Abstract
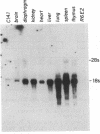
The high degree of polymorphism found among the class I genes of the murine major histocompatibility complex (H-2) has led to the postulation that specific genetic mechanisms are responsible for their diversity. These same genetic mechanisms are probably responsible for the high spontaneous mutation frequency seen in H-2 alleles. The bml mutation of the H-2Kb gene has been shown to be 7 base pair changes over a 13 base pair region that result in three amino acid substitutions in the C1 domain of the protein product. The clustering of base-pair changes has suggested that the bm1 mutation resulted from a recombinational event analogous to gene conversion between the H-2Kb gene and a "donor" gene sequence. A 23-base oligonucleotide complementary to the bm1 mutant sequences was synthesized and used to probe genomic DNA restriction digests of the parental H-2b haplotype as well as other H-2 haplotypes. Our results indicate that a potential donor gene sequence is present in the genomes of all of the five mouse strains studied. Of eight tissues that were tested by blot-hybridization analysis, the potential donor gene sequences are transcribed only in the liver. Models for the generation of polymorphism among the H-2 class I genes via subgene rearrangements are proposed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Arden B., Klein J. Biochemical comparison of major histocompatibility complex molecules from different subspecies of Mus musculus: evidence for trans-specific evolution of alleles. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2342–2346. doi: 10.1073/pnas.79.7.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brégégère F. A directional process of gene conversion is expected to yield dynamic polymorphism associated with stability of alternative alleles in class I histocompatibility antigens gene family. Biochimie. 1983 Apr-May;65(4-5):229–237. doi: 10.1016/s0300-9084(83)80274-0. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Kohn H. I., Melvold R. W. Spontaneous histocompatibility mutations detected by dermal grafts: significant changes in rate over a 10-year period in the mouse H-system. Mutat Res. 1974 Aug;24(2):163–169. doi: 10.1016/0027-5107(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Kress M., Cosman D., Khoury G., Jay G. Secretion of a transplantation-related antigen. Cell. 1983 Aug;34(1):189–196. doi: 10.1016/0092-8674(83)90149-6. [DOI] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy E., Roberts S., Evans E. P., Burtenshaw M. D., Costantini F. D. A foreign beta-globin gene in transgenic mice: integration at abnormal chromosomal positions and expression in inappropriate tissues. Cell. 1983 Sep;34(2):343–358. doi: 10.1016/0092-8674(83)90369-0. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Ramachandran K., Flavell R. A. A potential donor gene for the bm1 gene conversion event in the C57BL mouse. Nature. 1983 Dec 22;306(5945):792–795. doi: 10.1038/306792a0. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Nairn R., Yamaga K., Nathenson S. G. Biochemistry of the gene products from murine MHC mutants. Annu Rev Genet. 1980;14:241–277. doi: 10.1146/annurev.ge.14.120180.001325. [DOI] [PubMed] [Google Scholar]
- Ozato K., Evans G. A., Shykind B., Margulies D. H., Seidman J. G. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Wallace R. B. The complete amino acid sequence of the murine transplantation antigen H-2Db as deduced by molecular cloning. Immunogenetics. 1982;16(1):1–9. doi: 10.1007/BF00364437. [DOI] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Obata Y., Nathenson S. G., Reyes A. A., Ikuta S., Wallace R. B. Identification of the cloned gene for the murine transplantation antigen H-2Kb by hybridization with synthetic oligonucleotides. Mol Cell Biol. 1983 Apr;3(4):750–755. doi: 10.1128/mcb.3.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Tan Z. K., Ikuta S., Huang T., Dugaiczyk A., Itakura K. Solid-phase synthesis of polynucleotides. VIII: A simplified synthesis of oligodeoxyribonucleotides. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):383–391. doi: 10.1101/sqb.1983.047.01.045. [DOI] [PubMed] [Google Scholar]
- Tsao S. G., Brunk C. F., Pearlman R. E. Hybridization of nucleic acids directly in agarose gels. Anal Biochem. 1983 Jun;131(2):365–372. doi: 10.1016/0003-2697(83)90185-9. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C., Hämmerling G. J., Goronzy J. Recognition of H-2 domains by cytotoxic T lymphocytes. Nature. 1981 Aug 13;292(5824):627–629. doi: 10.1038/292627a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Nathenson S. G. Intramolecular organization of Class I H-2 MHC antigens; localization of the alloantigenic determinants and the beta 2 m binding site to different regions of the H-2 Kb glycoprotein. J Immunol. 1983 Mar;130(3):1419–1425. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]