Abstract
Recombinant DNA techniques have been used to quantitate the amount of nucleotide sequence divergence in the mitochondrial DNA population of individual normal humans. Mitochondrial DNA was isolated from the peripheral blood lymphocytes of five normal humans and cloned in M13 mp11; 49 kilobases of nucleotide sequence information was obtained from 248 independently isolated clones from the five normal donors. Both between- and within-individual differences were identified. Between-individual differences were identified in approximately 1/200 nucleotides. In contrast, only one within-individual difference was identified in 49 kilobases of nucleotide sequence information. This high degree of mitochondrial nucleotide sequence homogeneity in human somatic cells is in marked contrast to the rapid evolutionary divergence of human mitochondrial DNA and suggests the existence of mechanisms for the concerted preservation of mammalian mitochondrial DNA sequences in single organisms.
Full text
PDF
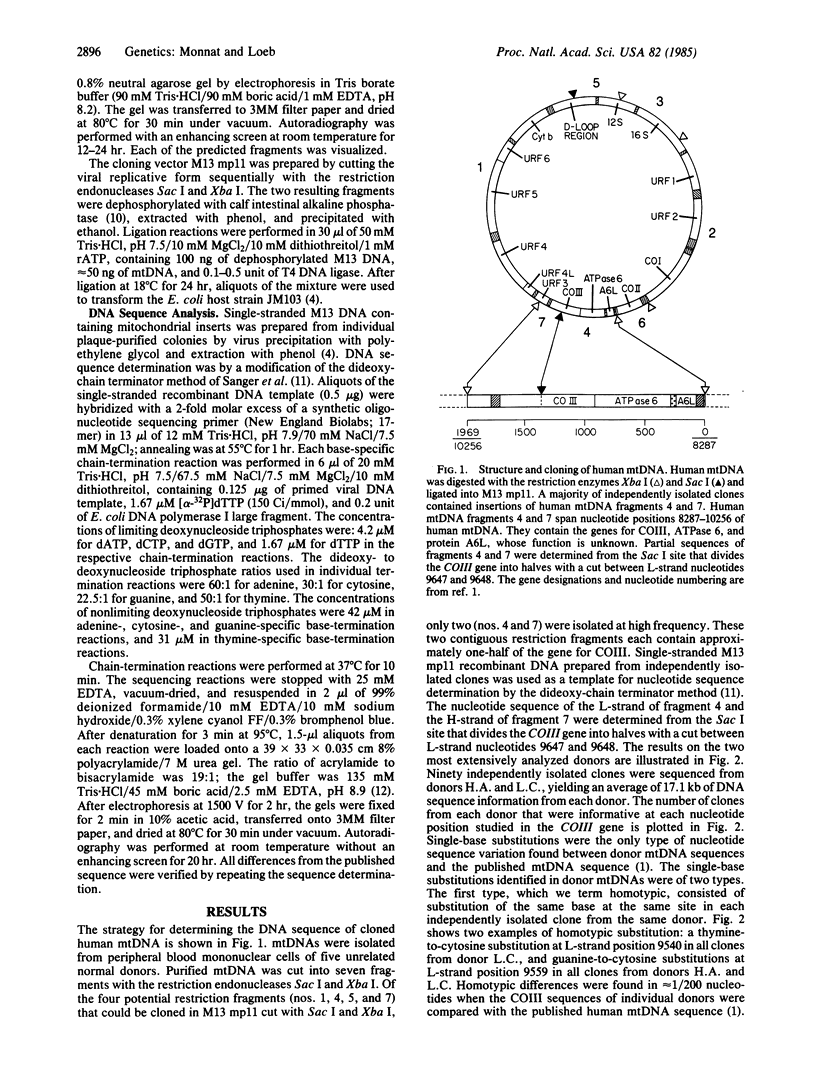
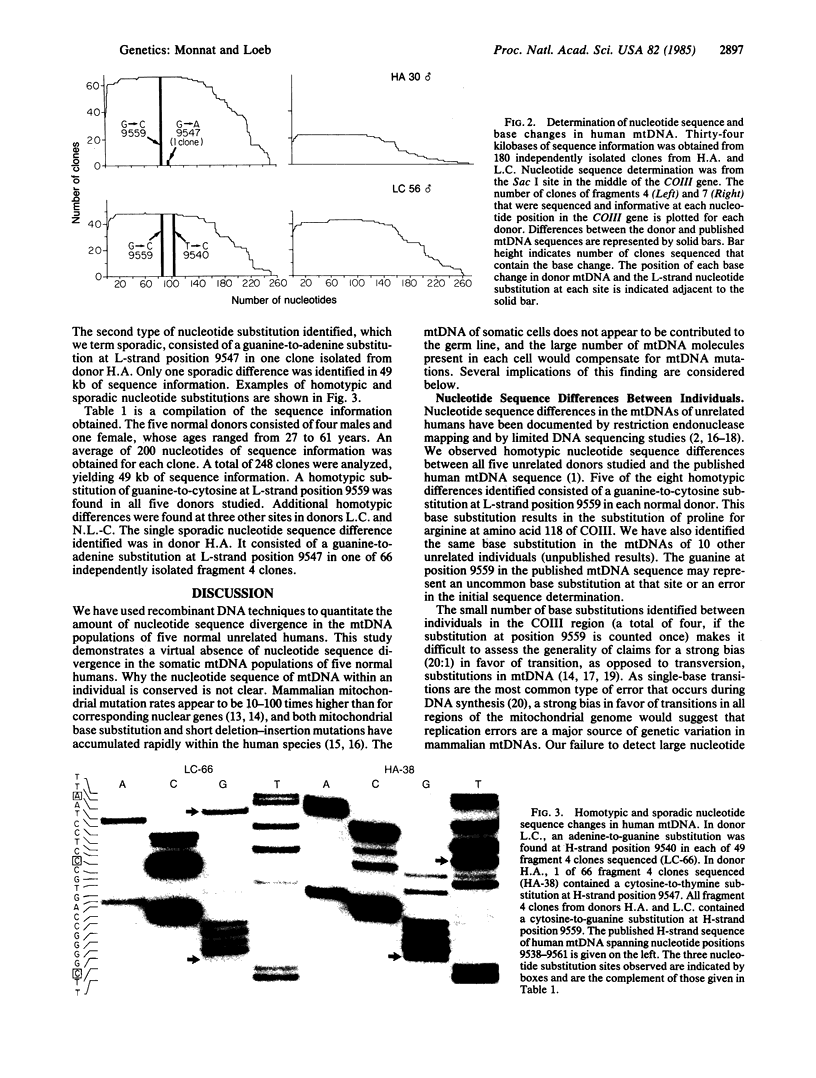
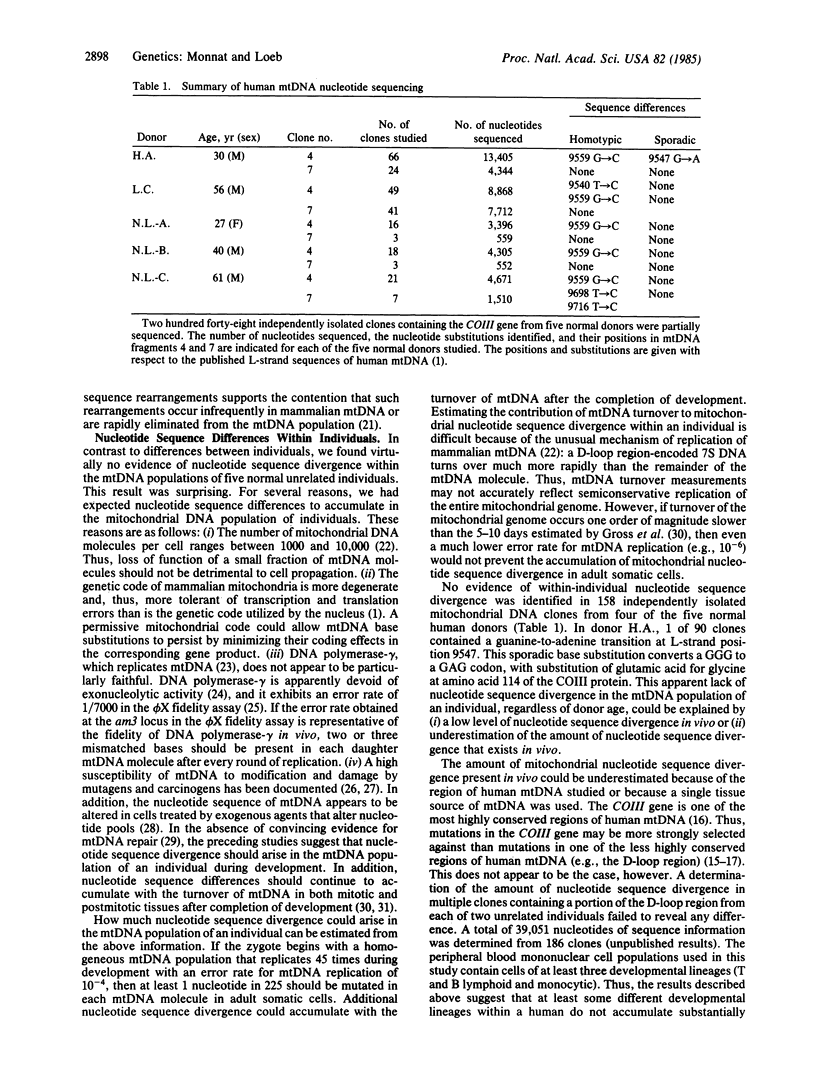

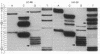
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquadro C. F., Greenberg B. D. Human mitochondrial DNA variation and evolution: analysis of nucleotide sequences from seven individuals. Genetics. 1983 Feb;103(2):287–312. doi: 10.1093/genetics/103.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Weinstein I. B. Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzo[a]pyrene. Science. 1980 Jul 11;209(4453):297–299. doi: 10.1126/science.6770466. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Bolden A., Noy G. P., Weissbach A. DNA polymerase of mitochondria is a gamma-polymerase. J Biol Chem. 1977 May 25;252(10):3351–3356. [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Mechanisms of evolution in animal mitochondrial DNA. Ann N Y Acad Sci. 1981;361:119–134. doi: 10.1111/j.1749-6632.1981.tb46515.x. [DOI] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Wilson A. C. Length mutations in human mitochondrial DNA. Genetics. 1983 Aug;104(4):699–711. doi: 10.1093/genetics/104.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Allen E. F., Forsberg S. A., Preparata R. M., Greening E. O. Genetic control of mutation rates in bacteriophageT4. Nature. 1969 Mar 22;221(5186):1128–1132. [PubMed] [Google Scholar]
- Drouin J. Cloning of human mitochondrial DNA in Escherichia coli. J Mol Biol. 1980 Jun 15;140(1):15–34. doi: 10.1016/0022-2836(80)90354-x. [DOI] [PubMed] [Google Scholar]
- Eigen M., Schuster P. The hypercycle. A principle of natural self-organization. Part A: Emergence of the hypercycle. Naturwissenschaften. 1977 Nov;64(11):541–565. doi: 10.1007/BF00450633. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B. D., Newbold J. E., Sugino A. Intraspecific nucleotide sequence variability surrounding the origin of replication in human mitochondrial DNA. Gene. 1983 Jan-Feb;21(1-2):33–49. doi: 10.1016/0378-1119(83)90145-2. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Getz G. S., Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969 Mar 25;244(6):1552–1562. [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Newbold J. E., Potter S. S., Edgell M. H. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974 Oct 11;251(5475):536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Klenow H., Henningsen I. Selective elimination of the exonuclease activity of the deoxyribonucleic acid polymerase from Escherichia coli B by limited proteolysis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):168–175. doi: 10.1073/pnas.65.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. Fidelity of mammalian DNA polymerases. Science. 