Abstract
Recombinant proteins and cytokines are under broad preclinical and clinical investigation to promote angiogenesis, but their success is limited by ineffective delivery, lack of long-term stability, and excessive cost. Mesenchymal stem/stromal cells (MSC) secrete bioactive trophic factors, and thus, may provide an effective alternative to address these challenges. Glycine-Histidine-Lysine (GHK) is a peptide fragment of osteonectin (SPARC), a matricellular protein with reported proangiogenic potential. We examined the capacity of GHK to upregulate secretion of proangiogenic factors from human MSC in culture and when covalently coupled to alginate hydrogels. GHK had no apparent cytotoxic effects on MSC in culture over a wide range of concentrations. We detected a dose-dependent increase in vascular endothelial growth factor (VEGF) concentration in media conditioned by GHK-treated MSC, which increased endothelial cell proliferation, migration, and tubule formation. We covalently coupled GHK to alginate using carbodiimide chemistry, and human MSC were entrapped in alginate hydrogels to assess VEGF secretion. Similar to monolayer culture, MSC responded to GHK-modified gels by secreting increased concentrations of VEGF and basic fibroblast growth factor (bFGF) compared to unmodified gels. The pre-treatment of MSC with antibodies to α6 and β1 integrins prior to entrapment in GHK-modified gels abrogated VEGF secretion, suggesting that the proangiogenic response of MSC was integrin-mediated. These data demonstrate that the proangiogenic potential of MSC can be significantly increased by the presentation of GHK with a biodegradable carrier, therefore increasing their clinical potential when used for tissue repair.
INTRODUCTION
Angiogenesis is an essential process to support normal tissue growth and wound healing, with an end result of supplying critical nutrients and waste removal for metabolically active tissues. Angiogenesis is regulated by multiple factors, including proangiogenic growth factors and cytokines, proteolytic enzymes, extracellular matrix, and cell adhesion molecules [1]. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are potent mitogenic agents and stimulators of angiogenesis [2], which act by recruiting endothelial cells for capillary formation. The activity of endogenous angiogenic growth factors has been exploited therapeutically by the systemic and local delivery of recombinant proteins to stimulate the ingrowth of new blood vessels at target sites [3, 4]. However, the efficacy of this approach is limited by potential challenges related to systemic toxicity, a lack of site-specific delivery, and short half-lives requiring the administration of supraphysiological concentrations of each factor. In addition to their multilineage potential, mesenchymal stem/stromal cells (MSC) secrete a host of bioactive molecules including VEGF and bFGF [5], and thus, may provide an effective alternative to the delivery of recombinant angiogenic proteins. Multiple studies have utilized MSC to drive angiogenesis and restore collateral perfusion [6-10].
Alginate hydrogels are used in a wide range of biomedical applications including bulking agents for tissue defects, the delivery of inductive molecules, and the transplantation of cells into a defect site [11]. This biocompatible matrix is highly tailorable by controlling the molecular weight, composition, and presence of instructive signals on the backbone of the polymer [12, 13]. The hydrophilic nature of alginate requires the incorporation of proteins or peptides to promote cell adhesion and instruct cell phenotype. Arginine-Glycine-Aspartic Acid (RGD), a small fragment of the ubiquitous fibronectin protein that enables cell adhesion of many cell types and promotes osteogenic differentiation of cells for the osteoblastic lineage, has been widely studied when covalently linked to an alginate backbone [14-16]. Although other peptides and proteins have been incorporated in alginate gels [17, 18], this approach has predominantly been directed toward enhancing cell adhesion or instructing cell phenotype.
The human tripeptide Glycine-Histidine-Lysine (GHK) stimulates growth of several mammalian cell types [19] and is used extensively in cosmetic products [20]. The GHK peptide sequence is present in osteonectin (secreted protein acidic and rich in cysteine, SPARC), an extracellular matrix protein that regulates endothelial function during tissue repair [21]. Endogenous plasma levels of GHK are dependent on age, with concentrations of 200 ng/ml at 20 years old that decrease to 80 ng/ml by the age of 60 [22]. This is in agreement with decreased concentrations of other factors in the systemic circulation that drive neovascularization and tissue repair [23]. GHK has distinct biological actions such as increasing synthesis of collagen, elastin, and proangiogenic factors, suppression of inflammation, stem cell proliferation, and enhancement of many other wound healing processes [22, 24]. Keratinocytes and fibroblasts exhibit increased proliferation and trophic factor secretion when exposed to formulations containing this peptide [22, 25-27]. GHK has a high affinity for ionic copper, and several reports suggest that its therapeutic action is achieved upon formation of a GHK-Cu complex [22, 26, 27].
We hypothesized that localized presentation of GHK would enhance trophic factor secretion by MSC and that this signal could be covalently incorporated into alginate gels to drive production of these factors by entrapped cells. The objectives of this study were to determine the potential toxicity of GHK on MSC, perform a detailed functional analysis of GHK-induced trophic factor secretion, and demonstrate the successful incorporation of GHK into alginate gels for potential use as a transplantation vehicle for MSC.
MATERIALS AND METHODS
Cell culture
Human bone marrow-derived MSC were received at passage 2 (D. Prockop, Institute of Regenerative Medicine, Texas A&M University) and used without further characterization. Cells were expanded in minimum essential alpha medium (α-MEM, Invitrogen) containing 10% fetal bovine serum (FBS, JR Scientific) and 1% penicillin/streptomycin (P/S, Mediatech), and MSC were used at passage 4-6 for experimental studies.
