could be used to treat a variety of diseases including cardiovascular, neurodegenerative, and musculoskeletal disease and diabetes. Although the role of MVs in regenerative medicine is still in its infancy, the use of MVs to stimulate tissue repair provides novel and exciting possibilities.
Acknowledgments
We are grateful to the U.S. National Institutes of Health (R01 GM098294), the Engdahl Family Foundation, and the Schulze Family Foundation for their support of our work related to this topic. KS would like to thank Drs. Robert Winn, Robert Belton, Richard Rovin, and Johnathan Lawrence, and the Upper Michigan Brain Tumor Center for supporting his previous work in the field of microvesicles. All authors have read the journal’s policy on conflicts of interest and have none to declare.
Abbreviations
- AKI
acute kidney injury
- BrdU
5-bromo-2′-deoxyuridine
- CPC
cardiomyocyte progenitor cell
- EGFRvIII
epidermal growth factor receptor variant III
- EMMPRIN
extracellular matrix metalloproteinase inducer
- EPC
endothelial progenitor cell
- ESC
embryonic stem cell
- FasL
Fas ligand
- HGF
hepatocyte growth factor
- HLSC
human liver stem cell
- iPSC
induced pluripotent stem cell
- MSC
mesenchymal stem cell
- MV
microvesicle
- MVB
multivesicular body
- siRNA
short interfering RNA
- TGF-β
transforming growth factor β
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–8. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484–94. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, Gruenberg J. The recycling endosome of madin-darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol Biol Cell. 2000;11:2775–91. doi: 10.1091/mbc.11.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fullekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann N Y Acad Sci. 2004;1014:164–9. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- 9.Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci. 2002;115:963–72. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- 10.Del Conde I, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201:871–9. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccin A, Murphy WG, Smith OP. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007;21:157–71. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Dale GL, Remenyi G, Friese P. Tetraspanin CD9 is required for microparticle release from coated-platelets. Platelets. 2009;20:361–6. doi: 10.1080/09537100903096692. [DOI] [PubMed] [Google Scholar]
- 13.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–87. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 14.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–83. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 15.Abusamra AJ, Zhong Z, Zheng X, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–73. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Andreola G, Rivoltini L, Castelli C, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber V, Fais S, Iero M, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology. 2005;128:1796–804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran S, Palanisamy V. Horizontal transfer of RNAs: Exosomes as mediators of intercellular communication. Wiley Interdiscip Rev RNA. 2012;3:286–93. doi: 10.1002/wrna.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–27. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 23.Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-micrornas. Nucleic Acids Res. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan A, Farber EL, Rapoport AL, et al. Transfer of micrornas by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Smith AG. Embryo-derived stem cells: Of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–62. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 29.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013 Jun 28; doi: 10.1016/j.biochi.2013.06.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: Considerations of stem cells, niches, and microvesicles. Stem Cell Rev. 2008;4:137–47. doi: 10.1007/s12015-008-9036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–8. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Yan X, Sun Z, et al. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev. 2007;16:695–706. doi: 10.1089/scd.2006.0118. [DOI] [PubMed] [Google Scholar]
- 36.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–96. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 37.Duffield JS, Park KM, Hsiao LL, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–55. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 39.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for AKT-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 41.Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–37. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Bruno S, Camussi G. Role of mesenchymal stem cell-derived microvesicles in tissue repair. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2413-z. [DOI] [PubMed] [Google Scholar]
- 43.Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: Role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 2010;19:7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- 44.Dorronsoro A, Robbins PD. Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther. 2013;4:39. doi: 10.1186/scrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratajczak MZ. The emerging role of microvesicles in cellular therapies for organ/tissue regeneration. Nephrol Dial Transplant. 2011;26:1453–6. doi: 10.1093/ndt/gfr165. [DOI] [PubMed] [Google Scholar]
- 46.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010;396:528–33. doi: 10.1016/j.bbrc.2010.04.135. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Zhu D, Huang L, et al. Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One. 2012;7:e46957. doi: 10.1371/journal.pone.0046957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 53.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–21. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 54.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–8. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 55.Borges FT, Melo SA, Ozdemir BC, et al. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–92. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Olson EN. AngiomiRs–key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19:205–11. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–92. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 58.Mummery CL, Davis RP, Krieger JE. Challenges in using stem cells for cardiac repair. Sci Transl Med. 2010;2:27p, s17. doi: 10.1126/scitranslmed.3000558. [DOI] [PubMed] [Google Scholar]
- 59.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by AKT-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 60.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Lai RC, Choo A, Lim SK. Derivation and characterization of human ESC-derived mesenchymal stem cells. Methods Mol Biol. 2011;698:141–50. doi: 10.1007/978-1-60761-999-4_11. [DOI] [PubMed] [Google Scholar]
- 62.Vrijsen KR, Sluijter JP, Schuchardt MW, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–70. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guan K, Hasenfuss G. Do stem cells in the heart truly differentiate into cardiomyocytes? J Mol Cell Cardiol. 2007;43:377–87. doi: 10.1016/j.yjmcc.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 64.Aguirre A, Sancho-Martinez I, Izpisua Belmonte JC. Reprogramming toward heart regeneration: Stem cells and beyond. Cell Stem Cell. 2013;12:275–84. doi: 10.1016/j.stem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 66.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for emmprin in tumor-stromal interactions. Oncogene. 2004;23:956–63. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 67.Belton RJ, Jr, Chen L, Mesquita FS, Nowak RA. Basigin-2 is a cell surface receptor for soluble basigin ligand. J Biol Chem. 2008;283:17805–14. doi: 10.1074/jbc.M801876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–54. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herrera MB, Fonsato V, Gatti S, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–18. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin H, Li Y, Buller B, et al. Exosome-mediated transfer of mir-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–64. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu YM, Gibbs KM, Davila J, et al. MicroRNA mir-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci. 2011;33:1587–97. doi: 10.1111/j.1460-9568.2011.07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 73.Calabrese JM, Seila AC, Yeo GW, Sharp PA. RNA sequence analysis defines Dicer’s role in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:18097–102. doi: 10.1073/pnas.0709193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyoshi N, Ishii H, Nagano H, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Katsman D, Stackpole EJ, Domin DR, Farber DB. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS One. 2012;7:e50417. doi: 10.1371/journal.pone.0050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 77.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. sup pp 1–13. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 78.Islam A, Shen X, Hiroi T, Moss J, Vaughan M, Levine SJ. The brefeldin A-inhibited guanine nucleotide-exchange protein, BIG2, regulates the constitutive release of TNFR1 exosome-like vesicles. J Biol Chem. 2007;282:9591–9. doi: 10.1074/jbc.M607122200. [DOI] [PubMed] [Google Scholar]
- 79.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: A novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–41. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marcus ME, Leonard JN. Fedexosomes: Engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals (Basel) 2013;6:659–80. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 82.Hartman ZC, Wei J, Glass OK, et al. Increasing vaccine potency through exosome antigen targeting. Vaccine. 2011;29:9361–7. doi: 10.1016/j.vaccine.2011.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microrna to breast cancer cells. Mol Ther. 2013;21:185–91. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012;14:892–9. doi: 10.1038/ncb2567. [DOI] [PubMed] [Google Scholar]
- 85.Inagawa K, Miyamoto K, Yamakawa H, et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:1147–56. doi: 10.1161/CIRCRESAHA.112.271148. [DOI] [PubMed] [Google Scholar]
- 86.Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song K, Nam YJ, Luo X, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]