Abstract
Pericytes are mural cells of the microcirculation, which have been shown to play key roles in regulating microvascular morphogenesis and stability, throughout each tissue bed and organ system assessed. Importantly, recent work has revealed that pericytes share several characteristics with mesenchymally- and adipose-derived stem cells suggesting that there may be lineage-related connections amongst bonafide pericytes and these vascular ‘progenitors,’ which can assume a perivascular position in association with endothelial cells. Hence, pericyte identity as a mediator of vascular remodeling may be confounded by its close relationships with its progenitors or pluripotent cell counterparts and yet demonstrates their potential utility as cell-based therapies for unmet clinical needs. Crucial to the development of such therapies will be a comprehensive understanding of the origin and fate regulating these related cell types as well as the unveiling of the molecular mechanisms by which pericytes and endothelial cells communicate. Such mechanistic inputs, which disrupt normal cellular ‘cross-talk’ during disease inception and progression, offer opportunities for intervention and will be discussed in the context of the vasculopathies accompanying tumor growth, diabetes, and fibrosis.
INTRODUCTION
Pericytes and Microvascular Remodeling
During vascular remodeling, the blood vessel responds to hemodynamic changes in order to adapt and restore homeostasis. Endothelial cells comprise the inner lining of vessels, while pericytes encompass blood microvessels such as blood capillaries, pre-capillary arterioles, pre-capillary venules, and collecting venules (1). Pericytes use cytoplasmic processes to surround the abluminal surface of the endothelial tube (2). They share and co-produce a basement membrane with endothelial cells, demonstrating that pericyte-endothelial interaction plays a key role in basement membrane formation, maintenance, and remodeling. Pericytes are in close proximity to endothelial cells and are typically 20 nm apart, with a single pericyte covering several endothelial cells incompletely (1, 2).
At distinct points in the basement membrane, pericytes and endothelial cells form specialized junctions with each other (1, 2). Peg-socket type contacts are formed by pericyte cytoplasmic fingers that are inserted into invaginations within the endothelium. Adherens junctions connect the cytoskeleton of pericytes and endothelial cells, mediating contact inhibition through contractile forces. Gap junctions between the cytoplasms of pericytes and endothelial cells enable passage of metabolites and ionic currents (3, 4).
Pericytes play an important role in regulation of endothelial cell proliferation and differentiation, contractility and tone, and stabilization and permeability (1–7). During angiogenesis, the formation of blood vessels from preexisting structures, nascent microvessels are composed of proliferative endothelium with an immature basement membrane. This event is followed by microvascular maturation through pericyte recruitment (2). Among the first cells to migrate to newly vascularized tissues, pericytes are located at the growing front of endothelial sprouts (1, 2). Pericyte investment of the vasculature is associated with resistance to capillary regression and suppression of endothelial growth. Thus, pericytes have a stabilizing effect on these newly formed microvessels (1–7).
Evolution of the Pericyte in History
Pericytes were first described by Charles-Marie Benjamin Rouget in 1873 as cells with contractile properties that surround the endothelial cells of small blood vessels (1). Krogh further investigated capillary recruitment and vascular tone and defined the cells adjacent to the endothelium that may be involved in these functions as Rouget cells. By 1923, Zimmermann devised the term “pericyte” due to the cell’s close proximity to endothelial cells and used light microscopy studies to further elucidate their morphology (2). Early immunocytochemistry studies revealed pericyte expression of proteins such as actin (8), tropomyosin (9), and myosin (10), among others, demonstrating their potential role as force-generating contractile elements in the regulation of vascular permeability and blood flow (Figure 1, Figure 2). Since then, this cell type has been studied in depth because of its crucial role in maintenance of vascular stability.
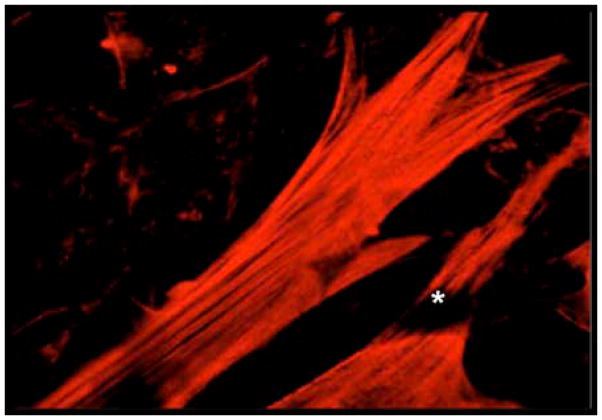
Figure 1. Discriminating between vascular cells using contractile protein isoform-specific antibodies.
Co-cultures of mural cells and vascular endothelial cells were fixed and permeabilized before labeling with anti-vascular smooth muscle actin-specific IgG. Note the brightly fluorescent mural cells with robust stress fibers and the darkened (negative) image of the endothelial cell (*)‘draping’ across the pericyte.
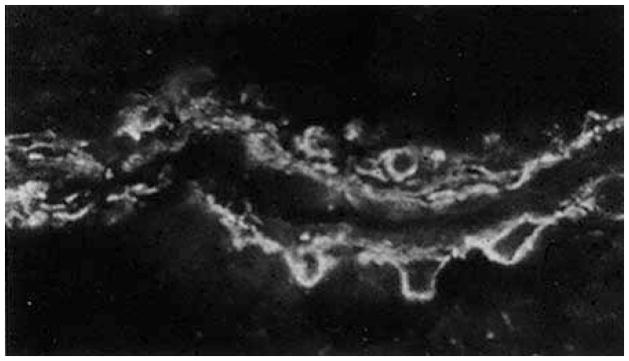
Figure 2. In situ localization of a mural cell-enriched cerebral microvessel.