Assessment of peptide cytotoxicity
MSC were seeded at 2.5×105 cells/cm2 in multi-well plates and allowed to adhere overnight. Media was refreshed with media supplemented with GHK peptide (G4GHKSP, Commonwealth Biotechnologies) at 1, 10, 100, or 500 ng/mL, together with ammonium tetrathiomolybdate (50 μg/mL, Acros Organics) to deplete endogenous copper from serum [26, 28]. Unless otherwise stated, all experiments were performed in the presence of CuCl2 (40 μM, Fisher Scientific) [29] to provide a more consistent concentration of copper than achievable with FBS. MSC were cultured for 3 days, and metabolic activity was measured using alamarBlue (AbD Serotec) according to the manufacturer’s instruction. Cells were collected in 1X passive lysis buffer (Promega), and protein concentration and DNA content were determined using the Micro BCA protein assay kit (Thermo Scientific) and Quant-iT Pico Green dsDNA assay kit (Invitrogen), respectively. Cell morphology was examined by brightfield microscopy on day 3 using a Nikon Eclipse TE2000-U and SpotRT digital camera. To determine apoptosis in GHK-exposed cells, MSC were collected in passive lysis buffer after incubation with GHK for 3 days, and caspase activity was quantified using the Caspase-Glo 3/7 luminescence assay (Promega). Luminescence was measured on a multifunctional plate reader (Synergy HTTR) and normalized to DNA content within each well [30].
Quantification of VEGF secretion by GHK-treated MSC
Conditioned media was collected from GHK-treated and untreated MSC. Media was refreshed 24 hours before collection on day 3, and the VEGF concentration in each media was measured using a Human VEGF Quantikine ELISA Kit (R&D Systems) as described by the manufacturer’s instructions and normalized to DNA.
Assessment of GHK enhancement of MSC proangiogenic potential
The proangiogenic potential of GHK-treated and untreated MSC was measured by determining the ability of conditioned media to stimulate endothelial cell proliferation, migration, and tubule formation. Human umbilical cord blood endothelial colony forming cells (ECFC, M. Yoder, Indiana University [31, 32]) were seeded at 7,500 cells/cm2 in a 24 well plate coated with rat tail collagen (50 μg/mL, BD Biosciences). Cells were maintained overnight in EGM-2 media (Lonza) supplemented with growth factors (SingleQuot), 10% FBS and 1% P/S and then washed with PBS to remove non-adherent cells. EGM-2 media was replaced with 400 μL of conditioned media collected from GHK-treated and untreated MSC and incubated for 72 hours. ECFC proliferation in response to media conditioned by GHK-treated MSC was also tested in the presence of a pan-VEGF antibody (1:100,000, ab1316, Abcam) by adding the antibody to the culture media for the 72 hour incubation period. After rinsing with PBS, ECFC were trypsinized and counted with a Z1 Dual Coulter Counter (Beckman Coulter).
Chemotaxis was measured using BD Falcon™ FluoroBlok™ transwell inserts for 24-well plates (3.0 μm pore size; BD Biosciences). Inserts coated with 0.1% bovine gelatin were placed into a 24-well plate containing conditioned media from GHK-treated or untreated MSC. ECFC (1×105 cells/well) were seeded on the transwell in 300 μL growth factor deficient EGM-2 (EGM lacking VEGF, bFGF, and IGF), and plates were incubated for 24 hours. Cells migrating through each transwell were stained with calcein (Invitrogen) and fluorescence was quantified using a microplate reader (Synergy HTTR) at 485/530 nm.
Tubulogenesis was determined by observing the capacity of ECFC to form tubules on Matrigel [32]. Briefly, ECFC were suspended in 500 μL of conditioned media, seeded on Matrigel (BD Biosciences) at 2×104 cells/cm2 and incubated for 24 hours in standard culture conditions. Tubule formation was observed by brightfield microscopy.
Covalent coupling of GHK onto alginate hydrogels
Peptides were covalently coupled to UltraPure MVG alginate (Pronova Biopolymers) with aqueous carbodiimide chemistry, purified, and sterilized as previously described for RGD incorporation [15, 16]. Hydrogels were formed with 22 μmoles of peptide per gram alginate. The alginate product was purified by dialysis against deionized water containing decreasing salt concentrations for five days, frozen, and lyophilized for 5-10 days until dry.
A 2.5% alginate solution was obtained by adding serum free α-MEM, and alginate gels were fabricated by the addition of calcium. MSC expressing green fluorescent protein (GFP-MSC, D. Prockop) were suspended in 800 μL of alginate solution and mixed with 160 μL α-MEM containing 40 μL of supersaturated CaSO4 solution (8.4 g of CaSO4 in 40 mL water). The solutions were mixed for 30 s using a 3-way stopcock to achieve a final alginate concentration of 2% with a final cell density of 5×106 cells/mL. The gel was cast between parallel glass plates with 2 mm thickness and incubated for 1 hour at 37°C. Hydrogel disks were cut out with an 8 mm biopsy punch and placed in α-MEM with 10% FBS. The presence and morphology of entrapped cells was determined using fluorescence microscopy. Conditioned media were collected on day 7 by refreshing the media 24 hours prior to collection. The content of conditioned media from MSC embedded in alginate gels at day 7 was examined using a RayBio® Human Angiogenesis Antibody Array G Series 1 (RayBiotech) according to the manufacturer’s instructions [10, 33], and detected signals were quantified by a gel documentation system. DNA content was measured after gel homogenization and quantified.
