Abstract
Objective(s): Estrogen receptor-alpha (ERα) mediates estrogen action in regulation of different levels of the hypothalamic-pituitary-testis axis. It has a key role in spermatogenesis. Estrogen receptor alpha knock-out (ER koα) male mice were infertile and severe impairment in spermatogenesis and seminiferous tubules was observed. Recently, it has been reported that microRNA (miRNA) mir-100 and let-7b were predicted to target ERα gene. MiRNA are small, endogenous, single stranded RNA molecules that regulate gene expression and have been implicated in various disease states. It has been proved that some miRNAs expression is tissue- and disease-specific, giving potential for identifying miRNAs as a diagnostic tool.
Materials and Methods: In this study, the change in the expression levels of mir-100, let-7b and ERα expression levels were evaluated in oligospermic infertile patients (n=43) compared to control fertile subjects (n=43). After washing and separating sperms, total RNA was isolated and then cDNA was synthesized. The expression levels of mir-100 and let-7b and ERα were evaluated by real time PCR.
Results: Mir-100, let-7b levels were significantly higher than those in control group (P=0.008 and P=0.009, respectively). We have found that, ERα level was significantly decreased in comparison with normal group (P< 0.0001).
Conclusion: Changes in mir-100, let-7b and ERα expression levels in oligospermic patients may be associated with the susceptibility and progression of infertility. The results of this study indicate that miRNA can have a key role in spermatogenesis and might have a diagnostic and prognostic value in men infertility.
Key Words: Fertility, Has-mir-100, Has-let-7b, MicroRNA
Introduction
Infertility is defined as a failure to bring baby after 12 or more months of consistently intercourse without contraception (1). More than 15% of couples are infertile, about 40% of which is related to male factors. Oligospermia and asthenospermia are common causes of infertility in males, however the molecular mechanisms causing these effect is not entirely clear. The causes are known in less than 35% of these cases and more than 60% related to genetic disease with unknown molecular mechanisms. In Oligospermia, the number of spermatozoa is reduced and in asthenospermia abnormality in sperm motility is seen (2). About 60-75% of infertility cases are idiopathic with or without abnormal semen analysis (3). It was seen that in bulls, spermatozoa with normal motility and morphology, the fertility of some bulls was reduced which may be due to molecular defects in the sperm cells (4).
More than hundreds of genes are involved in spermatogenesis. Molecular and cellular integrity of sperm cells is important for fertilization any deletion or mutation in the sequence of genes and inappropriate gene expression cause disorder in spermatogenesis and fertility (5, 6). Estrogen has a positive impact on the function of sperm by stimulating capacitation and fertilizing ability (7). As Also, it has a key role in modulating male reproductive tract. Cellular signaling of estrogen is mediated through the estrogen receptors (ERα) (8) that are present throughout the male reproductive tract and spermatozoa. ERα mediates estrogen action in regulation of different levels of the hypothalamic-pituitary-testis axis. ERα has an essential role in male fertility, it was proved that ER knockout (Era-/-) mice become infertile (9).
ERα gene expression is regulated by small noncoding RNAs (microRNA). Previous studies showed that mir-100 and let-7b were predicted to target ERα gene (10, 11). MicroRNAs (miRNAs) were first detected in human spermatozoa by Ostermeier et al they are abundant in spermatozoa (12) but their function in spermatogenesis and fertilization is unknown. miRNAs are small (18-25 nucleotides) noncoding regulatory RNAs which negatively regulate gene expression (13). They participate in the designation of cell fate, embryonic development, control of growth, differentiation, and the death of cells (14). There is a number of miRNA expressed in male mouse germ cells. miRNAs are involved in regulation of gene expression during mitotic, meiotic, and post-meiotic stages of spermatogenesis (15). Impaired biogenesis of miRNAs disrupts spermatogenesis and causes infertility in male mice (16). In the present study, we investigated the expression levels of mir-100, let-7b, their common target gene (ERα) and their correlation with oligospermic and normospermic control in men.
Materials and Methods
Study design
From infertile men (n=723) referred to Alzahra Infertility Center, Tabriz, Iran (age 27.5 + 4.8 years), 43 oligospermic infertile patients were selected. The written consent of the subjects was done according to medical ethics. Control samples (n=43) wereselected from normal volunteers who had a baby in the last two years and their semen analysis was normal. Two months before sampling, none of the control subjects nor patients treated with the drug and they didn’t have intercourse 3-5 days before sampling. This research was approved by the Ethics Committees of Tabriz medical University, Tabriz, Iran.
