Abstract
Objective(s): We aimed to characterize the phenotype and genotype of Bacillus spp isolated from diabetic patients’ eyes, by studying the drug sensitivity patterns with a disc-diffusion method.
Materials and Methods: Fifty eyes of 25 patients with type II diabetes mellitus, with at least 10 years of diabetes history, were included in the study. We analyzed the eyes for the presence of Bacillus spp.; presumptive isolates were identified by morphological, and biochemical tests, and confirmed by the VITEK system. Automated EcoRI ribotyping was performed with a RiboPrinter® Microbial Characterization System. We determined the antibiotic resistance of the isolates by the Kirby–Bauer disc diffusion test.
Results: Seven out of 25 patients were on insulin treatment; 7 on oral anti-diabetic medication; and 11 on combination therapy of insulin and oral medications. Among the 28 Bacillus spp isolates, 14 were B. cereus, 11 were B. pumilus, 2 were B. mojavensis and 1 was B. subtilis. Almost all the strains were either resistant or multiresistant, particularly towards cefuroxime, methicillin, and ceftazidime.
Conclusion: Diabetic patients seem to be more prone to B. cereus infections than healthy individuals. It would be greatly beneficial to understand and recognize the prevalence of microorganisms and their resistance patterns for better outcome in ocular surgeries.
Key Words: Antibiotic resistance, Automated ribotyping, Bacillus spp., Diabetic eyes
Introduction
Bacillus spp and coagulase-negative staphylococci are the most common causes of Gram-positive endogenous endophthalmitis (1). Bacteria of the genus Bacillus are ubiquitous in the natural environment. In the world of clinical microbiology, Bacillus spp have potentially important roles as “contaminants” as well as pathogens (2). The genus Bacillus comprises a very large and diverse group, the members of which are either aerobes or facultative anaerobes. Bacillus spp are Gram-positive rods that are capable of forming endospores; they usually produce catalase and tolerate extremes of temperature and moisture (3). Their phylogenetic taxonomy places them in the phylum Firmicutes, class Bacilli, order Bacillales, and family Bacillaceae. In the Bacillaceae family, no other related spp are as important as the Bacillus spp The order Bacillales includes most of the familiar Gram-positive human pathogens. Bacillus cereus is one of the most common causes of post-traumatic and post-operative endophthalmitis, particularly in the presence of retained intraocular foreign bodies (4). Bacillus spp is a major cause of rapid blinding in post-traumatic and endogenous endophthalmitis cases. The majority of patients with Bacillus endophthalmitis lose significant visual function or the eye itself in less than few days (5-9). B. pumilus, B. licheniformis, and B. subtilis are among the species comprising the B. subtilis group of aerobic spore-forming organisms, all of which are very similar (10). Despite intensive medical and surgical intervention, patients typically retain poor vision (1).
Compromised immunity is an important factor in the development of endogenous endophthalmitis. In a review, 56% of patients with endogenous bacterial endophthalmitis were also immunocompromised, and diabetes was the most common underlying disease involved (1). The increased risk of infection in diabetics has been well documented; however, no correlation has been shown between diabetes and post-operative or post-traumatic endophthalmitis. Links between underlying ocular diseases associated with diabetes (e.g., diabetic retinopathy) have not been established. B. cereus is by far the most common cause of Bacillus endophthalmitis. However, endophthalmitis can also becaused by B. thuringiensis, a bacterium that is commonly used for organic gardening and farming.
Bacillus thuringiensis is genetically and phenotypically similar to B. cereus (11, 12). For B. cereus and B. thuringiensis, the quorum-sensing transcriptional regulator—plcR—controls the expression of many extracellular virulence factors (13). Wild-type Bacillus causes severe intraocular inflammation in 12 hr. The ability of B. cereus toxins to induce the type of damage seen in endophthalmitis was shown in a mouse model of sterile endophthalmitis, in which bacterial supernatants from wild type and plcR-deficient B. cereus were examined. Supernatant from the wild-type B. cereus caused rapid loss of retinal function and more severe inflammation than did the supernatant from plcR-deficient B. cereus (14). In terms of individual toxins, those tested to date (hemolysin BL, phosphatidylinositol-specific phospholipase C, and phosphatidylcholine-specific phospholipase C) contributed little to the overall pathogenesis of experimental B. cereus endophthal -mitis (15, 16). Taken together, these data highlight the importance of quorum-sensing to the pathogenicity of Bacillus endophthalmitis (6-9).