The capacity of GHK-modified alginate to support cell adhesion was examined using gel punch outs prepared as described above. 1×106 GFP-expressing MSC suspended in 30 μL α-MEM with 10% FBS were pipetted on the top of each disk and allowed to adhere for 5 hours in standard culture conditions, after which 2 mL of complete media were added to each well. After rinsing to remove any unattached cells, the presence of cells on the surface was determined using fluorescence microscopy with images collected at days 1 and 3 post-seeding, and fluorescence was quantified using a fluorescent plate reader (Synergy HTTR) at 485/530 nm.
Validation of covalent linking of peptide to alginate gel
Peptide bonding to alginate was confirmed by FTIR and NMR. The KBr disc method was adopted in IR. Briefly, the lyophilized sample and KBr powder were mixed (approximately 1:150 in weight) and ground manually in an agate mortar with a pestle before the disc was pressed. The IR spectra were collected using a Nicolet™ iS10 FT-IR (Thermo Fisher Scientific, USA) spectrometer at room temperature immediately after the preparation of the discs. Each spectrum was acquired by the accumulation of 36 scans at a resolution of 4 cm−1.
1H nuclear magnetic resonance (NMR) was performed on the dried sample to quantify complete GHK conjugation. High-resolution, 1H NMR spectra were taken on a Avance DRX 800 (Bruker) spectrometer. Deuterated water (D2O) was used as a solvent, and the sample concentrations were varied between 2.5 and 3 wt%. An accurately measured volume of the sample solution (550 μL) was transferred to a 5 mm NMR tube. 1H NMR spectra were recorded at 800 MHz using a NMR spectrometer (Bruker Avance 600). Typically, 50 scans were collected into 32,000 data points over a spectral width of 0–16 ppm with a relaxation delay of 1 s and an acquisition time of 1.7 s. The spectra were phased corrected and integrated automatically using TOPSPIN.
Proangiogenic potential of MSC entrapped within GHK-modified alginate
MSC were serum-starved (0.5% FBS) overnight and pretreated for 30 min with antibodies (10 μg/mL) specific to integrin α6 (sc-6597, Santa Cruz Biotechnology), integrin β1 (sc-9970, Santa Cruz Biotechnology), integrin α2β1 (sc-59955, Santa Cruz) or a non-specific isotype control antibody (ab81032, Abcam). Cells were then rinsed with PBS, trypsinized and suspended in 2% alginate gels at 5×106 cells/mL.
Hydrogel disks were formed with an 8 mm biopsy punch and placed in 24 well plates containing growth media (α-MEM containing 10% FBS and 1% P/S). Media were collected on day 7 after refreshing the media 24 hours prior to collection. Cell content within the gel was measured by quantifying DNA. VEGF concentration within conditioned media was quantified by ELISA.
Statistical analysis
Data are represented as mean and standard deviation unless otherwise stated. Statistical analysis was performed using GraphPad Prism 6.0, and statistical significance was determined by one-way ANOVA with Tukey’s post testing. P values less than 0.05 were considered statistically significant.
RESULTS
GHK peptide is not cytotoxic to MSC
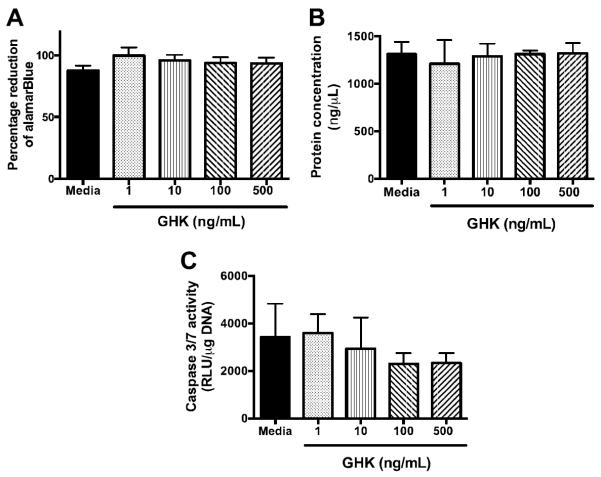
The potential cytotoxicity of the GHK peptide was assessed by quantifying changes in alamarBlue reduction, DNA content, and total protein. We did not detect differences in alamarBlue reduction as a function of peptide dosage compared to the MSC grown in untreated media (Fig. 1A). We observed consistent levels of intracellular protein for all conditions (Fig. 1B), and no statistically significant differences were detected in DNA content as a function of GHK dose (data not shown). We examined caspase activity as an early indicator of apoptosis to provide additional insight into potential effects of GHK on cellular viability. Similar to protein and DNA content, we did not observe significant increases in caspase activity as a function of GHK concentration (Fig. 1C). In fact, there was a trend for reduced caspase expression at higher GHK concentrations.
Figure 1.
Cell viability of MSC treated with increasing concentration of GHK for 3 days in monolayer culture: (A) metabolic activity assayed by percent reduction of alamarBlue; (B) protein concentration; (C) caspase 3/7 activity for n=4.