Exclusion criteria
The volunteers with infertile partner, infection in the genital tract, autoimmune disorders, reproductive tract abnormality, smoking, and alcohol and drug consumption were excluded from the study.
Isolation of spermatozoa from seminal fluid
Semen samples were collected in a sterile container and incubated at 37°C for 30 min to get the fluid. Then, semen analysis was performed according to WHO guidelines (2010). Sperms were purified by Goodrich methods (17). In brief, the samples were washed two times in 1×PBS buffer solution, then somatic cells were absent in SCLB solution ( 0.1% SDS, 0.5% TX-100 in DEPC water). The cells were counted, if somatic cells were present the process was repeated. Finally, the solution was frozen at –80°C.
RNA isolation
Total RNA was isolated using Exiqon miRCURY RNA isolation kit (Exiqon, Denmark) according to the manufacturer instructions. Quantity and quality of the isolated RNA was measured by Nanodrop 1000 (NanoDropND-1000spectrophotometer; Thermo Fisher Scientific, Waltham, MA). Total RNAs were reversed to cDNA using LNA universal RT miRNA PCR kit (Exiqon, Denmark). Briefly, 20 ng of total RNA was reverse transcribed. cDNA synthesis was performed by thermal cycler (Eppendorf, Germany) with the following parameter values; 60 min at 42°C, 5 min at 95°C and immediately cooled to 4°C until use.
Real-time PCR analysis
Quantitative real-time reverse transcriptase-PCR was carried out using the Corbett Rotor-Gene 6000 Real-Time PCR system (Qiagen, Germany). miRNAs quantification was performed using MiRCURY LNA Universal RT microRNA PCR system (Exiqon, Denmark). Mir-16 was used as the endogenous control miRNA. The relative expression level of ERα was measured by qPCR with primers (ERα: 5′-CCACATCAGTCACATGAGTAA-3′ and 5′-GTTCCATCAGCATCTACAG-3′) using SYBR Green PCR Kit (Qiagen, Germany). The expression levels were normalized to β-actin as housekeeping gene with the following primers (5′-TGGACTTCGAGCAAGAGATG-3′ and 5′-GAAGGAAGGCTGGAAGAGTG-3′). The reactions were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS software (version 18). The results were expressed as mean ± SD. Relative expression level of genes were calculated by the 2DDCq model (18). Unpaired Student's t-test was used to analyze the differences in gene expression between oligospermic and control group. Correlation analysis was performed using the Spearman rank correlation test. In all analysis, P-value < 0.05 was considered as significant.
Results
Expression level of mir-100, let-7b and ERα in oligospermic and control group
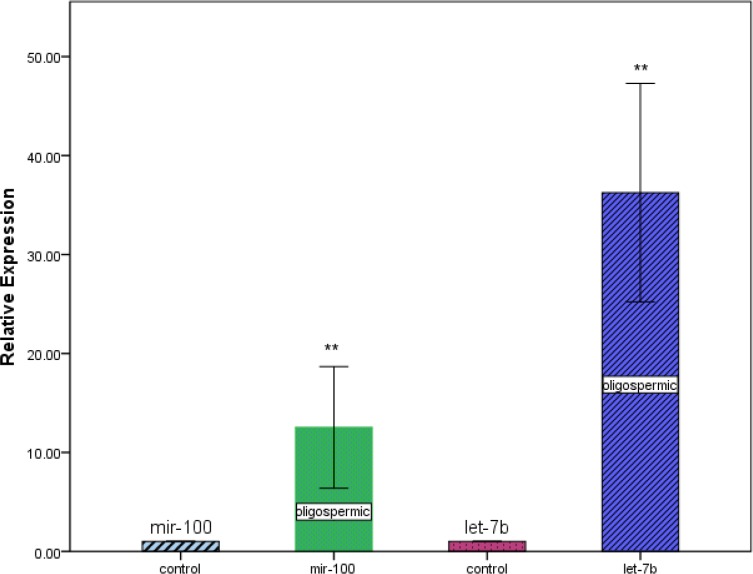
We determined the expression levels of mir-100, let-7b and ERα in oligospermic and control group. By real-time quantitative RT-PCR analysis, we found that, expression levels of mir-100 and let-7b were much higher in oligospermic than control group (P=0.008 and P=0.009, respectively, Figure 1). Inversely, expression level of ERα was significantly lower in oligospermic than control group (P<0.0001, Figure 2).