B. pumilus is highly resistant to extreme environmental conditions such as low or no nutrient availability, desiccation, irradiation, H2O2, and chemical disinfections (17). B. cereus, B. licheniformis, and B. pumilus may be more pathogenic in immunosuppressed hosts than other common Bacillus species (B. subtilis or B. megaterium) (18). However, B. pumilus has been rarely reported as a human pathogen (19). B. pumilus has toxic properties; it has cytopathic effects in Vero cells, hemolytic activity, the capacity for lecithinase production, and proteolytic action on casein (20). Little information has been published about B. mojavensis (21).
The present study has focused on the phenotype and genotype characterization of Bacillus spp obtained from the healthy conjunctiva of eyes of diabetic patients on the basis of their drug-sensitivity patterns assessed by the disc diffusion method.
Materials and Methods
Subject
A total of 25 patients, (15 women [60%] and 10 men [40%]; mean age: 59.54 ± 6.72 years) with Type II diabetes mellitus (n = 50 eyes) , with at least 10 years of diabetes history, and who had visited our ophthalmology department for routine diabetic control, without ocular infection or ocular allergic symptoms, were included in this study. Microbiologic sampling from conjunctival fornices was performed twice for both eyes with sterile cotton swabs, without topical anesthetic drops. Using sterile Stuart’s swabs, we obtained swabbed samples from the conjunctiva of each patient, which were then placed in Stuart’s transport medium and transferred to the microbiology laboratory. Informed consent was obtained from all the patients prior to conjunctival sampling.
Isolation of Bacillus spp
The conjunctival swabs were streaked on culture media. The following culture media: blood agar (5% sheep) and blood and mannitol egg-yolk-polymyxin agar (MYP-agar) were used. The culture media were incubated at 37°C to permit bacterial growth; the cultures were retained for 3 days to ascertain bacterial growth.
Bacterial identification
Colonies representing the most number of bacteria in each sample were subcultured in MYP-agar and blood agar by streaking on the same fresh medium and incubating at 37oC for 24–48 hr. The isolates were screened by colonial morphology, Gram staining, and spore formation. Further, we investigated the following parameters: coagulase activity, hemolysis, oxidation/fermentation of glucose, motility, catalase, oxidase, coagulase, growth in anaerobic medium, starch hydrolysis, casein hydrolysis, and gelatin hydrolysis (22). In addition, Voges–Proskauer and indol tests were done and nitrate reduction, starch hydrolysis, and mannitol utilization were also evaluated (23). The strains were further identified using the VITEK system (bioMerieux), according to the manufacturer instructions. The VITEK identification system is also a carbon-source utilization test. The reliability of these systems depends upon the number and diversity of bacteria in the databases.
Automated EcoRI ribotyping was performed with a RiboPrinter® Microbial Characterization System (Dupont Qualicon). The standard EcoRI DNA preparation kit was used based on the manufacturer protocol. Pure culture samples were obtained from agar plates incubated for 24–48 hr at 30°C. The microbial samples were subsequently analyzed according to the manufacturer instructions. The ribotype profiles of the isolates were compared with the reference DuPont identification database DUP2003. The identification of each isolate was done when the corresponding pattern matched one of the patterns of the DuPont Identification Library with a similarity of ≥0.85. The isolates were automatically grouped in ribogroups by the RiboPrinter® on the basis of the similarity of the respective ribotype patterns.
The generated Riboprinter® patterns were analyzed with the Finger Printing II software (DuPont Qualicon ,USA), and a dendrogram was generated by using the Unweighted Pair Group Method using arithmetic Averages (UPGMA) and Pearson correlation coefficients (optimization, 1.56%).
All strains were stocked in 10% glycerol and stored at -80°C. Working cultures were stored at 5°C and periodically transferred.
Antibiotic sensitivity testing
The antimicrobial resistance patterns of isolates were determined using the agar disc-diffusion method. Bacteria were suspended in sterile 0.85% saline to a turbidity matching that of a McFarland No. 2 standard (bioMe´rieux, Marcy l’Etoile, France), diluted 1:20, and streaked on Mueller–Hinton agar. Discs containing the following antibacterial agents were used: gatifloxacin (5 µg), cefuroxime (30 µg), ceftazidime (30 µg), vancomycin (30 µg), gentamicin (10 µg), amikacin (30 µg), ciprofloxacin (5 µg), lomefloxacin (10 µg), moxifloxacin (5 µg), and methicillin (10 U). Plates were incubated at 37°C for 24–48 hr depending on the organisms contained therein. The strains were characterized as sensitive, intermediate, or resistant based on the size of the inhibition zones around each disc, according to the National Committee for Clinical Laboratory Standards (CLSI) criteria (24).
