Abstract
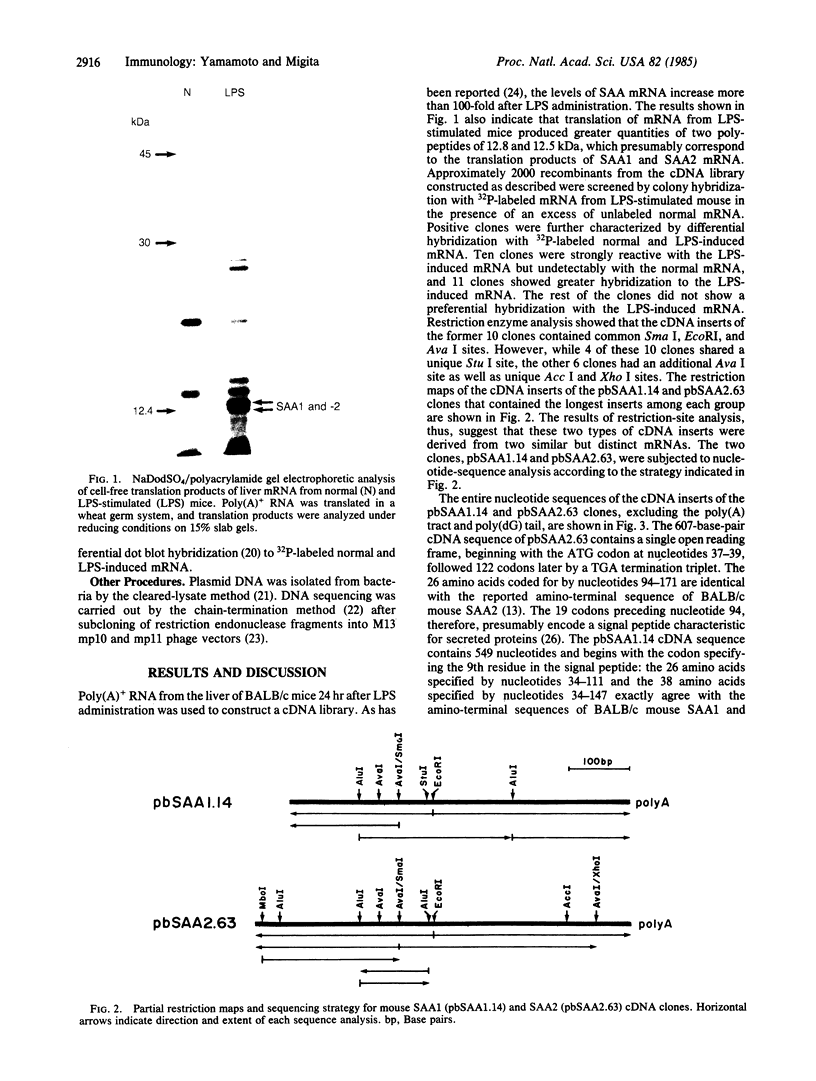
cDNA clones encoding two major mouse serum amyloid A proteins, SAA1 and SAA2, were isolated from a liver cDNA library of the lipopolysaccharide-stimulated BALB/c mouse, and their nucleotide sequences were determined. The insert of the SAA2 cDNA clone contained 607 nucleotides with a 5' untranslated region of 36 nucleotides, a signal peptide region corresponding to 19 amino acids, a mature protein region corresponding to 103 amino acids, and a 3' untranslated region of 202 nucleotides. The SAA1 cDNA insert contained 549 nucleotides specifying a part of a signal peptide region, a mature protein region, and a 3' untranslated region. A comparison of the nucleotide and deduced amino acid sequences of SAA1 cDNA with that of SAA2 cDNA showed a high degree of homology: 95% nucleotide sequence homology in the coding region (91% amino acid sequence homology) and 90% homology in the 3' untranslated region. One of nine amino acid differences between SAA1 and SAA2 predicted from the cDNA sequences was located in a putative proteolytic cleavage site for amyloid A protein formation: SAA2 had the Thr-Met sequence in this site, while SAA1 had the Thr-Ile sequence. This suggests that SAA1, which does not deposit as amyloid A protein, is also potentially susceptible to putative proteolytic enzymes. In addition, as compared with mouse SAA2, human SAA1, monkey and mink amyloid A protein, mouse SAA1 had two unique substitutions, which may play a role in differential deposition of mouse SAA isotypes in amyloid tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Natvig J. B., Sletten K., Husby G., Nordstoga K. Amyloid-related serum protein SAA from three animal species: comparison with human SAA. J Immunol. 1977 Jan;118(1):229–234. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausserman L. L., Herbert P. N., McAdam K. P. Heterogeneity of human serum amyloid A proteins. J Exp Med. 1980 Sep 1;152(3):641–656. doi: 10.1084/jem.152.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6860–6864. doi: 10.1073/pnas.77.11.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Greenwald M., Frangione B., Pras M., Franklin E. C. The amino acid sequence of duck amyloid A (AA) protein. J Immunol. 1977 Mar;118(3):1113–1118. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Hoffman J. S., Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. J Exp Med. 1984 Feb 1;159(2):641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., McCandliss R., Gross M., Sloma A., Familletti P. C., Tabor J. M., Evinger M., Levy W. P., Pestka S. Construction and identification of bacterial plasmids containing nucleotide sequence for human leukocyte interferon. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7010–7013. doi: 10.1073/pnas.77.12.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Stearman R. S., Peltzman C. G., Potter D. A. Induction of hepatic synthesis of serum amyloid A protein and actin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee D. C., Titani K., Ericsson L. H., Eriksen N., Benditt E. P., Walsh K. A. Amino acid sequence of amyloid-related apoprotein (apoSAA1) from human high-density lipoprotein. Biochemistry. 1982 Jul 6;21(14):3298–3303. doi: 10.1021/bi00257a008. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C., Frangione B., Greenspan J. Isolation and partial characterization of SAA-an amyloid-related protein from human serum. J Immunol. 1976 May;116(5):1415–1418. [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975 Apr;55(4):746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Feldmann R. J. Amphipathic helixes and plasma lipoproteins: a computer study. Biopolymers. 1977 Sep;16(9):2053–2065. doi: 10.1002/bip.1977.360160916. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Pownall H. J., Jackson R. L., Glenner G. G., Pollock P. S. Amyloid A: amphipathic helixes and lipid binding. Biochemistry. 1976 Jul 27;15(15):3187–3191. doi: 10.1021/bi00660a005. [DOI] [PubMed] [Google Scholar]
- Sletten K., Husby G. The complete amino-acid sequence of non-immunoglobulin amyloid fibril protein AS in rheumatoid arthritis. Eur J Biochem. 1974 Jan 3;41(1):117–125. doi: 10.1111/j.1432-1033.1974.tb03251.x. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., McAdam K. P., Ulevitch R. J. Interactions of bacterial lipopolysaccharide with acute-phase rabbit serum and isolation of two forms of rabbit serum amyloid A. J Immunol. 1982 Mar;128(3):1420–1427. [PubMed] [Google Scholar]
- Waalen K., Sletten K., Husby G., Nordstoga K. The primary structure of amyloid fibril protein AA in endotoxin-induced amyloidosis of the mink. Eur J Biochem. 1980 Mar;104(2):407–412. doi: 10.1111/j.1432-1033.1980.tb04441.x. [DOI] [PubMed] [Google Scholar]