Figure 1.
Relative expression levels of mir-100 and let-7b in oligospermic and control group. **P<0.01 compared with control group
Figure 2.
Relative expression levels of the estrogen receptor alpha (ERα) gene in control and oligospermic group. ***P<0.001 compared with control group
Correlation between expression levels of ERα and seminal plasma parameters
Correlation between expression levels of ERα and semen were analyzed using Spearman׳s rank correlation test (Table 1). Expression levels of ERα were strongly and positively correlated with those of sperm count, quick progressive, slow progressive and normal morphology (Spearman׳s correlation coefficient; +0.863, +0.723, +0.875 and +0.642, respectively) and negatively correlated with immotile (Spearman׳s correlation coefficient; -0.691).
Table 1.
Correlation analysis among estrogen receptor alpha, mir-100, let-7b and seminal plasma parameters
| Variable | Mir-100 | Let-7b | ERα |
|---|---|---|---|
| Volume | 0.469a | 0.105 | -0.092 |
| 0.171b | 0.772 | 0.799 | |
| Sperm count | -0.328 | 0.297 | 0.863* |
| 0.354 | 0.403 | 0.001 | |
| Quick progressive | -0.157 | 0.375 | 0.723* |
| 0.663 | 0.285 | 0.018 | |
| Slow progressive | -0.406 | 0.093 | 0.875* |
| 0.243 | 0.696 | 0.0009 | |
| Non-progressive | -0.105 | 0.377 | 0.551 |
| 0.772 | 0.281 | 0.098 | |
| Immotile | 0.171 | -0.367 | -0.691* |
| 0.630 | 0.296 | 0.026 | |
| Normal morphology | 0 | 0.453 | 0.642* |
| 1 | 0.188 | 0.045 | |
| pH | 0.151 | 0.333 | 0.345 |
| 0.676 | 0.346 | 0.328 |
a Spearman correlation coefficient
b Spearman s rank correlation test
* P<0.05 is considered significant
Correlation between expression levels of miRNAs and seminal plasma parameters
Relationship between expression levels of miRNAs and semen parameters, such as volume, sperm count, quick progressive, slow progressive, non-progressive, immotile, normal morphology and pH was evaluated using Spearman׳s rank correlation test (Table 1). Expression levels of miRNAs were not significantly correlated with those of other semen parameters.
Discussion
Spermatogenesis is an intricate process of germ cell development in which many genes are involved. Any defect in genes expression or their regulation, disrupts spermatogenesis and causes infertility (19, 20). miRNAs regulate gene expression by modification of special mRNA translation. Few studies have been conducted on miRNAs function in spermatogenesis and male fertility (21). In a study conducted on non-obstructive azoospermic infertile patients, significant change in miRNAs expression was seen compared to fertile control men (22). In that study, Lian et al showed that miRNAs have a regulatory role in spermatogenesis. In our study, we investigated mir-100, let-7b and their common target ERα gene expression in oligospermic infertile patients and compared them with normospermic fertile control by real-time PCR methods. Our result showed that mir-100 in oligospermic was significantly over expressed compared to control group. We also demonstrated that high mir-100 expression was associated with significant decreases in ERα gene expression level in oligospermic group. It was proved that mir-100 targets ERα gene and directly sets the level of the ERα gene (10, 23). Zhao et al reported significant inverse association between expression level of let-7b and ERα in breast cancer. Similarly, in the present study, we have shown that expression level of let-7b was increased unlike ERα expression, which was decreased. Recent studies demonstrated that, let-7b has an inhibitory effect on cell proliferation (11, 24). Guarducci et al showed that ERα promoter polymorphism were inversely associated with sperm count (25). As our results indicated, inhibition of germ cell proliferation by reducing the expression of ERα gene by let-7b is possible. In the male reproductive tract, there are higher levels of ERα in the efferent ductules (region of the male tract) than female reproductive system, it occupy one third of epididymis. It shows the importance of ERα in the male reproductive system and fertility. ERα regulates fluid reabsorption in the epididymis