The first successful human-to-human blood transfusion was performed in the 1820s by British obstetrician James Blundell to treat a woman with post-partum hemorrhage (1). Although there had been more than 200 years of research into blood, relatively little was known at that time, and the identification of blood groups by Landsteiner was still several decades away. Animal-to-human blood transfusion had been attempted, but the failure of these experiments suspended any significant progress in the field for almost 150 years. In 1816, John Henry Leacock established the importance of species compatibility for successful transfusion (2). Blundell followed Leacock’s work, and when faced with a patient who was exsanguinating, he extracted blood from the patient’s husband and transfused it, saving the woman’s life.
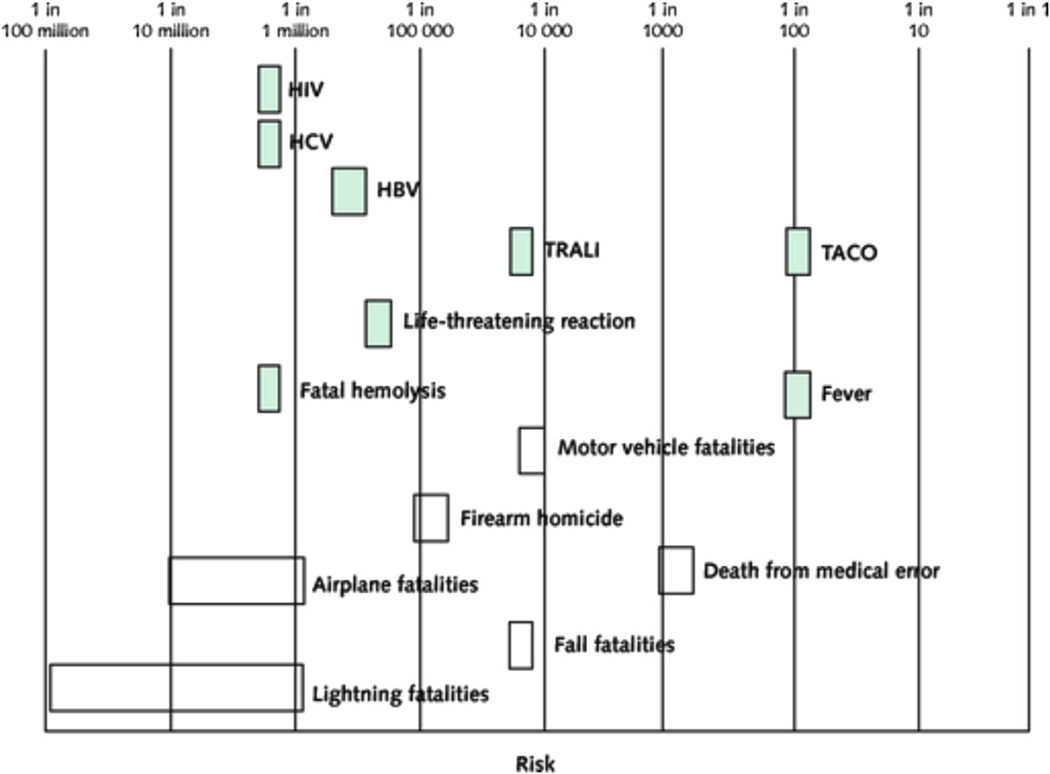
Since that first success in transfusion medicine, much progress has been made, particularly in the areas of blood compatibility and transfusion safety as it relates to the transmissibility of infectious agents. Risks associated with transfusion and the relative frequency of these events are shown in Figure 1. There has also been significant progress in determining the role of transfusion in children and adults with critical illness (3,4), as well as in patients with acute upper gastrointestinal hemorrhage (5). Randomized trials in these clinical settings have demonstrated that restrictive use of blood transfusion—defined in the trials as maintaining a hemoglobin value of 7 g/dl—is associated with either similar or better outcomes compared with liberal transfusion, defined as maintaining a hemoglobin value of 9 g/dl or higher. It is becoming clear that in many scenarios, a restrictive transfusion strategy is preferred. The one exception is in anemic patients with ischemic heart disease, including acute coronary syndrome (ACS), for whom guidelines indicate that the role of blood transfusion is not known (6). This is due to the lack of adequately powered randomized trial data supporting one strategy over the other.
Figure 1. Adverse Effects of RBC Transfusion Contrasted With Other Risks.
Risk is depicted on a logarithmic scale. Shaded bars represent the risk per red blood cell (RBC) unit transfused, and unshaded bars represent the risk for fatality per person per year for various life events. Reprinted, with permission, from Carson et al. (6).