Results
Among 25 patients,15 women (60%) and 10 men (40%), (mean age: 59.54 ± 6.72 years), 7 were on insulin treatment, 7 were using oral anti-diabetics, and 11 were using a combination of insulin and oral anti-diabetics. The mean HbA1c level was 8.3 ± 1.61. In addition to diabetes mellitus, 17 patients had hypertension, 12 had hypercholesterolemia, 2 had coronary artery disease, and 1 had breast cancer.
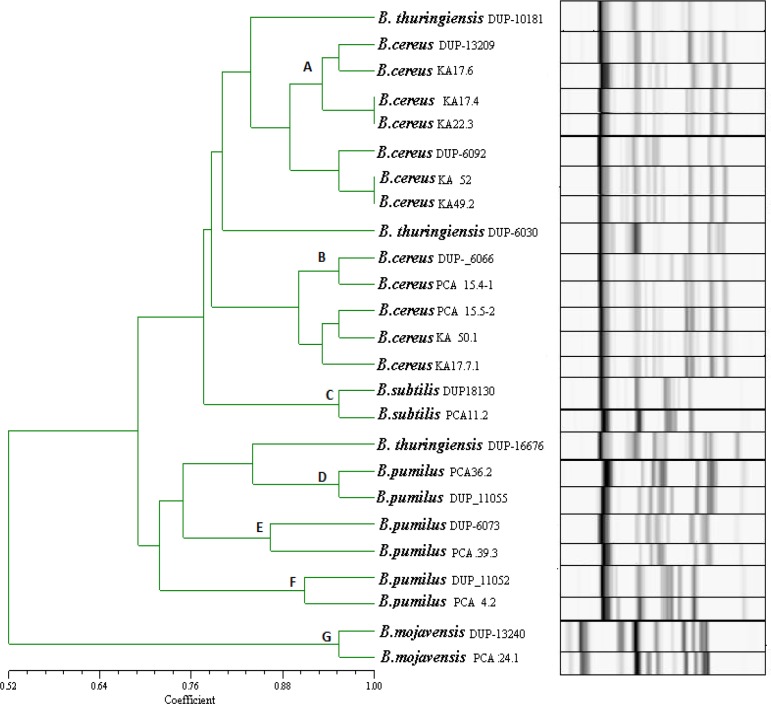
No growth was observed in 14 of 25 patients (56%). Bacillus spp were isolated from 15 of 50 eyes (isolates obtained from one eye in 7 patients, and both eyes in 4 patients); 5 of these isolates were isolated from men and 10 from women. In the present study, 28 isolates were recovered from diabetic patients and identified using phenotypic and genotypic tests. The Bacillus spp isolates were initially identified based on their colonial morphology on plates, cellular appearance as viewed by light microscopy, and production of ovoid terminal or subterminal spores. On the basis of the results of the carbohydrate fermentation reactions and physiological and morphological tests, these samples were identified as B. cereus, B. pumilus, B. mojevensis, and B. subtilis. The predominant microbial flora was B. cereus followed by B. pumilus. The results obtained by EcoRI ribotyping confirmed the presumptive classification of the isolates within the species as the Bacillus spp Based on the preset identification similarity threshold of 0.86; all the strains were automatically identified using the RiboPrinter®. B. cereus, B. pumilus, B. mojevensis, and B. subtilis were detected using the RiboPrinter® system. EcoRI ribotyping differentiated the isolates into 8 distinct ribotypes (Table 1). The similarity among these 8 ribogroups ranged from 0.78 to 0.97. The ribogroups belonged to 7 different DUP-IDs. These data show the high similarity inherent to the Bacillus strains isolated from the conjunctivas of patients with diabetes mellitus. B. cereus was the most common bacteria isolated, accounting for 14 out of the 28 isolates (50%). The other Bacillus spp isolated, included B. pumilus (11 isolates), B. subtilis (1 isolate), and B. mojevensis (2 isolates). The RiboPrinter® results are shown in Figure 1. The 7 DUP-IDs were allocated to the evolutionary lineages known for Bacillus spp In particular, DUP-IDs 13209 and DUP-IDs 6092 were classified as Lineage I; DUP-IDs 6066, as Lineage II; and DUPIDs 18130, as Lineage III; DUP-IDs 11055, as Lineage IV; DUP-IDs 6073, as Lineage V; DUP-IDs 11052, as Lineage VI; and DUP-IDs 13240, as Lineage VII (Table 1). No atypical profile or profiles belonging to all lineages were found. The dendrogram patterns generated from ribotype data produced 7 clusters (Figure 1). The threshold regarding the measure of similarity was fixed at 0.85%. Cluster A consisted of 5 isolates; Cluster B, 9 isolates; Cluster C, 1 isolate; Cluster D, 4 isolates; Cluster E, 5 isolates; and Clusters F and G consisted of 2 isolates each from diabetes patients. The results of the cluster analysis allowed us to confirm the existence of a widespread population of Bacillus spp characterized by the diffusion of highly similar strains in diabetes patients.