Frozen rat brain sections were prepared from perfusion-fixed specimens prior to treatment with fluorescently-labeled anti-smooth muscle actin IgG. Note the abundance of antibody-stained mural cells aligned along the length of this ‘muscular’ venule.
Recent research has highlighted the untapped potential of the pericyte as a critical modulator of vascular remodeling in disease states. Of particular interest is the ability of mesenchymal- and adipose-derived stem cells to exhibit pericyte-like properties under specific conditions. Thus, these cell types may serve as a potential progenitor pool from which pericytes can be cultivated, or could possibly be used to restore, replace, and rejuvenate damaged, remodeling and/or diseased tissues. This review will discuss the importance of pericyte-endothelial cell interactions in remodeling and re-stabilization of the vasculature, the use of stem cells as possible pericyte sources, and the vascular dysfunction that underlies the pathogenesis of diabetic retinopathy (DR), fibrotic diseases, and cancer.
PERICYTE ORIGIN
Pericytes were first evidenced by Clark et al in 1925, who observed the development of pericytes on the capillaries of tadpole larvae from connective tissue components (11). Lineage tracing studies using chick-quail chimeras and cell-specific markers later demonstrated that a majority of pericytes found in the cephalic region and central nervous system were derived from the neural crest (12, 13). Fate mapping analyses in mice using genetic reporters have also shown that cells from the mesothelium give rise to pericytes in the gut, liver, heart, and lung via epithelial-to-mesenchymal transition, delamination, and migration to the aforementioned organs (1, 14–16).
PERICYTE-ENDOTHELIAL CELL RATIOS VARY ACROSS TISSUES
The number and size of pericyte-endothelial contacts varies considerably in different tissues and in vessels of differing size. In general, pericytes are more abundant and have more extensive processes in venous capillaries and post-capillary venules (1, 2). Endothelial to pericyte ratios in normal tissues vary between 1:1 and 10:1, while pericyte coverage of the endothelial abluminal surface ranges between 70% and 10% (1, 2). The highest density of pericytes (endothelial cell: pericyte ratio 1:1) is in neural tissues, especially the retina, possibly due to its high metabolic activity and need for controlled blood flow (1, 2). This is in stark contradistinction to the skeletal musculature where the endothelial cell: pericyte ratio has been noted to be closer to 10:1 (1, 2). The differences in distribution and structure among pericytes demonstrate microvascular diversity, cellular plasticity, and the myriad of functions that pericytes play in regulating different organ systems (1–7).
MOLECULAR MEDIATORS OF VASCULAR REMODELING AND STABILITY ARE DEPENDENT ON ENDOTHELIAL CELL-PERICYTE INTERACTIONS
As variations in pericyte density can alter the microenvironment of the vasculature, soluble mediators synthesized or expressed by vascular and non-vascular cells facilitate remodeling through coordinated endothelial-pericyte interactions. Several key entities involved in coordinating endothelial-pericyte signaling are discussed below.
A) TGF-β
Transforming growth factor- β (TGF-β) is a family of multifunctional cytokines that is expressed by both endothelial cells and mural cells (1, 5). The mature molecule regulates induction of mural cells from mesenchymal cells during embryonic development and is involved in mural and endothelial proliferation and differentiation (1, 3, 5, 17). Ablation studies of the TGF-β1 signaling pathway in retinal pericytes reveal that TGF-β1 alters pericyte contractility and results in loss of pericytes from the ablumninal surface of the endothelium (18). Thus, TGF-β1 is required to sustain endothelial cell-pericyte interactions, which keep endothelial cells in growth arrest; without TGF-β1, the loss of pericytes leads to endothelial cell proliferation and angiogenesis at sites of injury. Recent work has shown that the the RhoGTPase pathway may play an even greater role in pericyte contractility than TGF-β1, with alterations in RhoGTP levels resulting in attenuation of pericyte contact-mediated endothelial growth arrest (19, 20).
B) Angiopoietin 1/Angiopoietin 2 and Tie-2 receptor
Angiopoietin-1 (Ang1) is expressed by perivascular mesenchymal cells including pericytes, while its receptor, Tie-2, is mainly expressed on endothelial cells. Ang1 reduces vascular permeability in skin, tumors, and in vitro models of the blood brain barrier. This molecule has also been shown to stabilize vessels via stimulation of pericyte recruitment (1,3,5). Though the underlying mechanism is unknown, Ang1 stimulates release of pericyte recruitment factors such as TGF-β1 and platelet-derived growth factor-B (PDGF-B) from endothelial cells. Angiopoietin 2 (Ang-2) is an antagonist of Tie-2 that is expressed by endothelial cells at sites of angiogenesis. It promotes vessel destabilization and is associated with poor pericyte coverage of the vasculature, as seen in diabetic retinopathy (1, 3, 5, 17, 21).
C) PDGF-B and PDGFR-β
PDGF-B is expressed by endothelial cells in angiogenic sprouts and remodeling arteries and stimulates migration and proliferation of platelet-derived growth factor receptor-β-(PDGFR-β) expressing pericytes (10). Knockout of either PDGF-B or PDGFR-β in mice causes reduced pericyte coverage of microvessels in various organs (5, 6). In mice deficient in PDGF-B or PDGFR-β, pericyte association with vessels is markedly reduced, leading to microvessel leakage, endothelial cell over-proliferation, and impaired blood flow (22,23). Thus, PDGF-B/PDGFR-β-dependent pericyte recruitment and investment is critical to vascular development.