GHK induces bioactive VEGF secretion by MSC
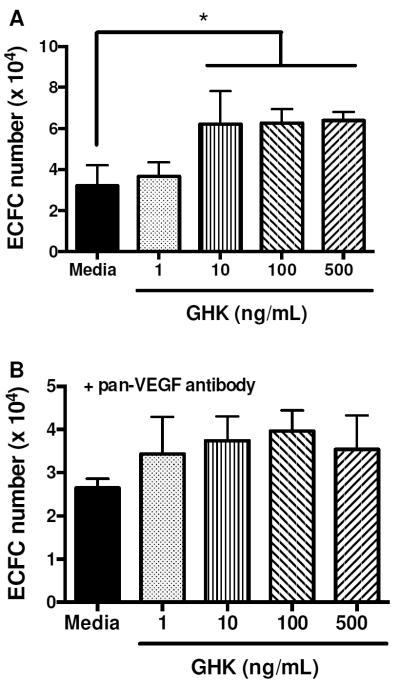
Compared to cells in untreated media, GHK-treated MSC secreted more VEGF in a dose dependent manner (Fig. 2). The mitogenic potential of the media collected from cells stimulated with GHK was examined by quantifying ECFC proliferation in response to conditioned media. We observed a trend for increased ECFC proliferation using conditioned media from MSC stimulated with higher GHK concentrations. Conditioned media from MSC treated with 10, 100 and 500 ng/mL GHK stimulated significantly greater ECFC proliferation compared to media from cells exposed to 1 ng/mL (Fig. 3A). The addition of conditioned media from GHK-treated cells at all dosages enhanced ECFC proliferation compared to untreated media alone. Upon the addition of a neutralizing VEGF antibody, statistical differences in ECFC proliferation were no longer detected, regardless of conditioned media content (Fig. 3B). GHK itself did not stimulate ECFC proliferation (data not shown).
Figure 2.
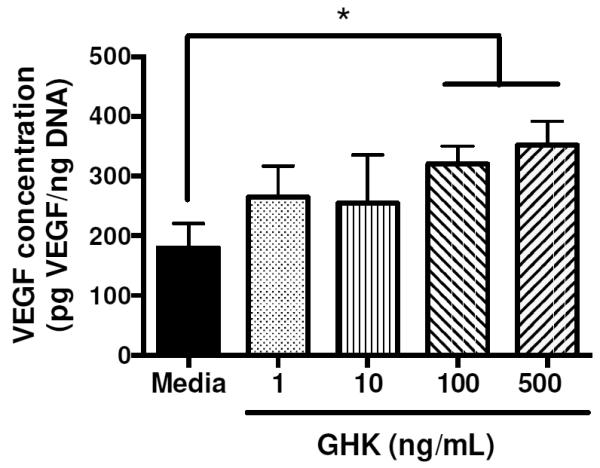
VEGF secretion in conditioned media from GHK-treated or untreated MSC at 3 days. *p<0.05 (n=3).
Figure 3.
Mitogenic activity of conditioned medium from GHK-treated MSC on ECFC. (A) ECFC proliferation exhibits a dose-dependent response to increasing GHK dosage on MSC. (B) ECFC proliferation is inhibited upon the addition of a pan-VEGF antibody. *p<0.05 (n=3).
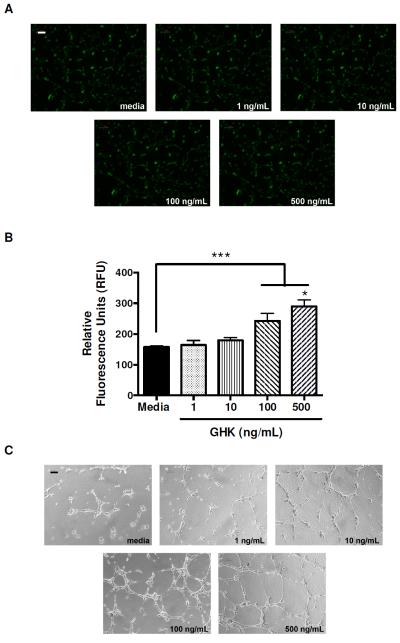
The proangiogenic potential of conditioned media from GHK-stimulated MSC was further examined by quantifying ECFC migration and tubule formation. Conditioned media from GHK-treated MSC acted as a chemoattractant for ECFC. We observed substantially more cells on the underside of the transwell after calcein staining (Fig. 4A), and fluorescence quantitation revealed a dose-dependent response of ECFC migration to conditioned media from GHK-stimulated MSC (Fig. 4B). ECFC adhered to the Matrigel surface and formed branching networks with multicentric junctions within 8 hours. The number of tube-like structures formed was visibly increased in the presence of conditioned media from MSC treated with higher GHK concentrations (Fig. 4C).
Figure 4.
ECFC migration and tubule formation in response to conditioned media from GHK-treated MSC. (A) Fluorescence microscopy images of ECFC that migrated through transwell. Images are taken at 40x magnification and are representative of three independent experiments. Scale bar represents 100 μm. (B) Quantification of fluorescence on underside of transwell following calcein staining of migrated ECFC. ***p<0.001 vs. media; *p<0.05 vs all other groups (n=3). (C) Phase contrast images of ECFC morphology on Matrigel reveal tubulogenesis as a function of GHK dose on MSC. Images are taken at 40x; scale bar represents 100 μm.