and is responsible for maintaining fluid osmolality and pH (26). In our study, reduced ERα expression was associated with little change in semen pH. Gunawan et al showed that a polymorphism in the coding region of ERα in exon 1 was related to sperm motility (27). Recent findings are consistent with our results. We proved that expression level of ERα in oligospermic was significantly down regulated compared to control group. Also, motility and pH in seminal fluid of oligospermic was lower than those in control. Our data showed that, spermatozoa with normal morphology was decreased in oligospermic group compared to control group. Moreover, our results showed significant positive correlation between the expression of ERα and the morphology. This is consistent with recent findings of Josepha and collegues. They proved that in ERαKO mice sperm maturation and capacity to fertilize were destroyed and contributed to infertility (28). ERα plays an important role in the balancing of sperm metabolism (29) and its dysfunction causes reduction of sperm density, sperm motility, and percentage of sperm with normal cell morphology (30). ERα in the acrosome of the spermatozoa is more than in other sections, acrosome contains Lysis enzymes which puncture the outer coat of the egg and allow the infiltration of sperm (31). Acrosome dysfunction impairs oocyte fertilization and causes male infertility (32). Oligospermic patients have a high frequency of defective sperm zona pellucid interaction (33, 34) and according to our finding, decrease in ERα may cause acrosome dysfunction.
Table 2.
Comparison of sperm parameters of control and oligospermic group
| Variable | Control (n=43) | Oligospermic(n=43) | P-value |
|---|---|---|---|
| Volume ml | 3.616 ± 0.1744 | 3.035 ± 0.2344 | 0.0498 |
| Sperm count × 106sperm/ml | 75.74 ± 2.840 | 9.465 ± 0.5834 | <0.0001 |
| Quick progressive % | 15.00 ± 1.581 | 2.000 ± 2.000 | 0.0009 |
| Slow progressive % | 25.00 ± 2.739 | 12.00 ± 1.225 | 0.0025 |
| Non progressive % | 31.00 ± 3.674 | 16.00 ± 2.449 | 0.0094 |
| Immotile % | 27.00 ± 2.000 | 72.00 ± 3.391 | <0.0001 |
| Normal morphology % | 26.53 ± 0.9188 | 10.47 ± 0.5213 | <0.0001 |
| pH | 7.353 ± 0.03840 | 7.316 ± 0.07306 | 0.6607 |
| Abstinence day | 3.800 ± 0.2494 | 3.600 ± 0.3055 | 0.6182 |
*P<0.05 is considered significant
Control, sperm count≥15 × 106sperm/ml; Oligospermic, sperm count < 15× 106 sperm/ml
It is possible that miRNA interfere with spermatogenesis through other genes. Possible targets for let-7b are SRC1, PTEN, MEST, AKT1 and AKT2. Both PTEN and AKT1 are common targets for mir-100 and let-7b. SRC1 (Steroid receptor co-activator 1) is a transcriptional co-activator of many transcription factors involving nuclear receptor. It is a transcriptional partner of a co-activator of ERα. SRC is involved in signaling pathways that lead to self-renewal or differentiation of spermatogonial stem cells (35). AKT1 is a serin/theronin kinase enzyme that has been proved to be the mediator of cellular growth, proliferation, survival, and metabolism in various cell types (36). Kim et al showed that in Akt2-/- male mice, apoptotic sperms in null mice were more than wild-type mice, and sperm motility and concentration were significantly lower in the null sperm (37). PTEN (phosphatase and tensin homologue deleted on chromosome ten) plays special roles in different cellular processes, including cell transformation, survival, proliferation, migration and mediate the differentiation of germ cells (38). Defect in MEST function or processing was correlated with low sperm counts. MEST hyper methylation was seen in idiopathic infertile men with sperm morphology below 5% normal spermatozoa and progressive sperm motility below 40%. It is a biomarker of sperm quality (39, 40).
Conclusion
We have defined efficacy of mir-100, let-7b and ERα in oligospermic infertile patients. Our study obtained more information on the molecular mechanism of infertility, and their possible regulatory role in spermatogenesis and fertilization. miRNAs might have a diagnostic and prognostic value in oligospermic infertile men.