1981 Aug 14;213(4509):765–767. doi: 10.1126/science.6454965. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Lu A. L., Clark S., Modrich P. Methyl-directed repair of DNA base-pair mismatches in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4639–4643. doi: 10.1073/pnas.80.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukage A., Bohn E. W., Wilson S. H. On the DNA polymerase III of mouse myeloma: partial purification and characterization. Biochemistry. 1975 Mar 11;14(5):1006–1020. doi: 10.1021/bi00676a020. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Niranjan B. G., Bhat N. K., Avadhani N. G. Preferential attack of mitochondrial DNA by aflatoxin B1 during hepatocarcinogenesis. Science. 1982 Jan 1;215(4528):73–75. doi: 10.1126/science.6797067. [DOI] [PubMed] [Google Scholar]
- Olivo P. D., Van de Walle M. J., Laipis P. J., Hauswirth W. W. Nucleotide sequence evidence for rapid genotypic shifts in the bovine mitochondrial DNA D-loop. Nature. 1983 Nov 24;306(5941):400–402. doi: 10.1038/306400a0. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M., Swift H. Mitochondrial nucleic acids and their relation to the biogenesis of mitochondria. Physiol Rev. 1970 Jul;50(3):376–427. doi: 10.1152/physrev.1970.50.3.376. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., Van Etten R. A., Clayton D. A. Isolation of mammalian mitochondrial DNA and RNA and cloning of the mitochondrial genome. Methods Enzymol. 1983;97:426–434. doi: 10.1016/0076-6879(83)97153-7. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Assignment of the chloramphenicol resistance gene to mitochondrial deoxyribonucleic acid and analysis of its expression in cultured human cells. Mol Cell Biol. 1981 Aug;1(8):697–710. doi: 10.1128/mcb.1.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]