Table 1.
EcoRI ribotyping profiles of the Bacillus spp isolated from conjunctiva of diabetic patients
| No | Sample no | Dupont ID label | Ribogroup | DUP number | Similarity |
|---|---|---|---|---|---|
| I | KA 17.7-1 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.85 |
| PCA 15.4-1 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.84 | |
| PCA 15.3 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.87 | |
| PCA 17.2 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.85 | |
| PCA 15.5-2 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.85 | |
| KA17.11 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.87 | |
| PCA 17.10 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.85 | |
| KA17.8 | B. cereus | EcoRI 425-114-S-4 | DUP-6066 | 0.87 | |
| II | KA 50.1 | B. cereus | EcoRI 425-126-S-5 | DUP-6066 | 0.83 |
| III | KA 22.3 | B. cereus | EcoRI 425-114-S-4 | DUP-13209 | 0.86 |
| KA 17.4 | B. cereus | EcoRI 425-114-S-4 | DUP-13209 | 0.84 | |
| IV | KA 17.6-1 | B. cereus | EcoRI 425-126-S-8 | DUP-13209 | 0.84 |
| KA 52 | B. cereus | EcoRI 425-126-S-8 | DUP-6092 | 0.87 | |
| V | KA 49.2 | B. cereus | EcoRI 425-114-S-8 | DUP-6092 | 0.85 |
| VI | PCA 36.1 | B. pumilus | EcoRI 425-122-S-2 | DUP-6073 | 0.90 |
| KA 17.5.1 | B. pumilus | EcoRI 425-122-S-2 | DUP-6073 | 0.93 | |
| KA 17.5.2 | B. pumilus | EcoRI 425-122-S-2 | DUP-6073 | 0.92 | |
| PCA 39.3 | B. pumilus | EcoRI 425-122-S-2 | DUP-6073 | 0.84 | |
| KA 35.1 | B. pumilus | EcoI 425-122-S-2 | DUP-6073 | 0.92 | |
| VII | PCA 4.2 | B. pumilus | EcoRI 425-129-S-4 | DUP-11052 | 0.93 |
| PCA 9.4 | B. pumilus | EcoRI 425-129-S-4 | DUP-11052 | 0.92 | |
| VIII | PCA 36.2 | B. pumilus | EcoRI 425-122-S-2 | DUP-11055 | 0.87 |
| PCA 35.1 | B. pumilus | EcoRI 425-122-S-2 | DUP-11055 | 0.78 | |
| PCA 31 | B. pumilus | EcoRI 425-122-S-2 | DUP-11055 | 0.81 | |
| KA 40 | B. pumilus | EcoRI 425-122-S-2 | DUP-11055 | 0.86 | |
| IX | PCA 24.1 | B. mojevensis | EcoRI 425-114-S-2 | DUP-13240 | 0.86 |
| PCA 34.1 | B. mojevensis | EcoRI 425-114-S-2 | DUP-13240 | 0.86 | |
| X | PCA 11.2 | B. subtilis | EcoRI 425-129-S-1 | DUP-18130 | 0.94 |
Figure 1.
Cluster analysis of the Bacillus spp isolates from conjunctiva. Dendrogram based on UPGMA cluster analysis
The ribotyping of 28 strains and other reference strains showed that Bacillus species can be easily distinguished using this genotype characterization method. The ribotyping of 15 strains and calculation of the similarity values between the isolates and the reference strains allowed us to identify of all strains, which yielded a fingerprint identical to that of B. cereus reference strains 6066, 13209, and 6092. The ribotyping of 11 strains yielded a fingerprint identical to that of B. pumilus reference strains 6073, 11052, and 11055. The similarity of the fingerprint patterns allowed the grouping of the isolates with reference strains and their identification on this basis.
PCA 24.1 and PCA 34.1 exhibited similar patterns to those provided by the B. mojevensis database (DUP-13240). Similarly, 11.2 showed patterns similar to those of the database for B. subtilis (DUP-18130). These findings show the high similarity among B. pumilus strains isolated from diabetic patients’ eyes.