Observational studies examining the association between red blood cell transfusion and outcomes in patients with ACS have shown either no benefit or an increased risk for mortality with transfusion above a nadir hematocrit of 24% (hemoglobin of 8 g/dl) (7,8) or a baseline hematocrit of 33% (9). In this issue of the Journal, Silvain et al. (10) provide some potential mechanistic insights into these findings. In a cross-sectional, observational, prospective study, they examined the association between red blood cell transfusion and measures of platelet reactivity using multiple assays, including vasodilator-stimulated phosphoprotein–1, thrombin receptor–activated peptide–1, and adenosine diphosphate–induced light transmission aggregometry. The population studied was diverse, including patients with ACS, patients with congestive heart failure, those receiving dual-antiplatelet therapy, as well as patients not receiving antiplatelet therapy. Silvain et al. found that transfusion was associated with a modest but significant increase in measures of platelet reactivity, which occurred in the presence of no change in inflammatory biomarkers. The increase in platelet reactivity was most robust in patients with ACS previously taking P2Y12 inhibitors, and there did not seem to be a strong relationship between the duration of red blood cell storage and the effect of platelet reactivity.
These new findings should be considered in the context of the known role of platelet activation and aggregation in the pathophysiology of ACS and ACS-related sequelae (11). Increased platelet reactivity has been described as a risk factor for adverse outcomes in patients with ACS and those undergoing percutaneous coronary intervention (12). The increased platelet reactivity induced by blood transfusion could explain the association between transfusion and the increased risk for recurrent myocardial infarction (MI) seen in the observational studies (7). In light of these mechanistic data, do the reported results further the case for withholding transfusion in patients with ACS?
To answer this question, it is important to review what is known about anemia, transfusion, and outcomes in the ACS population. As mentioned previously, the observational data show an association between transfusion above a hemoglobin level of 8 g/dl and either no effect on mortality or an increased risk for mortality. In contrast, anemia is an independent predictor of mortality in patients with ACS (13). Because ACS is a state in which the myocardium is deprived of oxygen, anemia could further exacerbate myocardial ischemia in patients with coronary stenoses (14). Increasing hemoglobin through transfusion should increase oxygen delivery and mitigate myocardial ischemia, but experimental studies have indicated no increase in tissue oxygenation with transfusion (15). This may be due to chemical changes in red cells that occur during storage (the so-called storage lesion), such as depletion of 2,3-diphosphoglycerate and nitric oxide, that diminish the ability of transfusion to deliver oxygen (16). The study of Silvain et al. (10) adds another potential mechanism by which transfusion may be harmful; however, some limitations of their study should be noted.
First, the effect of transfusion on platelet function may be transient, and platelet function may return to normal after a certain period of time. Thus, post-transfusion platelet function may not necessarily affect 30-day or longer term outcomes. Second, all of the patients in the study of Silvain et al. (10) were anemic, and there may be an interaction among the degree of anemia, the robustness of hemoglobin correction with transfusion, and platelet function. In other words, there may be a gradient of the effect on platelet function across patients with ACS on the basis of their anemia that was not explored in the study. Third, in a randomized trial comparing transfusion strategies among patients with acute upper gastrointestinal bleeding, patients assigned to liberal transfusion had a higher rate of recurrent hemorrhage (5), suggesting that other effects of transfusion on hemostatic mechanisms may offset increases in platelet reactivity. Finally, long-term clinical outcomes were not measured.
It is also important to review the limitations of the published data on transfusion and clinical outcomes in patients with ACS. As mentioned previously, the data are derived predominantly from observational analyses, and observational assessment of a therapy for which the indication for the treatment may itself be associated with adverse outcomes leads to “confounding by indication” that is unlikely to be overcome regardless of the statistical technique used (17). Only 2 small, randomized trials of red blood cell transfusion in patients with acute MI have been published (18,19). The CRIT Randomized Pilot Trial assigned 45 patients with MI and hematocrit ≤ 30% within 72 h of admission to a liberal versus a conservative transfusion strategy: maintaining hematocrit of 30% to 33% versus 24% to 27% (18). Although the trial was not powered for a specific end point, patients assigned to liberal transfusion had a significantly higher rate of the composite end point of in-hospital death, recurrent MI, or congestive heart failure compared with patients assigned to the restrictive arm (38% vs. 13%, p = 0.046). This is countered by the Myocardial Ischemia and Transfusion trial, which randomized 110 patients with ACS or stable angina undergoing percutaneous coronary intervention with hemoglobin values < 10 g/dl to either a liberal (maintaining hemoglobin ≥ 10 g/dl) or conservative (transfusion for symptoms or hemoglobin < 8 g/dl) transfusion strategy (19). In the liberal transfusion group, there was statistically insignificant lower rate of 30-day death, MI, or unscheduled revascularization, which was the primary end point, and a significantly lower rate of 30-day mortality. In addition, a subgroup analysis of the Transfusion Requirement in Critical Care trial also showed a trend toward reduced 30-day mortality among patients with cardiovascular disease assigned to the liberal transfusion strategy (20). These conflicting clinical trial data from studies using different populations, designs, and end points explain the inability of the guidelines to make any recommendation on the role of transfusion in this high-risk patient population.