GHK can be covalently linked to alginate
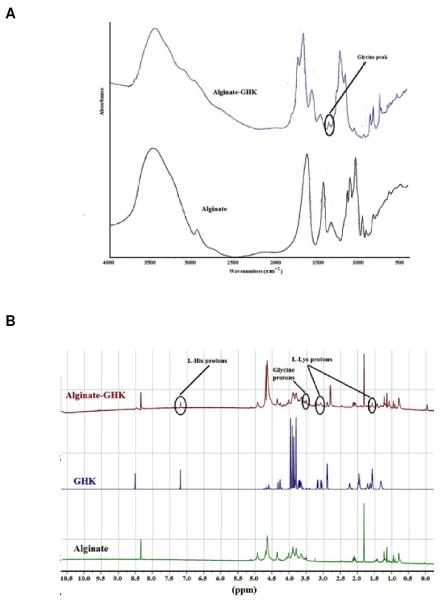
The tripeptide modification of alginate requires the successful formation of an amide bond. The secondary amide I band at 1700 cm−1 are noted in the alginate-GHK spectrum and are absent from the unmodified alginate (Fig. 5A). The modified alginate spectrum contains an amide II absorption band at 1567 cm −1, and a weak shoulder present at 1247 cm−1, which also corresponds to the amide II band. The C-N stretch for secondary amines is between 1350 cm−1 and 1280 cm−1 versus 1340 cm−1 through 1250 cm−1 for primary amines. The absorbance peak at 1340 cm−1 was attributed to glycine. The modified alginate spectrum contains a series of shoulders within this frequency range, one of which is assigned to the amide II band at 1250 cm−1, the remaining peaks may be a response of either the primary or secondary amines. The presence of a strong peak at 1600 cm−1 in both the alginate and GHK-modified alginate spectra indicate that this peak is assigned to the polymer’s structure and corresponds to the carboxylate functionality of alginate, which has two bands of absorption between 1650 cm−1 – 1550 cm−1 and at 1400 cm−1, both of which are present in the alginate and GHK-modified alginate spectra. However, the two shoulders flanking the 1600 cm−1 peak in the GHK-modified alginate spectrum are amide I and II bands. As there are no amides within unmodified alginate, it can be concluded that modification of the tripeptide to alginate did occur.
Figure 5.
Covalent linkage of GHK to alginate was confirmed. (A) Attenuated total reflection–Fourier transform infrared (ATR–FTIR) spectra of alginate and GHK modified alginate reveals unique peaks in GHK-modified alginate. (B) 1H NMR spectra of alginate, GHK peptide and GHK-modified alginate recorded at 800 MHz using D2O as a solvent.
By NMR analysis, we observed characteristic proton peaks of peptides at 3.2 and 1.6 ppm for lysine and the C-H ring protons from histidine at 7.2 ppm in the GHK-modified alginate 1H NMR spectrum (Fig. 5B). Absence of all these peaks in unmodified alginate confirms the GHK modification on the alginate backbone. A prominent singlet (with low peak intensity) at 3.55 ppm, which is assigned to glycine, also supported the GHK modification with alginate.
GHK peptide-modified alginate gels do not promote adhesion
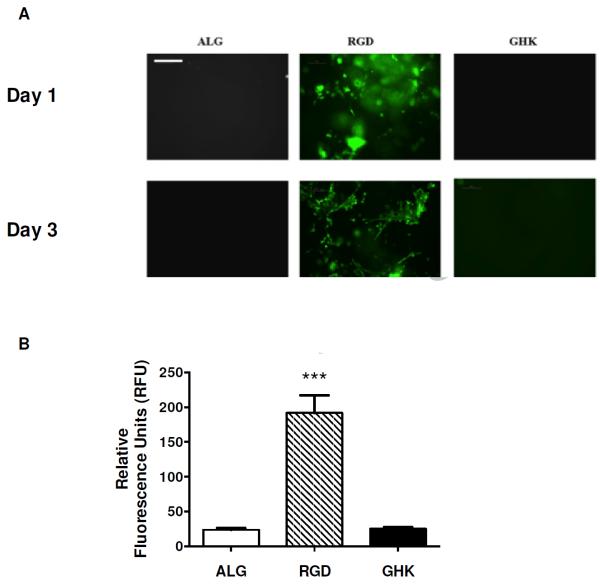
We determined the capacity of GHK to facilitate cell adhesion following covalent conjugation of the peptide to the hydrogel by culturing MSC on the gel surface for 3 days (Fig. 6A). As expected, MSC adhered and were elongated when cultured on the surface of RGD-modified alginate, while cells did not adhere to unmodified alginate hydrogels due to their hydrophilic nature [15]. Hydrogels containing the GHK peptide did not support MSC adhesion at 1 or 3 days. Quantification of visible fluorescence was in agreement with fluorescence microscopy, with maximum fluorescence values observed from MSC cultured on RGD-modified gels (Fig. 6B). There was no statistical difference in fluorescence values for MSC cultured on unmodified or GHK-modified alginate gels.
Figure 6.
MSC adhesion on peptide-modified alginate gels. (A) Fluorescence microscopy reveals that MSC did not attach on unmodified or GHK-modified alginate gels, but cells are visible and spread on RGD-modified gels after 1 and 3 days. Magnification is 100x; scale bar represents 200 μm. (B) Quantification of fluorescence from GFP-expressing MSC on gel surface after 3 days. ***p<0.001 vs. ALG or GHK (n=3).
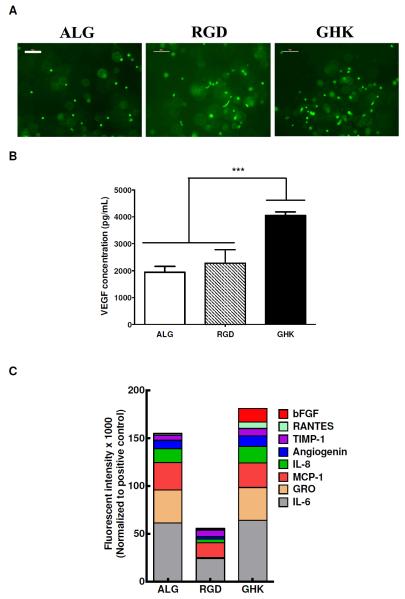
Additionally, we examined potential differences in morphology and cellular viability of MSC when entrapped in alginate hydrogels. Cells entrapped in unmodified alginate gels exhibited a rounded morphology, while MSC in GHK-modified alginate gels exhibited similar morphologies at day 7 (Fig. 7A). Many cells suspended in RGD-modified alginate gels exhibited an elongated cell shape at day 7. We did not observe statistically significant differences in DNA content for any gel formulation (data not shown).