Among the 28 isolates, 8 distinct EcoRI ribogroups were identified, and various resistance profiles were obtained. Table 1 summarizes the ribogroups found in this study. The antimicrobial susceptibility of the isolates was investigated using a panel of 10 different drugs, and the rates of resistant strains were as follows: methicillin 89.28%, gentamicin 3.57%, cefuroxime 71.43%, ceftazidime 71.43%, ciprofloxacin 3.57%, lomefloxacin 7.14%, and moxifloxacin 7.14%. No strain was resistant to amikacin, vancomycin, and gatifloxacin (Table 2). Among the ribogroups, the first group consisted of 14 isolates. The B. cereus KA 17.6-1 isolates belonging to the EcoRI-126-S-8 ribogroup were interestingly and strikingly related by their antibiotic resistance patterns, all being resistant to gentamicin, ciprofloxacin, lomefloxacin, and moxifloxacin.
Table 2.
Susceptibility prevalence for Bacillus spp isolates (mm)
| Strain | Ribogrup | Cefuroxime | Methicillin | Ceftazidime | Ciprofloxacin | Gentamicin | Amikacin | Vancomycin | Gatifloxacin | Lomefloxacin | Moxifloxacin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. cereus KA 17.4 | EcoRI-114-S-4 | R | R | R | 30 | 26 | 23 | 20 | 29 | 29 | 31 |
| B.cereus KA 17.8 | EcoRI-114-S-4 | R | R | R | 22 | 28 | 23 | 17 | 29 | 27 | 27 |
| B. cereus PCA 17.2 | EcoRI-114-S-4 | 11 | R | 13 | 27 | 26 | 23 | 19 | 28 | 33 | 32 |
| B.cereus KA 17.11 | EcoRI-114-S-4 | 7 | R | 13 | 40 | 36 | 25 | 20 | 30 | 33 | 34 |
| B. cereus PCA 15.3 | EcoRI-114-S-4 | 13 | R | 11 | 37 | 22 | 24 | 21 | 33 | 33 | 31 |
| B. cereus KA 17.10 | EcoRI-114-S-4 | 13 | R | 13 | 33 | 34 | 32 | 22 | 39 | 32 | 37 |
| B. cereus PCA 15.5-2 | EcoRI-114-S-4 | R | R | 10 | 36 | 29 | 41 | 24 | 37 | 24 | 43 |
| B. cereus KA17.7-1 | EcoRI-114-S-4 | R | R | 12 | 35 | 22 | 24 | 20 | 30 | 32 | 32 |
| B. cereus KA 49.2 | EcoRI-114-S-8 | R | R | 10 | 17 | 32 | 41 | 20 | 25 | 30 | 17 |
| B. cereus KA 22.3 | EcoRI-114-S-4 | 11 | 12 | R | 36 | 35 | 33 | 24 | 38 | 35 | 37 |
| B.cereus PCA 15.4-1 | EcoRI-114-S-4 | 12 | R | R | 50 | 25 | 41 | 21 | 28 | 27 | 40 |
| B. cereus PCA 7.2 | EcoRI-114-S-4 | 11 | R | R | 30 | 22 | 20 | 19 | 37 | 40 | 32 |
| B.cereus KA 52 H(+) | EcoRI-114-S-8 | R | 10 | 22 | 34 | 16 | 22 | 20 | 36 | 15 | 28 |
| B. cereus KA 17.6-1 | EcoRI-126-S-8 | 39 | 15 | 31 | R | R | 28 | 48 | 51 | R | R |
| B. pumilus PCA 35.1 | EcoRI-122-S-2 | 12 | 9 | R | 30 | 31 | 29 | 21 | 40 | 33 | 43 |
| B.pumilus KA 40 | EcoRI-122-S-2 | 13 | 21 | 28 | 34 | 21 | 39 | 42 | 43 | 30 | 45 |
| B. pumilus PCA 31 | EcoRI-122-S-2 | 15 | 13 | R | 30 | 25 | 22 | 16 | 35 | 27 | 43 |
| B. pumilus PCA 36.1 | EcoRI-122-S-2 | 42 | 14 | 24 | 32 | 58 | 34 | 49 | 38 | 36 | 40 |
| B. pumilus PCA 36.2 | EcoRI-122-S-2 | 40 | 15 | 25 | 32 | 56 | 35 | 48 | 38 | 37 | 39 |
| B. pumilus KA 17.5 -2 | EcoRI-122-S-2 | R | R | R | 29 | 22 | 32 | 23 | 35 | 30 | 33 |
| B. pumilus KA 17.5 -1 | EcoRI-122-S-2 | R | 10 | R | 29 | 23 | 33 | 23 | 36 | 31 | 32 |
| B. pumilus PCA 39.3 | EcoRI-122-S-2 | 12 | R | R | 34 | 22 | 22 | 21 | 34 | 32 | 33 |
| B. pumilus PCA 4.2 | EcoRI-129-S-4 | 25 | R | R | 27 | 25 | 31 | 36 | 18 | 37 | 40 |
| B. pumilus PCA 9.4 | EcoRI-129-S-4 | 14 | R | R | 36 | 20 | 26 | 27 | 40 | 33 | 38 |
| B.mojevensis PCA 34.1 | EcoRI-114-S-2 | 30 | 15 | 15 | 25 | 39 | 55 | 31 | 35 | R | 37 |
| B.mojevensis PCA 24.1 | EcoRI-114-S-2 | R | R | 10 | 31 | 50 | 26 | 19 | 31 | 40 | 38 |
| B. subtilis PCA 11.2- 1 | EcoRI-129-S-1 | 36 | 19 | 28 | 35 | 24 | 24 | 22 | 36 | 30 | R |