D) VEGF
The VEGF family is a group of potent endothelial mitogens and thus plays an active role in angiogenesis (5). Differentiation of mesenchymal cells to pericytes when co-cultured with endothelial cells is associated with increased VEGF production by mesenchymal cells and likely mediated by TGF-β. In turn, differentiated pericytes express VEGF, which potentiates endothelial cell survival and stability of microvessels (24). In contrast, PDGF and VEGF have an antagonistic relationship during neovascularization, with VEGF reducing pericyte coverage of the endothelium in PDGF-mediated angiogenesis. These opposing actions may be caused by the VEGF-mediated formation of a VEGF-R2/PDGFR-β complex that suppresses PDGFR-β signaling (25). The ability of VEGF to inhibit pericyte function and blood vessel maturation can be used in anti-VEGF therapies.
PERICYTE MARKERS CORROBORATE AMBIGUITY IN PERICYTE CHARACTERIZATION
Identification of pericytes within the microvasculature has been critical to the elucidation of pericyte-endothelial cross-talk. Pericytes express a wide variety of molecular markers, suggesting they may comprise, give rise to, or descend from a diverse population of progenitor or mural-like cells (1). Accurate identification of pericytes and this ‘cohort’ of vascular cells is dependent both on surface marker expression profiles together with a ‘mapping’ of their location along the abluminal surface of endothelial cells (1, 3). Widely accepted pericyte markers include α-smooth muscle actin (α-SMA), NG2, alkaline phosphatase (ALP), and PDGFR-β. Other markers associated with pericytes include desmin, vimentin, aminopeptidase N, CD13, CD133, and CD146 (1, 3, 4, 6, 7). Though numerous studies have elucidated key markers expressed by pericytes, a confounding problem to pericyte confirmation is the dynamic nature of these markers’ expression in development, disease, and specific tissues. Importantly, several cells possess perivascular capabilities as well as express the currently accepted ‘pericyte’ markers, which have been conclusively linked to pericytes encircling microvascular endothelial cells within capillary or venular basement membranes. In the following sections, the potential tissue- and organ-specific diversity of pericytes will be discussed in the context of cellular entities that have pericyte-like properties and functions.
STELLATE CELL: THE PERICYTE CELL OF THE LIVER
Stellate cells have been long regarded as specialized pericytes of the liver and are characterized by droplets of Vitamin A found in their cytoplasm. Comprising about five to eight percent of total cells in the normal liver, these desmin- and PDGFR-β positive cells are located in the perisinusoidal space, between the fenestrated endothelium (26). Believed to regulate microvascular hepatic flow, stellate cells foster sinusoidal constriction, and are therefore a possible therapeutic target for portal hypertension (27).
In response to liver injury, quiescent stellate cells lose their Vitamin A droplets and acquire a contractile phenotype with expression of α–SMA, as well as a myofibroblast-like phenotype. Prolonged activation of stellate cells has been implicated in liver fibrosis due to deposition of excess collagen, with TGF-β being a potent stimulator of collagen synthesis and fibrinogenesis. Fibrosis is also facilitated by stellate cell production of tissue inhibitors of metalloproteinases (TIMPs), leading to reduced degradation of excess matrix deposited during livery injury. Blockage of TIMP-1 with monoclonal antibodies ameliorates hepatic fibrosis induced by carbon tetrachloride. In addition, hepatic stellate cell apoptosis has been correlated with reversal of hepatic fibrosis (28). Based on these findings, it is evident that stellate cells play a key role in the pathogenesis of liver fibrosis, and are thus prime targets for therapies.
There is also evidence to suggest that hepatic stellate cells are important in the initiation and termination of liver regeneration. Hepatic stellate cells have been located in the canals of Hering, the stem cell niche of adult livers, and express certain stem cell markers such as CD133. These CD133+ stellate cells can develop into myofibroblast-like, endothelial-like, and hepatocyte-like cells in vitro (29). Fate mapping studies that traced GFP-labeled stellate cells in transgenic mice showed that quiescent hepatic stellate cells, after liver injury, could give rise to hepatic epithelial cells (30). A recent study showed the role of hepatic stellate cells in liver regeneration using a rat model of liver damage induced by 2-acetylaminofluorence/partial hepatectomy. In early stages of liver regeneration, conditioned media from activated stellate cells contained high levels of hepatocyte growth factor, activating liver progenitor cell proliferation. In later stages of liver regeneration, conditioned media from stellate cells showed high levels of TGF-β1, inhibiting hepatocyte proliferation and terminating liver regeneration (31). The dual role of the stellate cell as a positive and negative regulator of proliferation is likely due to changes in the ratios of hepatocyte growth factor and TGF-β1 in the microenvironment during early and late stages of liver regeneration. Therapies that alter cytokine profiles to lower TGF-β1 levels during late stages of liver regeneration may prove beneficial by modulating stellate cell activity.
As already mentioned, the stellate cell can reversibly respond to the pathophysiologic microenvironment, either enabling regeneration or hepatic fibrosis. In fact, the dynamics of stellate cell responsivity parallels microvascular pericyte phenotypic modulation, in which pericyte growth state and contractility is altered to regulate angiogenic stabilization or capillary endothelial cell proliferation. Similarly, hepatic stellate cell phenotypic switching plays a role in liver fibrosis through differentiation into myofibroblast-like cells, and in liver regeneration through differentiation into hepatocyte-like cells, which stimulate liver progenitor cell production. Thus, cell-based therapies modulating stellate cell activity can be beneficial in anti-fibrotics and liver remodeling after disease.
THE MESANGIAL CELL: A RENAL PERICYTE?