Figure 7.
Cells survive and exhibit increased angiogenic factor secretion when entrapped in GHK-modified alginate gels. (A) Fluorescence microscopy reveals MSC distribution within gels after 7 days. Magnification is 100x; scale bar represents 100 μm. (B) GHK presentation from alginate gels increases VEGF secretion by entrapped MSC into conditioned media, as determined by ELISA. ***p<0.001 (n=3). (C) Stacked column chart of fluorescence (×1000) of predominant angiogenic cytokines, as determined from MSC conditioned media using an angiogenic protein array. Data are presented as median subtracted background fluorescence intensity and normalized to positive controls and excluding inherent serum levels for pooled conditioned media for n = 4.
GHK peptide-modified alginate gels enhance MSC trophic factor secretion
We quantified the concentration of cell-secreted VEGF and other factors in conditioned media of unmodified and peptide-modified hydrogels (Fig. 7B). MSC entrapped in RGD-modified gels did not exhibit appreciable increases in VEGF secretion compared to unmodified alginate. After 7 days, VEGF concentrations in conditioned media from cells in GHK-modified hydrogels were significantly higher than media from unmodified alginate gels. Using an angiogenic protein array, we determined that numerous other potent proangiogenic factors were upregulated by MSC when entrapped in GHK-modified hydrogels after 7 days in culture (Fig. 7C). Specifically, we detected significant increases in bFGF and RANTES (CCL5) for MSC in GHK-modified gels compared to RGD-modified or unmodified alginate. We measured similar levels of all other factors for conditioned media of MSC in GHK- or unmodified alginate, yet all were substantially higher than conditioned media from cells in RGD-modified gels.
Proangiogenic response of MSC to GHK-modified hydrogels is integrin mediated
Using VEGF secretion as the criterion, we assessed the potential contribution of integrins to probe the mechanism of GHK stimulation on MSC (Fig. 8). Blocking of α2β1 integrin, known to facilitate cell adhesion and spreading, reduced VEGF secretion compared to MSC in GHK gels, but not to the level of MSC in unmodified gels. In contrast, simultaneous treatment of MSC with α6 and β1 blocking antibodies abrogated VEGF secretion similar to that observed with unmodified alginate and significantly lower than MSC pretreated with antibody to α2β1.
Figure 8.
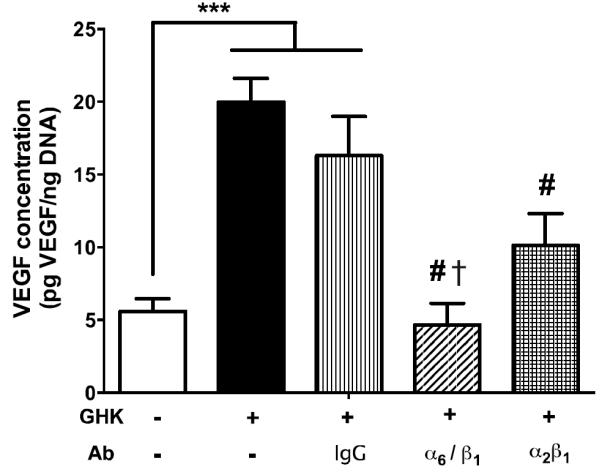
VEGF secretion stimulated by GHK is integrin-mediated. Blocking of integrin dimers suppressed VEGF secretion by MSC, with maximal suppression occurring for cells treated with α6 and β1 antibodies. VEGF secretion measured by ELISA using conditioned media from MSC entrapped in alginate hydrogels. ***p<0.001 vs. ALG. #p<0.001 vs. GHK. †p<0.05 vs. α2β1 (n=3).
DISCUSSION
The transplantation of stem/stromal cells into tissue defects represents a promising approach toward the revascularization of repair tissues. MSC secrete an endogenous cocktail of proangiogenic and anti-inflammatory factors that possess immense therapeutic potential [5], and methods to potentiate the secretion of proangiogenic cues have broad clinical applicability. A number of strategies are under investigation to enhance trophic factor secretion including genetic modification of cells to overexpress VEGF, culture of MSC in low oxygen, preconditioning of MSC in reduced oxygen during culture, and the presentation of phospholipids to stromal cells [9, 10, 30, 34, 35]. Herein, we presented an alternative biomaterials-based approach to increase secretion of bioactive proangiogenic cues (e.g., VEGF and bFGF) by the local presentation of GHK tripeptide from alginate hydrogels.
We examined the effect of GHK on cell survival over a range of concentrations in culture (1-500 ng/mL). We did not observe adverse effects on cellular proliferation, metabolic activity, or apoptosis over this broad range. Additionally, there were no visible changes in the cell morphology when treated with this peptide (data not shown). Together, these results demonstrate a lack of cytotoxicity associated with local presentation of this peptide to MSC. Furthermore, this particular peptide has been broadly utilized in the cosmetic industry for many years to promote skin repair and rejuvenation when used as a dermal cream [20, 22, 26].