Acknowledgment
This is reported of database from thesis entitled; Expression level of ERα-regulating microRNAs and their correlation with ERα gene expression in oligospermic infertile patients .registered in Tabriz University of Medical Science. The authors would like to thank Women’s Reproductive Health Research Center, Alzahra Hospital for granting of this work and School of Advanced Medical Science for technical support. Both of these centers are affiliated to Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1.Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99 doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Ford WC. Comments on the release of the 5th edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010;12:59–63. doi: 10.1038/aja.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, Weidner W. EAU guidelines on male infertility. Eur Urol. 2005;48:703–711. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Dejarnette JM. The effect of semen quality on reproductive efficiency. Vet Clin North Am Food Anim Pract. 2005;21:409–418. doi: 10.1016/j.cvfa.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Slezak R, Sasiadek M. [Chromosome Y microdeletions in the pathogenesis of male infertility] Pol Merkur Lekarski. 2002;13:229–233. [PubMed] [Google Scholar]
- 6.Guo T, Qin Y, Gao X, Chen H, Li G, Ma J, et al. The role of male chromosomal polymorphism played in spermatogenesis and the outcome of IVF/ICSI-ET treatment. Int J Androl. 2012;35:802–809. doi: 10.1111/j.1365-2605.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 7.Carreau S, Silandre D, Bois C, Bouraima H, Galeraud-Denis I, Delalande C. Estrogens: a new player in spermatogenesis. Folia histochemica et cytobiologica. 2007;45:5–10. [PubMed] [Google Scholar]
- 8.Ellmann S, Sticht H, Thiel F, Beckmann MW, Strick R, Strissel PL. Estrogen and progesterone receptors: from molecular structures to clinical targets. Cell Mol Life Sci. 2009;66:2405–2426. doi: 10.1007/s00018-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreau S, Genissel C, Bilinska B, Levallet J. Sources of oestrogen in the testis and reproductive tract of the male. International journal of andrology. 1999;22:211–223. doi: 10.1046/j.1365-2605.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Song T, Gong S, Zhong M, Su G. microRNA regulation of the expression of the estrogen receptor in endometrial cancer. Mol Med Rep. 2010;3:387–392. doi: 10.3892/mmr_00000269. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Deng C, Lu W, Xiao J, Ma D, Guo M, et al. let-7 microRNAs induce tamoxifen sensitivity by downregulation of estrogen receptor alpha signaling in breast cancer. Mol Med. 2011;17:1233–1241. doi: 10.2119/molmed.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostermeier GC, Goodrich RJ, Moldenhauer JS, Diamond MP, Krawetz SA. A suite of novel human spermatozoal RNAs. J Androl. 2005;26:70–74. [PubMed] [Google Scholar]
- 13.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development . 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 15.Ro S, Park C, Sanders KM, McCarrey JR, Yan W. Cloning and expression profiling of testis-expressed microRNAs. Dev Biol. 2007;311:592–602. doi: 10.1016/j.ydbio.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huszar JM, Payne CJ. MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biol Rep. 2013;88 doi: 10.1095/biolreprod.112.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodrich R, Johnson G, Krawetz SA. The preparation of human spermatozoal RNA for clinical analysis. Arch Androl. 2007;53:161–167. doi: 10.1080/01485010701216526. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan W. Male infertility caused by spermiogenic defects: lessons from gene knockouts. Mol Cell Endocrinol. 2009;306:24–32. doi: 10.1016/j.mce.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidari MM, Khatami M, Talebi AR. The POLG Gene polymorphism in Iranian varicocele-associated infertility patients. Iran J Basic Med Sci. 2012;15:739–744. [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav RP, Kotaja N. Small RNAs in spermatogenesis. Mol Cell Endocrinol. 2013:28. doi: 10.1016/j.mce.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Lian J, Zhang X, Tian H, Liang N, Wang Y, Liang C, et al. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod Biol Endocrinol. 2009;7:13. doi: 10.1186/1477-7827-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Shen K, Zeng JF, Yang JX, Cao DY, Cui QC. [Differential expression of microRNAs associated with estrogen receptor alpha and progesterone receptor in typeIand typeII endometrial adenocarcinomas. Zhonghua Fu Chan ke Za Zhi. 2009;44:765–770. [PubMed] [Google Scholar]