While the stellate cell is the matrix-producing pericyte of the liver, the mesangial cell is its cellular counterpart in the kidney. Mesangial cells can be considered a specialized subset of pericytes located in the glomerulus, while pericytes found in the tubular interstitium are referred to as peritubular pericytes (32). Mesangial cells express PDGFR-β and exhibit contractile properties and cytoskeletal architecture that anchors filaments to the glomerular basement membrane opposing podocyte foot processes. Under the influence of PDGF-B, mesangial cells migrate into the cleft of developing glomerular structures and assist in maintaining capillary structure and flow. Mesangial cell recruitment is inhibited in animals deficient in endothelial PDGF-B or mesangial PDGFR-β, resulting in hyper-dilated glomerular capillaries with aberrant capillary loops (33). Mesangial cells thus function as pericytes in that they regulate the microvasculature and maintain its stability.
Proliferation and migration of mesangial cells also occurs during glomerular remodeling after kidney injury. Initial kidney injury is marked by destruction of mesangial cells and is later followed by repopulation of mesangial cells. This proliferation of mesangial cells, if uncontrolled, can lead to hypercellularity and expansion of mesangial matrix, leading to glomerulosclerosis seen in aging and diseases such as diabetes and IgA nephropathy (34).
Like hepatic fibrosis, a hallmark of renal fibrosis is an over-accumulation of interstitial Type I collagen. Results of biochemical studies reveal that deposition of Collagen I is carried out by perivascular fibroblasts, as well as pericytes. When activated after injury, pericytes can escape from peritubular capillaries into the interstitium and differentiate into myofibroblasts that secrete matrix. Targeting pericytes may prove to be an efficient anti-fibrotic strategy and improve renal function in kidney disease (32).
THE PERICYTE-MESENCHYMAL STEM CELL CONUNDRUM
Though there is general agreement in the literature that stellate cells and mesangial cells are embryologically and functionally related to microvascular pericytes, there is much controversy regarding whether pericytes are descendants or antecedents of mesenchymal stem cells. Dar et al recently demonstrated that differentiating human pluripotent stem cells (hPSCs) could give rise to pericytes (35). The authors identified a population of CD31−CD73+CD90+CD105+ cells that expressed pericyte markers such as NG2, CD146, and PDGFR-β but did not express α-SMA. Injection of hPSC-derived pericytes into the ischemic hind limb of nude mice showed accelerated recovery of foot perfusion and a significantly larger proportion of perfused blood vessels and enhanced vascular density compared to control groups. Therefore, this study gives credence to the notion that pericytes may be derived from pluripotent progenitors.
It has been suggested that pericytes exhibit multilineage mesodermal potential and thus could be invaluable in cell-based therapeutics for regenerative medicine (36, 37). Pericytes and mesenchymal stem cells have been shown to share several molecular markers such as CD44, CD90, CD73, and CD105 (36, 37, 38, 39, 40). These studies have caused some to theorize that all MSCs are pericytes (41). One landmark study identified pericytes in various human organs such as skeletal muscle, pancreas, adipose tissue, and placenta that expressed specific pericyte markers such as CD146, NG2, and PDGFR-β. When cultured, these perivascular cells also expressed MSC markers including CD10, CD13, CD44, CD73, CD90, and CD105. This subset of pericytes differentiated in vitro into osteoblasts, adipocytes, and chondrocytes. Lastly, the study showed that perivascular cells natively express CD44, CD73, CD90, CD105, which are commonly expressed MSC surface components (37). The results suggest that MSCs may be derived from perivascular cells since pericytes not only inherently express some of these MSC markers, but may also be able to give rise to multiple cell types. Despite this, consensus regarding the relationships, origin or fate between/amongst pericytes and MSCs remains equivocal (42).
MSCs are perivascular in nature due to their close proximity to capillary beds, yet it does not immediately follow that these cells have been derived from pericytes. Adventitial cells, which line the outermost layer of all blood vessels but capillaries, have been shown to express MSC markers and are able to generate a progeny of multipotent cells, namely MSCs (43). This data suggests that MSCs are not exclusively derived from pericytes. A recent study used functional in vitro angiogenesis assays to distinguish pericytes from other MSCs (44). The researchers concluded that not all MSCs can be classified as pericytes, although these two cell types can share several similar markers. In this study, human placental-derived pericytes and basement membrane-derived MSCs were compared for the ability to carry out pericyte-like functions such as tube stabilization and increased sprouting. In vitro assays monitoring sprout formation in endothelial cells co-cultured with pericytes or mesenchymal cells showed that pericytes co-localized with sprouts and stimulated their formation, while mesenchymal cells migrated away from the endothelium. In vitro angiogenesis assays of placental-derived pericytes contributed to the formation of cord-like structures, which promoted endothelial network formation, while MSCs seemed to serve as a feeder layer for endothelial cells. Therefore, pericytes may be a subset of MSCs, but not all MSCs exhibit ‘classic’ pericyte behaviors.
ADIPOSE-DERIVED STEM CELLS EXHIBIT PERICYTE PHENOTYPE
While MSCs are excellent candidates for use in regenerative medicine, they are both more difficult to isolate and represent a small fraction of cells in the adult hematopoietic system. In contrast, adipose-derived stem cells (ASCs) are abundant and are easily extracted from a stromal vascular fraction from adipose tissue. Immunofluorescence and flow cytometry studies show that the majority of processed lipoaspirate from human tissue is of mesodermal or mesenchymal origin. Additionally, ASCs are capable of differentiating into adipogenic, chondrogenic, myogenic, and osteogenic cells in the presence of relevant induction factors (45). ASCs have been shown to express MSC markers such as CD105, CD106, CD44, and CD146 (46, 47, 48). Both MSCs and ASCs are represented in stromal fraction isolates and produce similar mitogens within their respective secretomes; however, notably, these cell populations are derived from different sources, i.e. the bone marrow and adipose tissue, respectively (47, 48).