Our initial studies in monolayer culture demonstrated that MSC secrete proangiogenic VEGF in a dose-dependent manner when exposed to GHK peptide in culture. These bioactive cues induced endothelial cell proliferation, migration, and tubule formation: three hallmarks of angiogenesis. These data are in good agreement with previous reports that this peptide augments fibroblast production of VEGF and bFGF [27].
In addition to its use in cosmetics, previous reports describe efforts to deliver GHK locally for stimulating bone repair [36, 37]. However, GHK was physically associated with collagen gels, thereby allowing the rapid diffusion away from the implant site. The local, sustained presentation of GHK to promote angiogenesis has not been reported. In these studies, our goal was to covalently attach the GHK peptide to the backbone of alginate to provide a sustained depot of this peptide for neighboring cells. We used RGD-modified alginate as a control for our chemistry, and we confirmed the successful chemical reaction for both RGD and GHK bonding using NMR. In nearly all studies, RGD-stimulated MSC secreted reduced concentrations of proangiogenic cues. The presentation of RGD peptide or RGD mimetics has been reported as to act as an antagonist to angiogenesis [38-40].
The covalent bonding of peptides to the backbone of polymers such as alginate or poly(ethylene glycol) facilitates changes in the cellular cytoskeleton upon integrin engagement with the ligand, causing desired changes in the genotype and phenotype of participating cells. Although adhesion is the primary function of the various integrins, they are also important signaling proteins [41]. We demonstrated that GHK does not support MSC adhesion when incorporated onto the alginate backbone, yet we still detected increased VEGF secretion compared to MSC in unmodified alginate gels. We observed partial reduction in VEGF secretion for MSC treated with α2β1 integrin-blocking antibodies, but a complete block of VEGF expression occurred with α6 and β1 integrin-specific antibodies. These data demonstrate that α6 or β1 (or both but individually) play an important role in regulating VEGF release from GHK-stimulated MSC. The α6β1 integrin is widely regarded as a receptor for the laminin family of extracellular matrix proteins. However, others have mapped SPARC’s β1-binding site to residues 113-130, a region that contains the GHK peptide sequence [42, 43]. These results suggest that SPARC may interact with β1 integrins and α6 integrins (known to engage type 1 collagen). The role of integrins on GHK-mediated angiogenesis merits further investigation.
CONCLUSIONS
These data demonstrate the utility of conjugating the proangiogenic peptide GHK to a biomaterial to potentiate MSC trophic factor secretion. Importantly, this strategy does not require additional culture time to enhance secretion of these cues, as is necessary for preconditioning paradigms [9, 44]. Furthermore, this approach boasts an improved safety profile compared to the prolonged ex vivo culture required to generate genetically modified cells that overexpress growth factors [34]. The safety of this pharmacological and materials-based approach is further supported by data demonstrating no appreciable cytotoxicity over a large dose range. This strategy effectively enhances the proangiogenic capacity of MSC and will have applications in wound healing, treatment of cardiovascular disease, and regenerative medicine.
Acknowledgements
This work was supported by the Department of the Army (W81XWH-10-1-0984) and the National Institutes of Health (NIH) Grant R21AG036963 to JKL. BYB was supported by the California Institute for Regenerative Medicine University of California, Davis Stem Cell Training Program (CIRM T1-00006, CIRM TG2-01163). We appreciate technical assistance from Dr. Jerry Dallas at the UC Davis NMR facility and NSF grant # DBIO722538.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–66. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 3.Ribatti D, Baiguera S. Phase II angiogenesis stimulators. Expert Opin Investig Drugs. 2013;22:1157–66. doi: 10.1517/13543784.2013.813933. [DOI] [PubMed] [Google Scholar]
- 4.Mitsos S, Katsanos K, Koletsis E, Kagadis GC, Anastasiou N, Diamantopoulos A, et al. Therapeutic angiogenesis for myocardial ischemia revisited: basic biological concepts and focus on latest clinical trials. Angiogenesis. 2012;15:1–22. doi: 10.1007/s10456-011-9240-2. [DOI] [PubMed] [Google Scholar]
- 5.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–5. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy GP, Ahsan T, O'Brien T, Barry F, Nerem RM. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng Part A. 2009;15:2459–70. doi: 10.1089/ten.TEA.2008.0341. [DOI] [PubMed] [Google Scholar]
- 7.He J, Decaris ML, Leach JK. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A. 2012;18:1520–8. doi: 10.1089/ten.tea.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capoccia BJ, Robson DL, Levac KD, Maxwell DJ, Hohm SA, Neelamkavil MJ, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–51. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2007–1104. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binder BY, Sondergaard CS, Nolta JA, Leach JK. Lysophosphatidic acid enhances stromal cell-directed angiogenesis. PLoS One. 2013;8:e82134. doi: 10.1371/journal.pone.0082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearney CJ, Mooney DJ. Macroscale delivery systems for molecular and cellular payloads. Nat Mater. 2013;12:1004–17. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 12.Alsberg E, Kong HJ, Hirano Y, Smith MK, Albeiruti A, Mooney DJ. Regulating bone formation via controlled scaffold degradation. J Dent Res. 2003;82:903–8. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 13.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 14.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci U S A. 2002;99:12025–30. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 16.Bhat A, Hoch AI, Decaris ML, Leach JK. Alginate hydrogels containing cell-interactive beads for bone formation. FASEB J. 2013;27:4844–52. doi: 10.1096/fj.12-213611. [DOI] [PubMed] [Google Scholar]