B. cereus KA17.6.1 strains belonging to the EcoRI-126-S-8 ribogroups showed different antibiotic resistance patterns as compared to the other B. cereus strains. This strain was found to be resistant towards ciprofloxacin, gentamicin, lomefloxacin, and moxifloxacin. All of the B. pumilus ribogroups were resistant to cefuroxime, methicillin, and ceftazidime, while B. subtilis was resistant to moxifloxacin, and B. mojevensis PCA 34.1 was resistant to lomefloxacin.
Over 85.71% of the isolates were multiresistant to at least 2 drugs. Of these, 14.29% isolates were resistant to 2, 67.86% to 3, and 3.57% to 5 antibiotics. Only 1 strain of the 28 was susceptible to all the tested antibiotics.
Discussion
With increasing numbers of diabetic patients worldwide, there has been a significant rise in the prevalence of diabetes-related eye problems. Few reports have examined the specific prevalence of Bacillus spp in this context. In contrast to previously published data, our study has shown the incidence of Bacillus spp in a significant number (30%) of diabetic eyes. B. cereus, B. pumilus, B. mojevensis, and B. subtilis were isolated. The predominant microbial flora was B. cereus followed by B. pumilus. Tekeli et al studied the flora of conjunctivas from diabetic patients but did not provide any information on Bacillus (25). Bilen et al isolated Bacillus spp from 8/132 (6.06%) diabetic patients and 1/50 (2.0%) non-diabetic patients (26). Coşkun et al. isolated B. subtilis (3.6%) from normal conjunctival flora (27). We isolated B. subtilis (3.57%) at similar frequencies in our study. Among the 28 isolates, 2 were identified to have B. mojevensis. This bacterium is not a pathogen and exhibits antimicrobial activity (28). The eyelids and conjunctival flora protect against pathogenic microbial colonization (29). Normal bacteriologic flora inhibits the growth and invasion of pathogenic bacteria by restricting their nutrition, limiting the space available for growth and secreting enzymes and antimicrobial substrates.
Notably, ribotyping with the RiboPrinter® is an automated process that requires little preparation and has a rapid turnaround time. The reference DuPont identification database lists all the profiles of the reference strains for each species and is used for the comparative identification of each isolate. Eight ribogroups were identified by RiboPrinter® analysis.
The antimicrobial susceptibilities of the clinical isolates were investigated using a panel of 10 drugs. Almost all the strains were either resistant or multiresistant, particularly towards cefuroxime, methicillin, and ceftazidime. Methicillin resistance was probably because of the capacity of the bacteria to produce ß-lactamases. None of the strains was resistant to amikacin, vancomycin, and gatifloxacin. Other investigators have also reported similar antibiotic susceptibilities for clinical isolates of Bacillus spp (30-32). However, toxicity to retinal cells has been reported following amikacin use (33). Only six (21.43%) out of the 28 tested isolates were susceptible to ceftazidime. Previous studies have also reported a low prevalence of ceftazidime resistance among Bacillus spp (31). Twenty-seven (96.43%) out of the 28 tested isolates were sensitive to ciprofloxacin. In a similar study, the authors reported 97.1% inhibition of B. cereus by 1 µg/ml ciprofloxacin (34, 35). In another study, 15 out of 16 Bacillus spp isolates expressed high-level ciprofloxacin sensitivity. B. cereus is often resistant to all ß-lactams, and serious infections are best treated with vancomycin or clindamycin, with or without an aminoglycoside. All the strains tested were inhibited by gentamicin except for B. cereus KA17.6.1. Bacillus spp was susceptible to aminoglycosides. Our results are similar to those obtained by Citron and Appleman (34).