Of interest, ASCs are believed to play an important role in microvascular support by differentiating into pericytes (49, 50). Traktuev et al. identified a certain group of CD34+ ASCs as pericyte-like cells due to their expression of NG2, α-SMA, and PDGFR- β (46). This population of cells was shown to localize in vessels at the interface between endothelium and adipocytes and supported endothelial survival, potentially mediated by secretion of proteins such as VEGF, FGF, and hepatocyte growth factor. Co-culture of ASCs and endothelial cells produced stabilized cell networks and ASCs were observed to make abluminal connections with EC tube networks on Matrigel. Another study showed that injection of ASCs into a mouse ischemic hindlimb model augmented angiogenic score, evidenced by increased blood flow and capillary density (47). Thus, ASCs can play pericyte-like roles in normalizing or restoring vascularization in disease and may be a more amenable source of cells compared to MSCs for personalized cellular medicine.
Several groups, including our own, have shown the potential role of adipose derived stem cells in regenerative medicine through the adoption of pericyte-like phenotype and function. One group studied the role of human adipose-derived stem cells (hASCs) in a rat model of mesenteric inflammation and vascular remodeling (51). Ten days post-injection of hASCs, these cells were visible in inflamed mesenteric tissue and about 20% were confirmed to localize to the abluminal surface of the endothelium and express pericyte markers such as NG2 and α-SMA. In addition, total vascular length significantly increased in tissues treated with hASCs compared to controls, indicating that these cells could enhance microvascular density during angiogenesis. Similarly, our lab has recently identified hASCs that are capable of vascular integration and maintenance of pericyte phenotype for approximately two months post-injection in a murine model of oxygen-induced retinopathy (52). We demonstrated that hASCs could both prevent the vascular dropout typically observed in this model, and foster regrowth into the ablated area. Treatment of hASCs with TGF-β1 enhanced and fostered hASC pericyte function in a manner similar to the effects of TGF-β1 in native, retinal pericytes. Our subsequent studies have demonstrated that hASCs reduce the devastating capillary loss by 79% in the Akimba mouse model of severe diabetic retinopathy. These studies strongly demonstrate the pericyte-like properties of ASCs, and the critical role ASCs can play in vascular protection and regeneration. This is supported by emergent clinical studies where such autologous cells are being used (53).
Here, we highlighted the similarities and differences of the stellate cell, mesangial cell, MSC, and ASC with the pericyte. These cells share many molecular markers (Figure 3), showing that they may carry out similar functions and be related to one another (28, 33, 48, 54). However, these cell types also have unique roles throughout the body that extend beyond the classical definition of the pericyte. Cells such as hepatic stellate and mesangial cells are pericytes that play a role in modulation of the vasculature in their respective organs, and also mediate physiologic and pathologic fibrosis. Mesenchymal stem cells and adipose-derived stem cells are capable of differentiating into pericytes, as well as other cell types, and can assist in microvascular remodeling in disease. Thus, this field of study is complex and will require extensive research to elucidate the interrelatedness of these cell populations. We posit that the pericyte likely represents a “cohort” of cells that share a perivascular phenotype, similar molecular marker profiles, and can modulate the microvasculature.
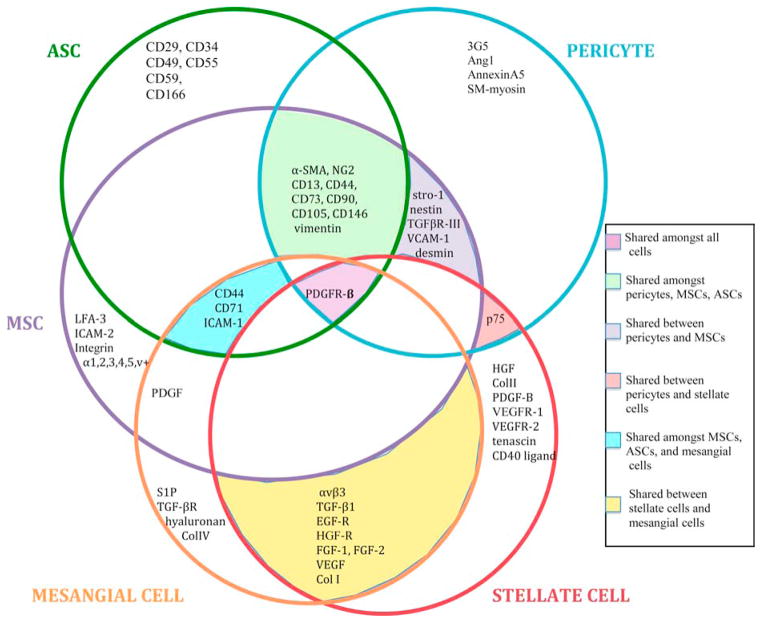
Figure 3. The pericyte cohort: Characterization of molecular marker profiles of stellate cells, mesangial cells, MSCs, ASCs, and pericytes.
The Venn Diagram above compares molecular markers expressed by stellate cells, mesangial cells, MSCs, ASCs, and pericytes. These markers are important in several important signaling pathways such as adhesion, receptor-ligand binding, and extracellular matrix formation. The similarities in function and marker profile between these cells support the notion that they are related and comprise a family or “cohort” of cells. As discussed, this cohort is comprised of mural cells (pericytes and vascular smooth muscle cells), perivascular cells (adipose- and mesenchymal-derived stem cells), and interstitial myofibroblast-like cells (mesangial and stellate cells).
Note: This diagram does not portray all molecular markers expressed by these cell types, and is reviewed elsewhere (28, 33, 48, 54).