- 17.Rafat M, Rotenstein LS, Hu JL, Auguste DT. Engineered endothelial cell adhesion via VCAM1 and E-selectin antibody-presenting alginate hydrogels. Acta Biomater. 2012;8:2697–703. doi: 10.1016/j.actbio.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Tsur-Gang O, Ruvinov E, Landa N, Holbova R, Feinberg MS, Leor J, et al. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials. 2009;30:189–95. doi: 10.1016/j.biomaterials.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Pickart L, Freedman JH, Loker WJ, Peisach J, Perkins CM, Stenkamp RE, et al. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature. 1980;288:715–7. doi: 10.1038/288715a0. [DOI] [PubMed] [Google Scholar]
- 20.Mazurowska L, Mojski M. Biological activities of selected peptides: skin penetration ability of copper complexes with peptides. J Cosmet Sci. 2008;59:59–69. [PubMed] [Google Scholar]
- 21.Rivera LB, Bradshaw AD, Brekken RA. The regulatory function of SPARC in vascular biology. Cell Mol Life Sci. 2011;68:3165–73. doi: 10.1007/s00018-011-0781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickart L. The human tri-peptide GHK and tissue remodeling. Journal of biomaterials science. 2008;19:969–88. doi: 10.1163/156856208784909435. [DOI] [PubMed] [Google Scholar]
- 23.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 24.Wegrowski Y, Maquart FX, Borel JP. Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ Life Sci. 1992;51:1049–56. doi: 10.1016/0024-3205(92)90504-i. [DOI] [PubMed] [Google Scholar]
- 25.Huang PJ, Huang YC, Su MF, Yang TY, Huang JR, Jiang CP. In vitro observations on the influence of copper peptide aids for the LED photoirradiation of fibroblast collagen synthesis. Photomed Laser Surg. 2007;25:183–90. doi: 10.1089/pho.2007.2062. [DOI] [PubMed] [Google Scholar]
- 26.Maquart FX, Pickart L, Laurent M, Gillery P, Monboisse JC, Borel JP. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ FEBS Lett. 1988;238:343–6. doi: 10.1016/0014-5793(88)80509-x. [DOI] [PubMed] [Google Scholar]
- 27.Pollard JD, Quan S, Kang T, Koch RJ. Effects of copper tripeptide on the growth and expression of growth factors by normal and irradiated fibroblasts. Arch Facial Plast Surg. 2005;7:27–31. doi: 10.1001/archfaci.7.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Henry NL, Dunn R, Merjaver S, Pan Q, Pienta KJ, Brewer G, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71:168–75. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 29.Lowndes SA, Sheldon HV, Cai S, Taylor JM, Harris AL. Copper chelator ATN-224 inhibits endothelial function by multiple mechanisms. Microvas Res. 2009;77:314–26. doi: 10.1016/j.mvr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Binder BY, Genetos DC, Leach JK. Lysophosphatidic acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Eng Part A. 2013 Oct 16; doi: 10.1089/ten.tea.2013.0487. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decaris ML, Lee CI, Yoder MC, Tarantal AF, Leach JK. Influence of the oxygen microenvironment on the proangiogenic potential of human endothelial colony forming cells. Angiogenesis. 2009;12:303–11. doi: 10.1007/s10456-009-9152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29:1727–37. doi: 10.1002/stem.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang J-A, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 36.Pohunkova H, Stehlik J, Vachal J, Cech O, Adam M. Morphological features of bone healing under the effect of collagen-graft-glycosaminoglycan copolymer supplemented with the tripeptide Gly-His-Lys. Biomaterials. 1996;17:1567–74. doi: 10.1016/0142-9612(95)00310-x. [DOI] [PubMed] [Google Scholar]
- 37.Pohunkova H, Adam M. Reactivity and the fate of some composite bioimplants based on collagen in connective tissue. Biomaterials. 1995;16:67–71. doi: 10.1016/0142-9612(95)91098-j. [DOI] [PubMed] [Google Scholar]
- 38.Buerkle MA, Pahernik SA, Sutter A, Jonczyk A, Messmer K, Dellian M. Inhibition of the alpha-nu integrins with a cyclic RGD peptide impairs angiogenesis, growth and metastasis of solid tumours in vivo. Br J Cancer. 2002;86:788–95. doi: 10.1038/sj.bjc.6600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheu JR, Yen MH, Kan YC, Hung WC, Chang PT, Luk HN. Inhibition of angiogenesis in vitro and in vivo: comparison of the relative activities of triflavin, an Arg-Gly-Asp-containing peptide and anti-alpha(v)beta3 integrin monoclonal antibody. Biochim Biophys Acta. 1997;1336:445–54. doi: 10.1016/s0304-4165(97)00057-3. [DOI] [PubMed] [Google Scholar]
- 40.Trabocchi A, Menchi G, Cini N, Bianchini F, Raspanti S, Bottoncetti A, et al. Click-chemistry-derived triazole ligands of arginine-glycine-aspartate (RGD) integrins with a broad capacity to inhibit adhesion of melanoma cells and both in vitro and in vivo angiogenesis. J Med Chem. 2010;53:7119–28. doi: 10.1021/jm100754z. [DOI] [PubMed] [Google Scholar]
- 41.Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–45. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 42.Funk SE, Sage EH. Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J Cell Physiol. 1993;154:53–63. doi: 10.1002/jcp.1041540108. [DOI] [PubMed] [Google Scholar]
- 43.Lane TF, Iruela-Arispe ML, John RS, Sage EH. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol. 1994;125:929–43. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18:1545–52. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]