Studies on the antibiotic resistance of B. pumilus and B. mojevensis are limited, because the organism is not pathogenic to humans or animals. However, some recent studies have revealed that several Bacillus species including B. pumilus can cause infections, ranging from skin infections to life-threatening bacteremia in immunocompromised individuals (18). Thus, more studies need to be performed to understand the human health significance of B. pumilus, genetic basis of infections, and resistance to antimicrobials (19, 36).
Conclusion
Microorganisms on conjunctival flora may represent the source of infection in certain situations such as ocular surgery, malnutrition, and immunocompromise. Bacillus spp cultured from ocular tissues or fluids should not be dismissed as contaminants.
However, we believe that in a high-risk population, species identification may be useful in diagnosing recurrent bacteremia, as evidenced by isolation of the same species, as opposed to contamination resulting from two species. Moreover, our findings of five B. cereus and three B. pumilus infections suggest that these species may be more pathogenic than other common species, such as B. subtilis or B. megaterium, in immunosuppressed hosts.
We recommend culturing swabbed samples from diabetic patients to check for endophthalmitis. In culture-negative diabetic patients, the possibility of a Bacillus infection should be considered. Since a significant percentage of Bacillus isolates are penicillin resistant, we recommend that initial empiric antibiotic treatment of these infections consist of gatifloxacin, vancomycin, and amikacin.
Acknowledgment
The authors don’t have any conflict of interest.
References
- 1.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 2.Drobniewski FA. Bacillus cereus and related spcies. Clin Microbiol Rev. 1993;6:324–338. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granum PE, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol Lett. 1997;157:223–228. doi: 10.1111/j.1574-6968.1997.tb12776.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhagat N, Nagori S, Zarbin M. Post-traumatic infectious endophthalmitis. Surv Ophthalmol. 2011;56:214–249. doi: 10.1016/j.survophthal.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 5.O’Day DM, Smith RS, Gregg CR, Turnbull PC, Head WS, Ives JA, et al. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- 6.Ho PC, O’Day DM, Head WS. Fulminating panophthalmitis due to exogenous infection with Bacillus cereus: report of 4 cases. Br J Ophthalmol. 1982;66:205–208. doi: 10.1136/bjo.66.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David DB, Kirkby GR, Noble BA. Bacillus cereus endophthalmitis. Br J Ophthalmol. 1994;78:577–580. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das T, Choudhury K, Sharma S, Jalali S, Nuthethi R. The Endophthalmitis Research Group. Clinical profile and outcome in Bacillus endophthalmitis. Ophthalmology. 2001;108:1819–1825. doi: 10.1016/s0161-6420(01)00762-x. [DOI] [PubMed] [Google Scholar]
- 9.Das T, Kunimoto DY, Sharma S, Jalali S, Majji AB, Nagaraja RT, et al. Relationship between clinical presentation and visual outcome in postoperative and posttraumatic endophthalmitis in South Central India. Indian J Ophthalmol. 2005;53:5–16. doi: 10.4103/0301-4738.15298. [DOI] [PubMed] [Google Scholar]
- 10.Fritze D. Bacillus identification— traditional approaches. In: Berkeley R, Heyndrickx M, Logan N, De Vos P, editors. Applications and Systematics of Bacillus and Relatives. Blackwell Science Ltd a Blackwell Publishing company; 2002. [Google Scholar]
- 11.Callegan MC, Kane ST, Cochran DC, Ramadan RT, Chodosh J, McLean C, et al. Virulence factor profiles and antimicrobial susceptibilities of ocular Bacillus isolates. Curr Eye Res. 2006;31:693–702. doi: 10.1080/02713680600850963. [DOI] [PubMed] [Google Scholar]