ROLE OF PERICYTE-ENDOTHELIAL CELL INTERACTIONS IN DISEASE
Aberrations in pericyte-endothelial cell interactions are a possible focal point wherein microvascular dysfunction and vasculopathy accompanying disease progression may originate. As discussed below, perturbations in endothelial-pericyte signaling may indeed represent a key mechanism by which the microvasculature becomes dysregulated, unstable, and ultimately pathogenic in such disease states such as diabetes, fibrosis, and cancer.
A) Diabetic Retinopathy
The total prevalence of diabetes in the United States is 25.8 million, about 8.3% of the population (55). The worldwide incidence of diabetes is 150 million and is projected to increase to 300 million by 2025 (56). DR is a common pathology seen in those with diabetes that results in loss of visual acuity due to macular edema and is the most common cause of adult blindness in Western civilization (55). Retinal pericyte loss can occur in diabetics, and is the hallmark finding in early stages of DR (57). The consequences of pericyte loss in the retina were studied in a mouse model of endothelium-restricted PDGF-BB ablation (58). Vascular aberrations such as irregular capillary density, microaneurysms, and acellular vascular areas due to endothelial cell loss were all associated with decreased pericyte density. In regions of increased pericyte density, a dense and highly irregular vascular network was observed, with increased endothelial cell proliferation and penetration of vessels into the choroid and vitreous. This is characteristic of the proliferative stage of DR (PDR), which is further characterized by thickening of the basement membrane and hemorrhage of retinal blood vessels (58). Causes of pericyte dropout in DR include decline in PDGF-β expression, up-regulation of Angiopoietin-2, and increased production of reactive oxygen species that damage the vasculature (58, 59).
While pericyte loss and the associated instability in the microvasculature of the retina has been proposed as a possible mechanism by which DR develops, recent work has revealed that the frank loss of pericytes may not represent an early event responsible for reversing retinal capillary growth arrest. Indeed, more subtle perturbations in pericyte signaling, contractile phenotype and endothelial cross-talk might be responsible for angiogenic switching and the emergent pathological vasculopathy that ensues following decades of diabetic insult. In fact, dysregulated pericyte mechanical stiffness and altered contractility has now been implicated as the key, early event in the microvascular pathogenesis observed in DR. For example, we demonstrate that perturbations in pericyte cytoskeletal signaling and cytoskeletal-membrane interactions that give rise to significant alterations in pericyte mechanical stiffness, which in turn alters pericytes’ ability to sustain capillary endothelial growth arrest (60). Therapeutics that target these key cytoskeletal and plasma membrane-associated molecules may prove to be promising avenues in modulating pericyte contractility, hence preventing or reversing diabetes-induced endothelial cell proliferation and angiogenesis.
B) Fibrotic Disease
Fibrotic diseases are believed to be potentiated by myofibroblasts, which, in turn, are believed to originate from an assortment of cell types, including fibroblasts, epithelial and endothelial cells, smooth muscle cells, pericytes, hepatic perisinusoidal cells, and MSCs (61, 62). Myofibroblasts play important roles in late stages of wound healing where they are involved in fibrosis and excessive scarring, and are linked to the expression and accumulation of various extracellular matrix proteins such as fibrillar and non-fibrillar collagens, fibronectin, laminin, and tenascin (63). In response to TGF-β, specialized extracellular matrix proteins such as fibronectin, and the damaged mechanical environment, cells are able to differentiate into myofibroblasts and acquire a contractile phenotype, evidenced by expression of α-SMA. In vivo studies show that when wound healing culminates in re-epithelialization, myofibroblast apoptosis occurs (64). The specific mechanisms are unclear, but may include growth factor signals or changes in the biomechanical forces in the extracellular matrix. In pathological fibrosis, failure of apoptosis leads to myofibroblast survival, expansion and excess matrix production, ultimately resulting in tissue contraction and pathological scar formation (64, 65).
Fibrosis is extremely common in patients with chronic kidney disease, a disease defined by a reduction in the density of peritubular capillaries. It is characterized by deposition of excess extracellular matrix proteins such as Collagen I and Collagen III between nephrons (66). This phenomenon is a strong predictor of declining renal function, which can eventually lead to end stage renal disease. Peritubular pericytes and specialized pericytes of the kidney such as mesangial cells may play a substantial role in mediating this process by acquiring a myofibroblast phenotype.
In kidney fibrosis, it has been shown that pericytes are the primary source of myofibroblasts. Lin et al (2008) used the unilateral ureteric obstruction (UUO) model of kidney fibrosis in ColI/GFP-expressing mice to track pericytes. These experiments revealed that migration and differentiation of pericytes contribute to the myofibroblast population in kidney fibrosis (67). Humphreys et al (2010) used lineage-restricted expression of bacterial Cre recombinase in transgenic or knock-in mice to genetically track renal epithelial cells and interstitial stromal cells in the UUO model of kidney fibrosis. The authors identified a population of CD73+, PDGFR-β+, α-SMA- subset of interstitial pericytes derived from the FoxD1 lineage, which is specific to kidney stromal cells and not endothelial or epithelial cells. This cell population was able to differentiate into α-SMA+ myofibroblasts in in vivo mice models of fibrosis. (68). A possible mechanism for this involves pericyte activation via injury-induced changes in molecules such as PDGF, pericyte detachment from peritubular capillaries and migration to the interstitium, and differentiation into myofibroblasts. This, in turn, leads to scar formation through collagen deposition. The association between pericyte dysfunction and pathogenesis of renal fibrosis strongly suggests that a pericyte-targeted therapeutic approach might be an innovative treatment strategy (67, 68). Linked to this, LeBleu and colleagues (2013) used a pre-clinical animal mouse model to track pericytes in vivo, only to reveal that NG2+ pericytes and PDGFR-β+ pericytes were devoid in the renal interstitum and do not contribute to the renal myofibroblast population. Further, the investigators showed that deletion of pericytes does not alter myofibroblast recruitment or improve kidney fibrosis, while demonstrating that myofibroblast accumulation in kidney fibrosis might be attributed to the local proliferation of resident tissue fibroblasts or bone-marrow derived mesenchymal cells (69). While such animal modeling of human disease processes may not accurately reflect pathogenesis observed in patients, it is also possible that the marrow-derived mesenchymal cells could have a pericyte-like origin, which then differentiated into myofibroblasts. Nonetheless, these results not only raise questions regarding the origin of myofibroblasts in renal fibrosis, but also reflect upon the ambiguity regarding the origin or identity of tissue- and organ-specific pericytes. Further experimentation is needed to explore the discrepancies noted above and assess the role of pericytes in myofibroblast recruitment in fibrotic disease.