They also underscore the urgent need for a definitive randomized trial of transfusion strategies in patients with ACS. Large registries indicate that up to 10% of patients with ACS receive blood transfusions during their hospitalizations, translating into about 130,000 patients with ACS receiving transfusions annually. Given the lack of data in this population, it is impossible to know how many of these transfusions are inappropriate. To date, there remains equipoise in the field, with no strong evidence for or against red blood cell transfusion in patients with ACS.
In this context, the biases of physicians caring for patients with ACS run deep. A survey of Canadian physicians performed before the landmark Transfusion Requirement in Critical Care trial of transfusion strategies in critically ill patients (3) showed that the majority believed that the appropriate transfusion “trigger” for patients with acute ischemic heart disease was a hemoglobin level of 10 g/dl (hematocrit of 30%) (21). That is, such patients should receive transfusions if their hemoglobin levels fall below 10 g/dl. In contrast, European practice guidelines for patients with unstable angina or non–ST-segment elevation MI published more recently have recommended that transfusion be withheld unless a patient is symptomatic from anemia or the hemoglobin level is less than 8 g/dl (22). Ostensibly, these recommendations are based on the published observational data, which have the limitations outlined earlier. This disparity crystallizes the design of a definitive randomized trial of transfusion strategies in ACS. Such a trial should be adequately powered to determine whether a maintaining a hemoglobin level of 8 or 10 g/dl reduces major adverse cardiac events in patients with ACS. Given the impact of “bleeding-avoidance strategies” on decreasing bleeding and transfusion rates after percutaneous coronary intervention (23), the trial will necessarily enroll patients with multiple comorbidities, including frailty, who are likely much sicker and at higher risk for adverse outcomes than the patients enrolled in prior ACS trials. Although such a study may be challenging to undertake, pilot studies have shown that a transfusion strategy trial is feasible in this setting (18,19).
It has been almost 200 years since the first landmark event in transfusion medicine. The time for determining the role of blood transfusion in patients with ACS—patients who are at the highest risk for bleeding and stand to benefit the most from strategies that mitigate cardiac ischemia—is now.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Ellis H. James Blundell, pioneer of blood transfusion. Br J Hosp Med. 2007;68:447. doi: 10.12968/hmed.2007.68.8.24500. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt PJ, Leacock AG. Forgotten transfusion history: John Leacock of Barbados. BMJ. 2002;325:1485–1487. doi: 10.1136/bmj.325.7378.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebert PC, Wells G, Blajchman MA, et al. for the Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 4.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Medicine. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 7.Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 8.Alexander KP, Chen AY, Wang TY, et al. Transfusion practice and outcomes in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008;155:1047–1053. doi: 10.1016/j.ahj.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 10.Silvain J, Abtan J, Kerneis M, et al. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2013.11.029. 00:000–000. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 12.Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–1223. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- 14.Hebert PC, Hu LQ, Biro GP. Review of physiologic mechanisms in response to anemia. CMAJ. 1997;156:S27–S40. [Google Scholar]
- 15.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–1634. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 16.Rao SV, Califf RM. Is old blood bad blood? Am Heart J. 2010;159:710–712. doi: 10.1016/j.ahj.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Hebert PC, Fergusson DA. Do transfusions get to the heart of the matter? JAMA. 2004;292:1610–1612. doi: 10.1001/jama.292.13.1610. [DOI] [PubMed] [Google Scholar]
- 18.Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study) Am J Cardiol. 2011;108:1108–1111. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–971. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Hebert PC, Wells G, Martin C, et al. Variation in red cell transfusion practice in the intensive care unit: a multicentre cohort study. Crit Care. 1999;3:57–63. doi: 10.1186/cc310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 23.Subherwal S, Peterson ED, Dai D, et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: a report from the National Cardiovascular Data CathPCI Registry. J Am Coll Cardiol. 2012;59:1861–1869. doi: 10.1016/j.jacc.2011.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]