- 12.Callegan MC, Gilmore MS, Gregory M, Ramadan RT, Wiskur BJ, Moyer AL, et al. Bacterial endophthalmitis: Therapeutic challenges and host–pathogen interactions. Prog Retin Eye Res. 2007;26:189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agaisse H, Gominet M, Okstad , OA , Kolsto AB, Lereclus D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol Microbiol . 1999;32:1043–1053. doi: 10.1046/j.1365-2958.1999.01419.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramadan R, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res . 2006;31:1–11. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 15.Callegan MC, Booth MC, Jett BD, Gilmore MS. Pathogenesis of gram-positive bacterial endophthalmitis. Infect Immun . 1999;67:3348–3356. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callegan MC, Engelbert M, Parke DW II, Jett BD, Gilmore MS. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002;15:111–124. doi: 10.1128/CMR.15.1.111-124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev . 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzol PA. Clinical features and therapeutic interventions in 17 cases of bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol. 1987;25:672–674. doi: 10.1128/jcm.25.4.672-674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tena D, Martinez-Torres JA, Perez-Pomata MT, Sáez-Nieto JA, Rubio V, Bisquert J. Cutaneous infection Due to Bacillus pumilus: report of 3 cases. Clin Infect Dis. 2007;44:40–42. doi: 10.1086/511077. [DOI] [PubMed] [Google Scholar]
- 20.From C, Pukall R, Schumann P, Hormazábal V, Granum PE. Toxin-producing ability among Bacillus spp outside the Bacillus cereus group. Appl Environ Microbiol. 2005;71:1178–1183. doi: 10.1128/AEM.71.3.1178-1183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacon CW, Hinton DM. Endophytic and biological control potential of Bacillus mojavensis and related species. Biol Control. 2002;23:274–284. [Google Scholar]
- 22.Claus D, Berkeley RCW. Genus Bacillus Cohn 1872, 174 AL. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey’s manual of systematic bacteriology. Baltimore, USA: Williams and Wilkins; 1986. pp. 1105–1138. [Google Scholar]
- 23.Priest FG, Goodfellow M, Todd C. A numerical classification of the genus Bacillus. J Gen Microbiol. 1988;134:1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- 24.PA: Wayne; CSLI– Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing-2007; document M100 S-17. Seventeenth information supplement. [Google Scholar]
- 25.Tekeli O, Tekeli A, Hoşal B, Özenci H, Gürsel E. Conjunctival flora of patients with diabetes mellitus. Med Network Oftalmol. 1997;4:246–248. [Google Scholar]
- 26.Bilen H, Ateş O, Satam N, Uslu H, Akcay G, Baykal O. Conjunctival flora in patients with Type 1 or Type 2 diabetes mellitus. Adv Ther. 2007;24:1028–1035. doi: 10.1007/BF02877708. [DOI] [PubMed] [Google Scholar]
- 27.Coşkun M, Altıntaş AGK, Simavlı H, Anayol MA, Toklu Y, Çelikbilek N, et al. Normal konjonktival floranın analizi ve florokinolonlar ile penisilin türevlerine karşı antibiyogram duyarlılığının incelenmesi. Glokom-Katarakt. 2007;2:167–170. [Google Scholar]
- 28.Choi SM, Park MH, Jung TS, Moon JH, Kim KM, Kang JS. Characterization of Bacillus mojavensis KJS-3 for industrial applications. Arch Pharm Res. 2011;34:289–298. doi: 10.1007/s12272-011-0215-z. [DOI] [PubMed] [Google Scholar]
- 29.Birinci H, Birinci A, Şahin M, Öge F, Öge İ. Comparison of conjunctival flora in diabetic patients using insulin and controls. Turk J Ophthalmol. 1998;28:144–146. [Google Scholar]
- 30.Andrews JM, Wise R. Susceptibility testing of Bacillus species. J Antimicrob Chemother. 2002;49:1039–1046. doi: 10.1093/jac/dkf063. [DOI] [PubMed] [Google Scholar]
- 31.Miller JJ, Scott IU, Flynn Jr HW, Smiddy WE, Murray TG, Berrocal A, et al. Endophthalmitis Caused by Bacillus Species. Am J Ophthalmol. 2008;145:883–888. doi: 10.1016/j.ajo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Fekete T. Bacillus species (not anthracis) Clin microbiol Newsletter. 2009;31:87–92. [Google Scholar]
- 33.Campochiaro PA, Conway BP. Aminogloycoside toxicity—a survey of retinal specialists. Implications for ocular use. Arch Ophthalmol. 1991;109:946–950. doi: 10.1001/archopht.1991.01080070058035. [DOI] [PubMed] [Google Scholar]
- 34.Citron DM, Appleman MD. In vitro activities of daptomycin, giprofloxacin, and other antimicrobial agents against the cells and spores of clinical isolates of Bacillus species. J Clin Microbiol. 2006;44:3814–3818. doi: 10.1128/JCM.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber DJ , Saviteer SM, Rutala WA, Thomann CA. In vitro susceptibility of Bacillus spp to selected antimicrobial agents. Antimicrob Agents Chemother. 1988;32:642–645. doi: 10.1128/aac.32.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Özkocaman V, Özcelik T, Ali R, Özkalemkas F, Özkan A, Özakin C, et al. Bacillus spp among hospitalized patients with haematological malignancies: clinical features, epidemics and outcomes. J Hosp Infect. 2006;64:169–176. doi: 10.1016/j.jhin.2006.05.014. [DOI] [PubMed] [Google Scholar]