C) Cancer
Since the groundbreaking work of Folkman and colleagues, it has been well recognized that tumor growth is dependent on an ‘angiogenic switch’ and pathologic neovascularization (70). Tumor vessels display abnormalities such as fragility and leakage, which results in hemorrhage and increased interstitial fluid pressure. These pathologic vessels are often dilated, tortuous, and highly permeable (71). As a result, inefficient blood flow due to poor organization of tumor vasculature leads to ischemia and necrosis, which are hallmarks of rapidly growing tumors (5).
Understanding tumor vascular microenvironment is crucial to identifying the key aspects or rate limiting steps required for the escape from tumor dormancy and cancer progression (71, 72). In tumor-associated microvessels, pericytes can be observed near the growing front of blood vessels, where angiogenesis is most active. However, pericyte investment of such tumor microvessels is aberrant, since pericyte coverage is diminished and associations between pericytes and endothelial cells are perturbed (72, 73). The exact causes of abnormal pericyte behavior are unknown, but it is suggested that tumor hypoxia stimulates a signaling cascade that leads to vasodilation via endothelial cell release of nitric oxide. This, in turn, leads to increased vascular permeability via VEGF-A and Ang-2 expression, and subsequent basement membrane remodeling. In particular, VEGF has been shown to be important in dissociation of pericytes and endothelial cells under hypoxic conditions. Ultimately an immature vasculature that lacks organization and stability is created (72, 74). Interestingly, researchers have recently shown a potential benefit in combining anti-cancer agents with anti-pericyte agents. Targeting of pericytes leads to dissociation of pericytes from the tumor vasculature and destroys the blood supply of the tumor (75). For example, tyrosine kinase inhibitors, such as Gleevec, used in synergy with VEGF inhibitors more efficiently blocked tumor angiogenesis than VEGF inhibitors alone in several experimental models via inhibition of PDGFR-β (72, 74, 75). Disruption of PDGF signaling results in aberrant pericyte-endothelial interactions and reduces tumor vascularity, making endothelial cells more susceptible to anti-angiogenic chemotherapy. Hilden (2013) discusses the varied effects of PDGFR kinase inhibitors as therapies in different tumor types in ongoing clinical trials. Development of resistance to PDGFR inhibitors indicates these therapies may be short-lived or most effective when used in conjunction with other agents. Many PDGFR kinase inhibitors inhibit other kinases as well, suggesting their effectiveness may stem from effects on multiple tumor signaling pathways (76).
CONCLUSION: PERICYTES AS POTENTIAL TARGETS IN CELLULAR THERAPY
Pericytes play a fundamental role in the remodeling and stability of the vasculature. Though definitive pericyte characterization within various tissues and organ systems remains incomplete, these mural or perivascular cells represent key microvascular components. The mesangial cell and hepatic stellate cell are specialized pericytes of the kidney and liver, respectively, and can become myofibroblast-like. When considering the perivascular basis of the stem cell niche, mesenchymal stem cells and adipose-derived stem cells share several similar properties as pericytes, but the origin and fate of these related cell populations remains equivocal. Further research should be directed towards elucidating the similarities between these cell populations by studying the mechanisms by which they modulate the microvasculature and how they develop in the perivascular niche. Disruption of pericyte-endothelial interactions plays a role in the pathogenesis of diseases such as diabetic retinopathy, fibrotic disease, and cancer progression. Cellular-based therapies that either augment or inhibit pericyte-endothelial interactions may prove helpful in prevention or protection against disease inception or progression. Thus, deriving pericytes from mesenchymal and adipose stem cell populations could be a novel approach to deriving autologous cell populations capable of fostering microvascular stabilization or activation, respectively. Ultimately, a more detailed understanding of the pericyte’s origin and fate may be crucial in the development of innovative cell-based therapies for unmet clinical needs and regenerative medicine.
Acknowledgments
We are grateful to Dr. Tatiana Demidova-Rice and Dr. Jennifer Durham for their critical reading of this manuscript. This work was supported by the following grants: NIH EY 15125, EY 022063 (IMH).