- 24.Xu D, Tan J, Zhou M, Jiang B, Xie H, Nie X, et al. Let-7b and microRNA-199a inhibit the proliferation of B16F10 melanoma cells. Oncol Lett. 2012;4:941–946. doi: 10.3892/ol.2012.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guarducci E, Nuti F, Becherini L, Rotondi M, Balercia G, Forti G, et al. Estrogen receptor alpha promoter polymorphism: stronger estrogen action is coupled with lower sperm count. Hum Rep. 2006;21:994–1001. doi: 10.1093/humrep/dei439. [DOI] [PubMed] [Google Scholar]
- 26.Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS. Estrogen and its receptors in efferent ductules and epididymis. J Androl. 2011;32:600–613. doi: 10.2164/jandrol.110.012872. [DOI] [PubMed] [Google Scholar]
- 27.Gunawan A, Kaewmala K, Uddin MJ, Cinar MU, Tesfaye D, Phatsara C, et al. Association study and expression analysis of porcine ESR1 as a candidate gene for boar fertility and sperm quality. Anim Reprod Sci. 2011;128:11–21. doi: 10.1016/j.anireprosci.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol Reprod. 2010;82:958–967. doi: 10.1095/biolreprod.109.080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guido C, Perrotta I, Panza S, Middea E, Avena P, Santoro M, et al. Human sperm physiology: estrogen receptor alpha (ERalpha) and estrogen receptor beta (ERbeta) influence sperm metabolism and may be involved in the pathophysiology of varicocele-associated male infertility. J Cell Physiol. 2011;226:3403–3412. doi: 10.1002/jcp.22703. [DOI] [PubMed] [Google Scholar]
- 30.Safarinejad MR, Shafiei N, Safarinejad S. Association of polymorphisms in the estrogen receptors alpha, and beta (ESR1, ESR2) with the occurrence of male infertility and semen parameters. J Steroid Biochem Mol Biol. 2010;122:193–203. doi: 10.1016/j.jsbmb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Vigil P, Toro A, Godoy A. Physiological action of oestradiol on the acrosome reaction in human spermatozoa. Andrologia. 2008;40:146–151. doi: 10.1111/j.1439-0272.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 32.Hartmann M. Acrosome reaction and fertility. Int J Androl. 1995;18:53–55. [PubMed] [Google Scholar]
- 33.Liu DY, Baker HW. High frequency of defective sperm-zona pellucida interaction in oligozoospermic infertile men. Hum Reprod. 2004;19:228–233. doi: 10.1093/humrep/deh067. [DOI] [PubMed] [Google Scholar]
- 34.Liu de Y, Liu ML, Garrett C, Baker HW. Comparison of the frequency of defective sperm-zona pellucida (ZP) binding and the ZP-induced acrosome reaction between subfertile men with normal and abnormal semen. Hum Reprod. 2007;22:1878–1884. doi: 10.1093/humrep/dem087. [DOI] [PubMed] [Google Scholar]
- 35.Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stabile V, Russo M, Chieffi P. 17beta-estradiol induces Akt-1 through estrogen receptor-beta in the frog (Rana esculenta) male germ cells. Reproduction. 2006;132:477–484. doi: 10.1530/rep.1.01107. [DOI] [PubMed] [Google Scholar]
- 37.Kim ST, Omurtag K, Moley KH. Decreased spermatogenesis, fertility, and altered Slc2A expression in Akt1-/- and Akt2-/- testes and sperm. Reprod Sci. 2012;19:31–42. doi: 10.1177/1933719111424449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIver SC, Stanger SJ, Santarelli DM, Roman SD, Nixon B, McLaughlin EA. A unique combination of male germ cell miRNAs coordinates gonocyte differentiation. PloS one. 2012;7:e35553. doi: 10.1371/journal.pone.0035553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poplinski A, Tuttelmann F, Kanber D, Horsthemke B, Gromoll J. Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int J Androl. 2010;33:642–649. doi: 10.1111/j.1365-2605.2009.01000.x. [DOI] [PubMed] [Google Scholar]
- 40.Kerjean A, Dupont JM, Vasseur C, Le Tessier D, Cuisset L, Paldi A, et al. Establishment of the paternal methylation imprint of the human H19 and MEST/PEG1 genes during spermatogenesis. Hum Mol Genet. 2000;9:2183–2187. doi: 10.1093/hmg/9.14.2183. [DOI] [PubMed] [Google Scholar]