Abbreviations
- TGF-β
Transforming growth factor-β
- Ang1
Angiopoietin-1
- Ang2
Angiopoietin-2
- PDGF-B
Platelet-derived growth factor-B
- PDGFR-β
Platelet-derived growth factor receptor- β
- VEGF
Vascular endothelial growth factor
- α-SMA
α-smooth muscle actin
- NG2
Neuron-glial antigen 2
- ALP
Alkaline phosphatase
- TIMP
Tissue inhibitors of metalloproteinases
- hPSC
Human pluripotent stem cell
- MSC
Mesenchymal stem cell
- ASC
Adipose-derived stem cell
- DR
Diabetic retinopathy
- PDR
Proliferative diabetic retinopathy
- UUO
Unilateral ureteric obstruction
Footnotes
The authors have no financial or personal conflicts of interest that may influence the material presented in this review and recognize Translational Research’s policy on such disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Sims DE. The pericyte – a review. Tissue Cell. 1986;18(2):153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 3.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 4.Díaz-Flores L, Gutiérrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24(7):909–69. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 5.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623–9. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55(3):261–8. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314(1):15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 8.Herman IM, D’Amore PA. Microvascular pericytes contain muscle and non-muscle actins. J Cell Biol. 1985;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Bio. 1985;100(5):1379–86. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J Cell Bio. 1985;100(5):1387–95. doi: 10.1083/jcb.100.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark ER, Clark EL. The development of adventitial (Rouget) cells on the blood capillaries of amphibian larvae. Am J Anat. 1925;35(2):239–64. [Google Scholar]
- 12.Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ Res. 1998;82(9):221–31. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- 13.Etchevers HC, Vincent C, Le Dourain NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128(7):1059–68. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 14.Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53(3):983–95. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Que J, Wilm B, Hasegawa H, Wang F, Bader DM, Hogan BLM. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A. 2008;105(43):16626–30. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilm B, Ipenberg A, Hastie ND, Burch JBE, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132(23):5317–28. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 17.Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(5):630–8. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 18.Sieczkiewicz GJ, Herman IM. TGF-β 1 signaling controls retinal pericyte contractile protein expression. Microvasc Res. 2003;66(3):190–6. doi: 10.1016/s0026-2862(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 19.Kutcher ME, Kolyada AY, Surks HK, Herman IM. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Path. 2007;171(2):693–701. doi: 10.2353/ajpath.2007.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res. 2009;77(3):235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammes HP, Lin J, Wagner P, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53(4):1104–10. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 22.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15(4):215–28. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodeling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 24.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264(1):275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg JI, Shields DJ, Barillas SG, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–13. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41(8–9):1639–42. doi: 10.1016/j.biocel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat M, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman S. Hepatic stellate cells: multifunctional and enigmatic cells of the liver. Physiol Review. 2008;88(1):125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kordes C, Sawitza I, Müller-Marbach A, et al. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352(2):410–7. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Jung Y, Omenetti A, et al. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adults mouse livers. Stem Cells. 2008;26(8):2104–13. doi: 10.1634/stemcells.2008-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Zhang W, Zhou Q, et al. HSCs play a distinct role in different phases of oval cell-mediated liver regeneration. Cell Biochem Funct. 2012;30(7):588–96. doi: 10.1002/cbf.2838. [DOI] [PubMed] [Google Scholar]
- 32.Stefánska AM, Péault B, Mullins JJ. Renal pericytes: multifunctional cells of the kidney. Pflugers Arch. 2013;465(6):767–73. doi: 10.1007/s00424-013-1263-7. [DOI] [PubMed] [Google Scholar]
- 33.Schlöndorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20(6):1179–87. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 34.Abboud HE. Mesangial cell biology. Exp Cell Res. 2012;318(9):979–85. doi: 10.1016/j.yexcr.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Dar A, Domev H, Ben-Yosef O, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125(1):87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 36.Crisan M, Corselli M, Chen WCW, Péault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16(12):2851–60. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perviascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30(6):1104–9. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 39.Ringe J, Kaps C, Burmester G, Sittinger M. Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Naturwissenschaften. 2002;89(8):338–51. doi: 10.1007/s00114-002-0344-9. [DOI] [PubMed] [Google Scholar]
- 40.Minguell J, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226(6):507–20. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 41.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229–30. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 43.Corselli M, Chan C, Sun B, Yap S, Rubin JP, Péault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299–308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blocki A, Wang Y, Koch M, et al. Not all MSCs can acts as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev. 2013;22(17):2347–55. doi: 10.1089/scd.2012.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 46.Traktuev D, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchyme surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 47.Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose Tissue-Derived Stromal Cells as Novel Option for Regenerative Cell Therapy. J Atheroscler Thromb. 2006;13(2):77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 48.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adhered stromal (hADAS) cells. Stem Cells. 2005;23(3):412–23. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 50.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Pierce SM. IFATS Collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of perivascular phenotype. Stem Cells. 2008;26:2682–90. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendel TA, Clabough EBD, Kao DS, et al. Pericytes Derived from Adipose-Derived Stem Cells Protect Against Retinal Vasculopathy. PLoS One. 2013;8(5):e65691. doi: 10.1371/journal.pone.0065691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129(6):1277–90. doi: 10.1097/PRS.0b013e31824ecae6. [DOI] [PubMed] [Google Scholar]
- 54.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. 2011. [Google Scholar]
- 56.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23(7):1496–508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- 57.Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92(22):1037–45. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Enge M, Bjarnegård M, Gerhardt H, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO. 2002;21(16):4307–16. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammes HP, Lin J, Renner O, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51(10):3107–12. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 60.Kotecki M, Zeiger AS, Van Vliet KJ, Herman IM. Calpain- and talin-dependent control of microvascular pericyte contractility and cellular stiffness. Microvasc Res. 2010;80(3):339–48. doi: 10.1016/j.mvr.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coen M, Gabbiani G, Bochaton-Piallat M. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31(11):2391–6. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 62.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat M, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79(9):944–56. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 65.Kis K, Liu X, Hagood JS. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13(e27) doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20(3):297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 67.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascuar fibrobalsts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173(6):1617–27. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphreys BD, Lin S, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeBleu VS, Taduri G, O’Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19(8):1047–53. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folkman J. Tumor angiogenesis; therapeutic implications. New Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 71.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160(3):985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85(8):593–8. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 73.Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of Mural Cell Recruitment to Tumor Vessels. Circulation. 2002;105(1):112–7. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- 74.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15(1):102–11. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal. 2013;11:97. doi: 10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]